Surface Modification of Flax Yarns by Enzymatic Treatment and Their Interfacial Adhesion with Thermoset Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Biochemical Enzymatic Treatment
2.3. Characterization of Wetting Properties
2.4. Tensile Characterization of Flax Yarns
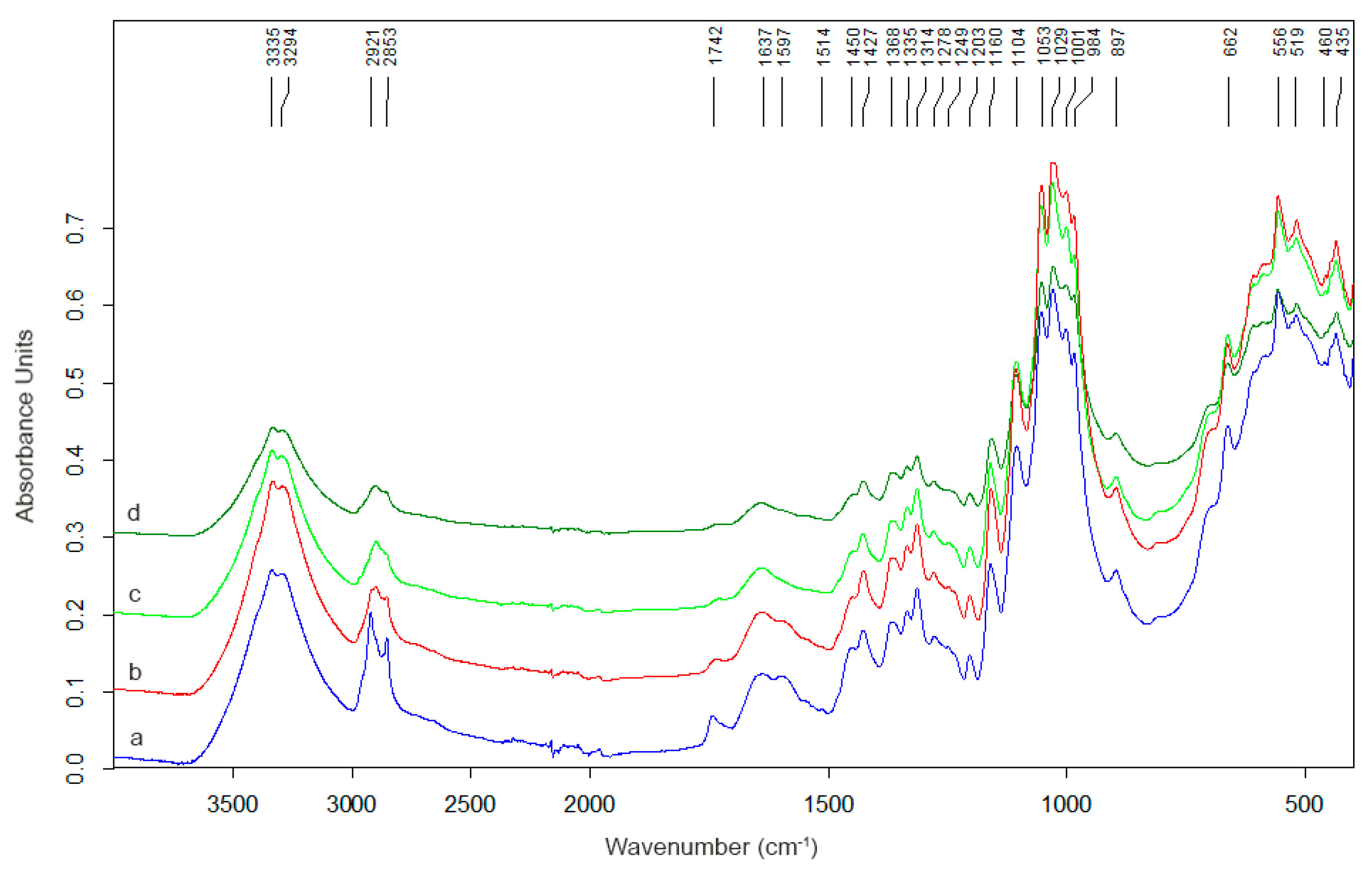
2.5. Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
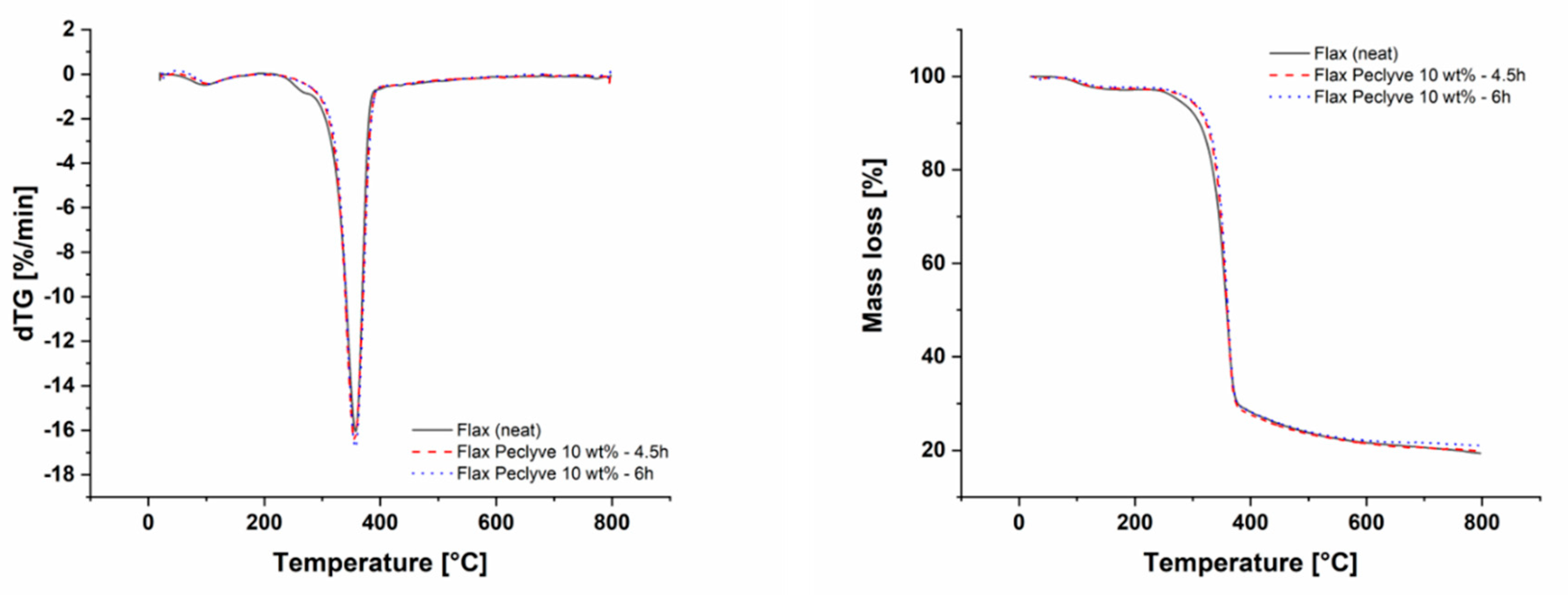
2.6. Thermogravimetric Analysis (TGA)
2.7. Single Yarn Fragmentation Test
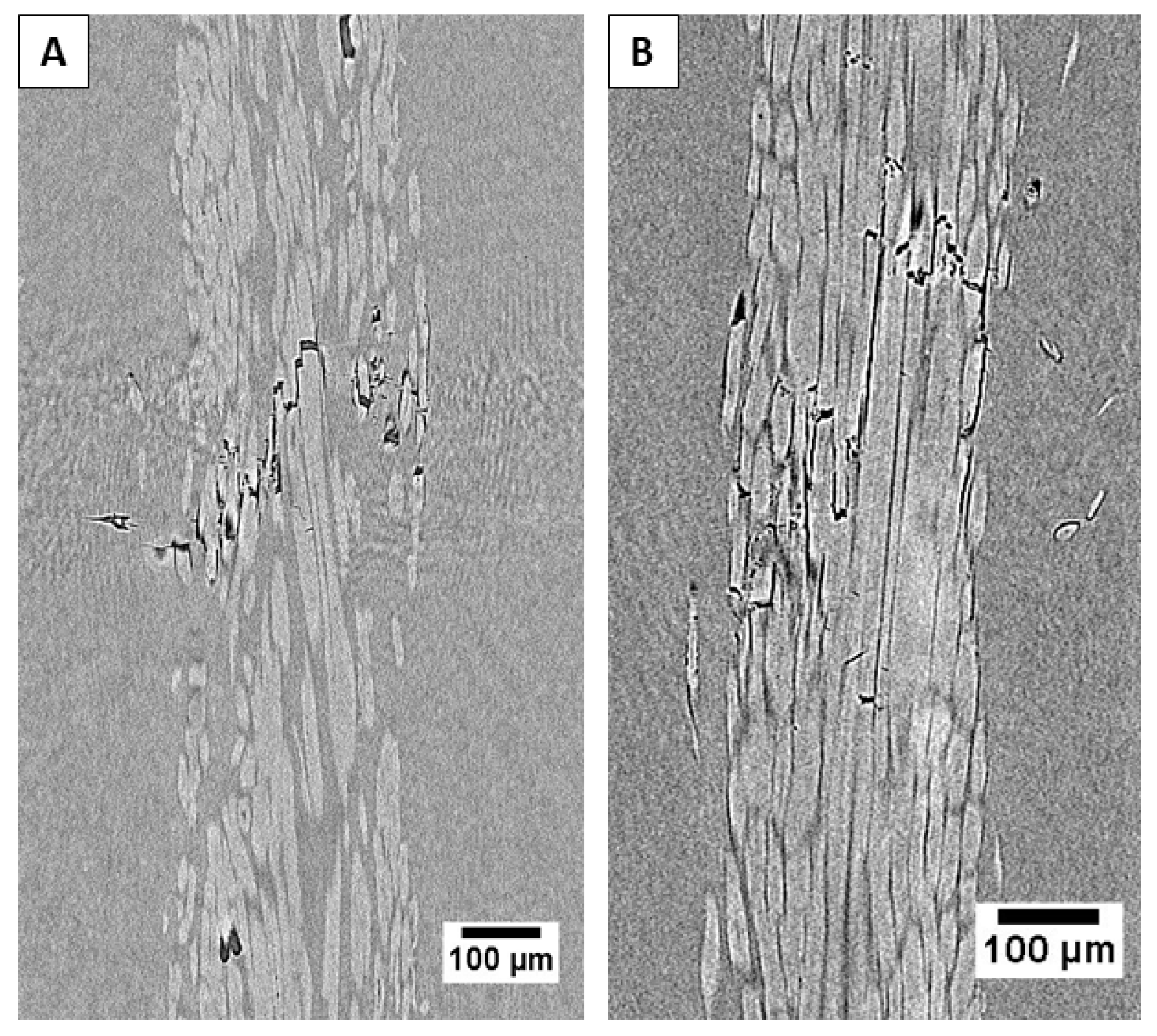
2.8. X-Ray Microtomography
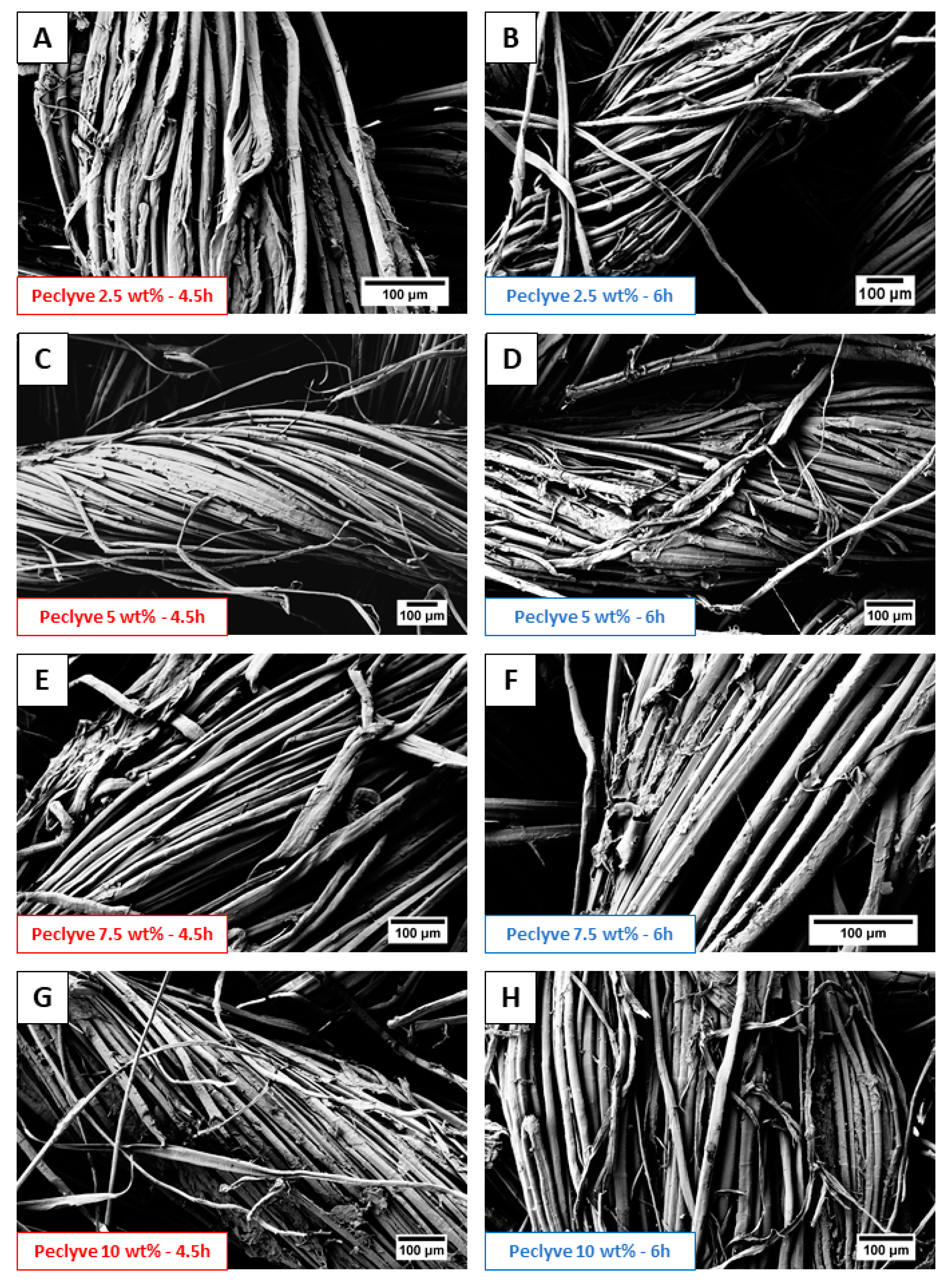
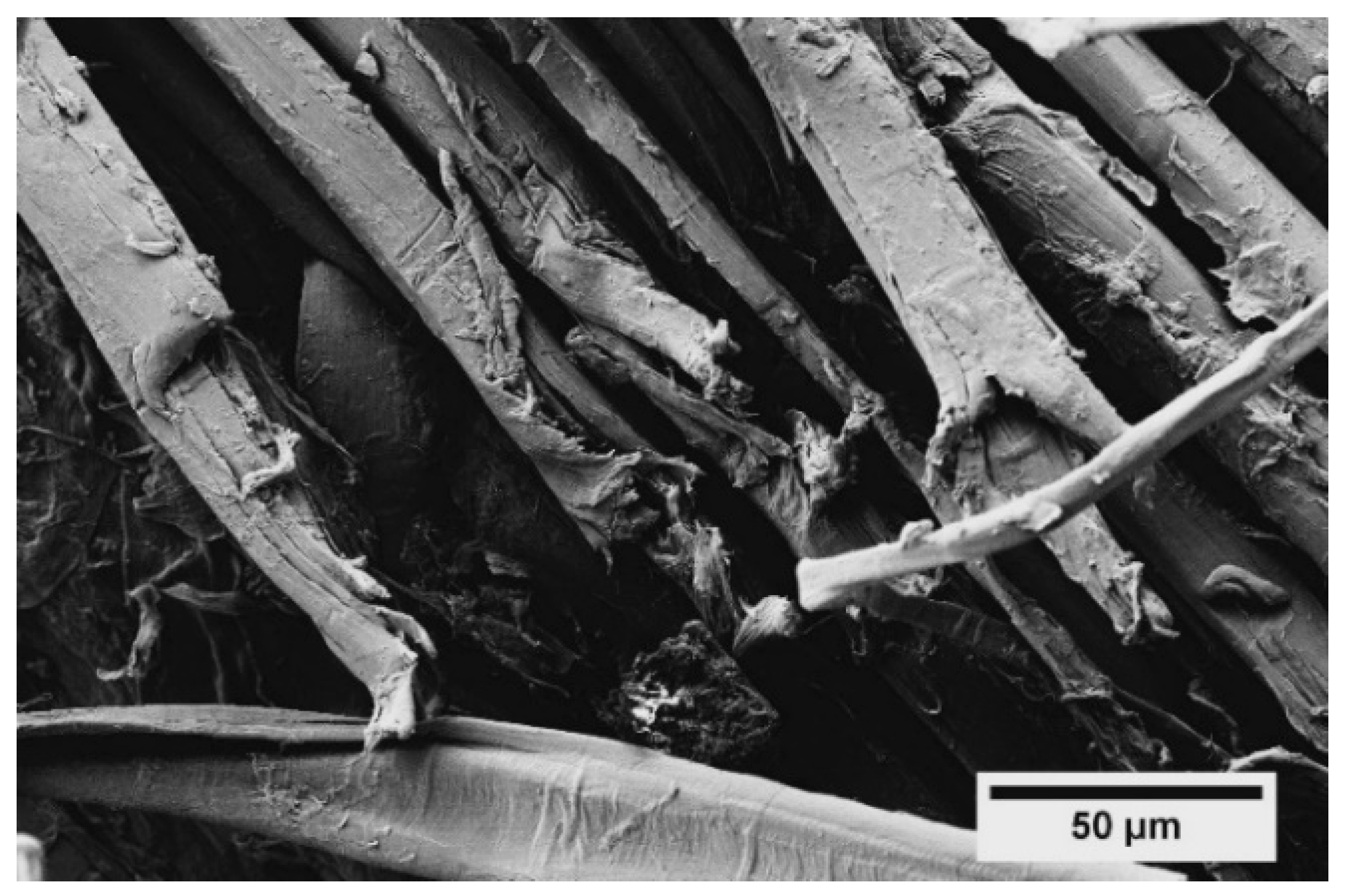
2.9. Optical and FE-SEM Observations
3. Results and Discussion
3.1. Characterization of Flax Yarns After Enzymatic Treatment
3.2. Fibre/Matrix Interfacial Assessment by Single Yarn Fragmentation Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bourmaud, A.; Beaugrand, J.; Shah, D.U.; Placet, V.; Baley, C. Towards the design of high-performance plant fibre composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef]
- Bledzki, A.; Mamun, A.; Lucka-Gabor, M.; Gutowski, V. The effects of acetylation on properties of flax fibre and its polypropylene composites. Express Polym. Lett. 2008, 2, 413–422. [Google Scholar] [CrossRef]
- Huo, S.; Thapa, A.; Ulven, C.A. Effect of surface treatments on interfacial properties of flax fiber-reinforced composites. Adv. Compos. Mater. 2013, 22, 109–121. [Google Scholar] [CrossRef]
- Amiri, A.; Ulven, C.; Huo, S. Effect of chemical treatment of flax fiber and resin manipulation on service life of their composites using time-temperature superposition. Polymers 2015, 7, 1965–1978. [Google Scholar] [CrossRef]
- Tserki, V.; Zafeiropoulos, N.E.; Simon, F.; Panayiotou, C. A study of the effect of acetylation and propionylation surface treatments on natural fibres. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1110–1118. [Google Scholar] [CrossRef]
- Bozaci, E.; Sever, K.; Sarikanat, M.; Seki, Y.; Demir, A.; Ozdogan, E.; Tavman, I. Effects of the atmospheric plasma treatments on surface and mechanical properties of flax fiber and adhesion between fiber-matrix for composite materials. Compos. Part B Eng. 2013, 45, 565–572. [Google Scholar] [CrossRef]
- Seghini, M.C.; Touchard, F.; Sarasini, F.; Chocinski-Arnault, L.; Tirillò, J.; Bracciale, M.P.; Zvonek, M.; Cech, V. Effects of oxygen and tetravinylsilane plasma treatments on mechanical and interfacial properties of flax yarns in thermoset matrix composites. Cellulose 2019, 1–20. [Google Scholar] [CrossRef]
- Yuan, X.; Jayaraman, K.; Bhattacharyya, D. Plasma treatment of sisal fibres and its effects on tensile strength and interfacial bonding. J. Adhes. Sci. Technol. 2002, 16, 703–727. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Erkens, L.; Fangueiro, R.; Souto, A.P. Surface modification of banana fibers by DBD plasma treatment. Plasma Chem. Plasma Process. 2012, 32, 259–273. [Google Scholar] [CrossRef]
- Yuan, X.; Jayaraman, K.; Bhattacharyya, D. Effects of plasma treatment in enhancing the performance of woodfibre-polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1363–1374. [Google Scholar] [CrossRef]
- Wang, W.; Xian, G.; Li, H. Surface modification of ramie fibers with silanized CNTs through a simple spray-coating method. Cellulose 2019, 26, 8165–8178. [Google Scholar] [CrossRef]
- Sarker, F.; Karim, N.; Afroj, S.; Koncherry, V.; Novoselov, K.S.; Potluri, P. High-performance graphene-based natural fiber composites. ACS Appl. Mater. Interfaces 2018, 10, 34502–34512. [Google Scholar] [CrossRef]
- Wang, H.; Xian, G.; Li, H. Grafting of nano-TiO2 onto flax fibers and the enhancement of the mechanical properties of the flax fiber and flax fiber/epoxy composite. Compos. Part A Appl. Sci. Manuf. 2015, 76, 172–180. [Google Scholar] [CrossRef]
- Foruzanmehr, M.; Vuillaume, P.Y.; Elkoun, S.; Robert, M. Physical and mechanical properties of PLA composites reinforced by TiO2 grafted flax fibers. Mater. Des. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Pickering, K.L.; Li, Y.; Farrell, R.; Lay, M. Interfacial Modification of Hemp Fiber Reinforced Composites Using Fungal and Alkali Treatment. J. Biobased Mater. Bioenergy 2007, 1, 109–117. [Google Scholar]
- Gulati, D.; Sain, M. Fungal-modification of natural fibers: A novel method of treating natural fibers for composite reinforcement. J. Polym. Environ. 2006, 14, 347–352. [Google Scholar] [CrossRef]
- Schirp, A.; Loge, F.; Aust, S.; Swaner, P.; Turner, G.; Wolcott, M. Production and characterization of natural fiber-reinforced thermoplastic composites using wheat straw modified with the fungusPleurotus ostreatus. J. Appl. Polym. Sci. 2006, 102, 5191–5201. [Google Scholar] [CrossRef]
- Gustavsson, M.T.; Persson, P.V.; Iversen, T.; Martinelle, M.; Hult, K.; Teeri, T.T.; Brumer, H. Modification of cellulose fiber surfaces by use of a lipase and a xyloglucan endotransglycosylase. Biomacromolecules 2005, 6, 196–203. [Google Scholar] [CrossRef]
- George, M.; Mussone, P.G.; Alemaskin, K.; Chae, M.; Wolodko, J.; Bressler, D.C. Enzymatically treated natural fibres as reinforcing agents for biocomposite material: Mechanical, thermal, and moisture absorption characterization. J. Mater. Sci. 2016, 51, 2677–2686. [Google Scholar] [CrossRef]
- George, M.; Mussone, P.G.; Bressler, D.C. Surface and thermal characterization of natural fibres treated with enzymes. Ind. Crops Prod. 2014, 53, 365–373. [Google Scholar] [CrossRef]
- Francois, C.; Placet, V.; Beaugrand, J.; Pourchet, S.; Boni, G.; Champion, D.; Fontaine, S.; Plasseraud, L. Can supercritical carbon dioxide be suitable for the green pretreatment of plant fibres dedicated to composite applications? J. Mater. Sci. 2020, 55, 4671–4684. [Google Scholar] [CrossRef]
- Seghini, M.C.; Touchard, F.; Chocinski-Arnault, L.; Placet, V.; François, C.; Plasseraud, L.; Bracciale, M.P.; Tirillò, J.; Sarasini, F. Environmentally friendly surface modification treatment of flax fibers by supercritical carbon dioxide. Molecules 2020, 25, 438. [Google Scholar] [CrossRef] [PubMed]
- De Prez, J.; Van Vuure, A.W.; Ivens, J.; Aerts, G.; Van de Voorde, I. Effect of enzymatic treatment of flax on fineness of fibers and mechanical performance of composites. Compos. Part A Appl. Sci. Manuf. 2019, 123, 190–199. [Google Scholar] [CrossRef]
- De Prez, J.; Van Vuure, A.W.; Ivens, J.; Aerts, G.; Van de Voorde, I. Flax treatment with strategic enzyme combinations: Effect on chemical fiber composition and ease of fiber extraction. Biotechnol. Rep. 2019, 23, e00358. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Meyer, A.S.; Fernando, D.; Silva, D.A.S.; Daniel, G.; Thygesen, A. Effect of pectin and hemicellulose removal from hemp fibres on the mechanical properties of unidirectional hemp/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 724–735. [Google Scholar] [CrossRef]
- De Prez, J.; Van Vuure, A.W.; Ivens, J.; Aerts, G.; Van de Voorde, I. Enzymatic treatment of flax for use in composites. Biotechnol. Rep. 2018, 20, e00294. [Google Scholar] [CrossRef]
- Stuart, T.; Liu, Q.; Hughes, M.; McCall, R.D.; Sharma, H.S.S.; Norton, A. Structural biocomposites from flax-Part I: Effect of bio-technical fibre modification on composite properties. Compos. Part A Appl. Sci. Manuf. 2006, 37, 393–404. [Google Scholar] [CrossRef]
- Li, Y.; Pickering, K.L. Hemp fibre reinforced composites using chelator and enzyme treatments. Compos. Sci. Technol. 2008, 68, 3293–3298. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Jaszkiewicz, A.; Erdmann, K. Polypropylene composites with enzyme modified abaca fibre. Compos. Sci. Technol. 2010, 70, 854–860. [Google Scholar] [CrossRef]
- Seghini, M.C.; Touchard, F.; Sarasini, F.; Chocinski-Arnault, L.; Mellier, D.; Tirillò, J. Interfacial adhesion assessment in flax/epoxy and in flax/vinylester composites by single yarn fragmentation test: Correlation with micro-CT analysis. Compos. Part A Appl. Sci. Manuf. 2018, 113, 66–75. [Google Scholar] [CrossRef]
- Joffe, R.; Andersons, J.; Wallström, L. Interfacial shear strength of flax fiber/thermoset polymers estimated by fiber fragmentation tests. Proc. J. Mater. Sci. 2005, 40, 2721–2722. [Google Scholar] [CrossRef]
- Kelly, A.; Tyson, W.R. Tensile properties of fibre-reinforced metals: Copper/tungsten and copper/molybdenum. J. Mech. Phys. Solids 1965, 13, 329–350. [Google Scholar] [CrossRef]
- Boisset, C.; Petrequin, C.; Chanzy, H.; Henrissat, B.; Schulein, M. Optimized mixtures of recombinant Humicola insolens cellulases for the biodegradation of crystalline cellulose. Biotechnol. Bioeng. 2001, 72, 339–345. [Google Scholar] [CrossRef]
- De Prez, J.; Van Vuure, A.W.; Ivens, J.; Aerts, G.; Van de Voorde, I. Flax treatment with strategic enzyme combinations: Effect on fiber fineness and mechanical properties of composites. J. Reinf. Plast. Compos. 2020, 39, 231–245. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Konczewicz, W.; Zimniewska, M.; Al-Deyab, S.S.; El-Newehy, M.H. Enhancing hydrophilicity of bioscoured flax fabric by emulsification post-treatment. Carbohydr. Polym. 2010, 82, 195–201. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, X.; Gao, W.; Chen, J. Characterization of bioscoured cotton fabrics using FT-IR ATR spectroscopy and microscopy techniques. Carbohydr. Res. 2006, 341, 2170–2175. [Google Scholar] [CrossRef]
- Haque, M.M.; Hasan, M.; Islam, M.S.; Ali, M.E. Physico-mechanical properties of chemically treated palm and coir fiber reinforced polypropylene composites. Bioresour. Technol. 2009, 100, 4903–4906. [Google Scholar] [CrossRef]
- Abidi, N.; Manike, M. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2018, 88, 719–730. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Wang, Z.; Zheng, C.; Dong, X.; Su, Z.; Sun, P.; Wang, D.; Han, C.C.; Xu, D. Morphology and mechanical behavior of isotactic polypropylene (iPP)/syndiotactic polypropylene (sPP) blends and fibers. Polymer (Guildf.) 2005, 46, 5956–5965. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tajvidi, M.; Takemura, A. Effect of fiber content and type, compatibilizer, and heating rate on thermogravimetric properties of natural fiber high density polyethylene composites. Polym. Compos. 2009, 30, 1226–1233. [Google Scholar] [CrossRef]
- Van de Velde, K.; Baetens, E. Thermal and mechanical properties of flax fibres as potential composite reinforcement. Macromol. Mater. Eng. 2001, 286, 342–349. [Google Scholar] [CrossRef]
- Bateman, D.F.; Basham, H.G. Degradation of Plant Cell Walls and Membranes by Microbial Enzymes. In Physiological Plant Pathology; Springer: Berlin/Heidelberg, Germany, 1976; pp. 316–355. [Google Scholar]
- Brebu, M.; Vasile, C. Thermal degradation of lignin-a review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Saleem, Z.; Rennebaum, H.; Pudel, F.; Grimm, E. Treating bast fibres with pectinase improves mechanical characteristics of reinforced thermoplastic composites. Compos. Sci. Technol. 2008, 68, 471–476. [Google Scholar] [CrossRef]
- Gassan, J.; Bledzki, A.K. Alkali treatment of jute fibers: Relationship between structure and mechanical properties. J. Appl. Polym. Sci. 1999, 71, 623–629. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Effect of various chemical treatments on the fibre structure and tensile properties of industrial hemp fibres. Compos. Part A Appl. Sci. Manuf. 2011, 42, 888–895. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Mechanical properties of alkali treated plant fibres and their potential as reinforcement materials II. Sisal fibres. J. Mater. Sci. 2006, 41, 2497–2508. [Google Scholar] [CrossRef]
- Fraser, W.A.; Ancker, F.H.; Dibenedetto, A.T.; Elbirli, B. Evaluation of surface treatments for fibers in composite materials. Polym. Compos. 1983, 4, 238–248. [Google Scholar] [CrossRef]
- Kim, B.W.; Nairn, J.A. Observations of fiber fracture and interfacial debonding phenomena using the fragmentation test in single fiber composites. J. Compos. Mater. 2002, 36, 1825–1858. [Google Scholar] [CrossRef]
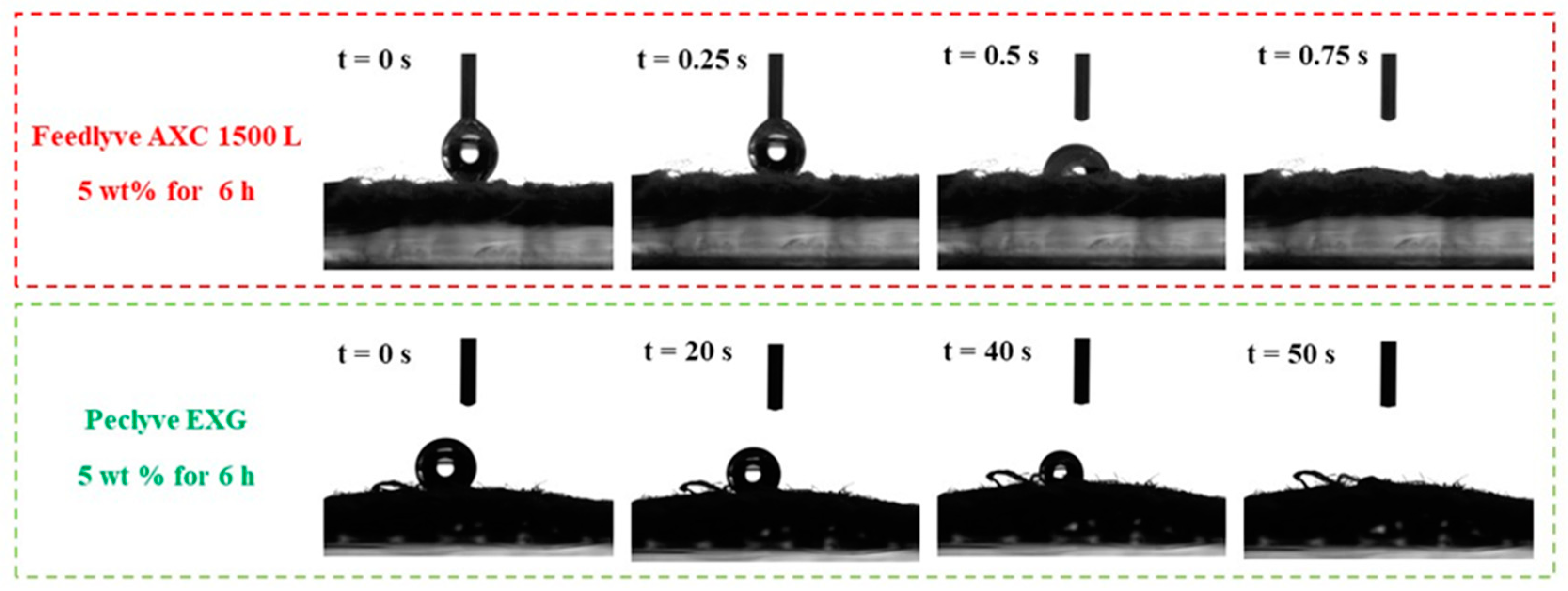


| Enzyme Solution | Biological Source | Main Activities | Enzyme Load [wt %] | Time [h] | T [°C] | pH |
|---|---|---|---|---|---|---|
| Feedlyve AXC 1500 L | Trichoderma longibrachiatum | Endo 1,4-β xylanase | 0.5–2.5–5 | 2–3–6 | 50 | 5.5 |
| Peclyve EXG | Aspergillus niger | Pectinase Xyloglucanase | 0.5–2.5–5–7.5–10 | 2–3–4.5–6 | 50 | 5 |
| Flax Fabric | Absorption Time t [s] | Absorption Time t after 2 h Treatment [s] | Absorption Time t after 3 h Treatment [s] | Absorption Time t after 6 h Treatment [s] |
|---|---|---|---|---|
| Neat | T < 1 | - | - | - |
| Peclyve 0.5 wt % | - | 1 | 1.2 | 5.5 |
| Peclyve 2.5 wt % | - | 1.4 | 2.2 | 1.5 |
| Peclyve 5 wt % | - | 1.5 | 35 | 50 |
| Feedlyve 0.5 wt % | - | t < 2 | t < 1 | 1 |
| Feedlyve 2.5 wt % | - | t < 2 | 1 | t < 1 |
| Feedlyve 5 wt % | - | 1 | t < 1 | t < 1 |
| Flax Yarn | Treatment Time | Cellulose Crystallinity index (CI) |
|---|---|---|
| Neat | - | 1.01 |
| Peclyve 2.5 wt % | 3 h | 1 |
| Peclyve 2.5 wt % | 4.5 h | 1.15 |
| Peclyve 2.5 wt % | 6 h | 1.24 |
| Peclyve 10 wt % | 3 h | 1.36 |
| Peclyve 10 wt % | 4.5 h | 1.54 |
| Peclyve 10 wt % | 6 h | 1.54 |
| Maximum Degradation Temperature [°C] | Temperature of 10% Weight Loss [°C] | |
|---|---|---|
| Flax yarn (neat) | 357.2 | 311.3 |
| Peclyve 10 wt %-4.5 h | 355.7 | 322.8 |
| Peclyve 10 wt %-6 h | 356.7 | 325.5 |
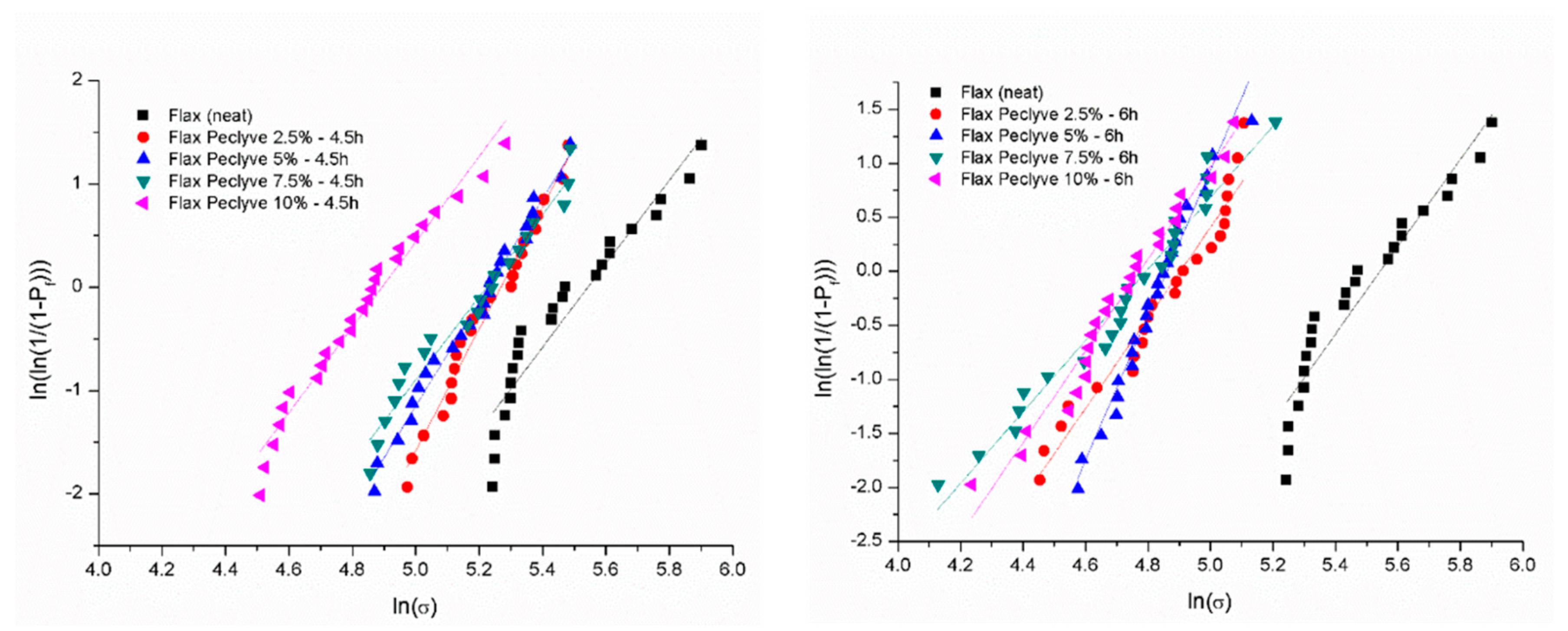
| Flax Yarn | Fmax [N] | Diameter [µm] | σf [MPa] | εf [%] | Characteristic Strength σ0 [MPa] | Weibull Modulus m |
|---|---|---|---|---|---|---|
| Neat | 19.8 ± 4.8 | 327 ± 95 | 236 ± 57 | 3.4 ± 0.42 | 257.4 | 5 |
| Peclyve 2.5 wt %-4.5 h | 20.2 ± 3.5 | 376 ± 69 | 181 ± 31 | 3.55 ± 0.49 | 194 | 6.8 |
| Peclyve 5 wt %-4.5 h | 16.9 ± 3.6 | 353 ± 59 | 172 ± 36 | 2.99 ± 0.47 | 186 | 5.5 |
| Peclyve 7.5 wt %-4.5 h | 16.2 ± 4 | 348 ± 77 | 170 ± 41 | 2.95 ± 0.41 | 186 | 4.8 |
| Peclyve 10 wt %-4.5 h | 14.8 ± 3.5 | 390 ± 78 | 123 ± 29 | 3.04 ± 0.47 | 134 | 5 |
| Peclyve 2.5 wt %-6 h | 14.1 ± 3.4 | 382 ± 61 | 123 ± 29 | 3.03 ± 0.59 | 134 | 4.6 |
| Peclyve 5 wt %-6 h | 13.7 ± 2.1 | 379 ± 63 | 121 ± 19 | 3.19 ± 0.55 | 129 | 7.6 |
| Peclyve 7.5 wt %-6 h | 11.7 ± 3.6 | 370 ± 74 | 108 ± 33 | 2.93 ± 0.6 | 120 | 3.5 |
| Peclyve 10 wt %-6 h | 11.1 ± 2.8 | 362 ± 63 | 107 ± 27 | 3.02 ± 0.54 | 118 | 4.3 |
| Matrix | Flax Yarn | lc [µm] | ldebonding [µm] |
|---|---|---|---|
| Neat | 2687 ± 631 | 444 ± 49 | |
| Epoxy | Peclyve 5 wt %-4.5 h | 2628 ± 531 | 353 ± 89 |
| Peclyve 10 wt %-6 h | 2511 ± 203 | 365 ± 46 | |
| Neat | 3942 ± 807 | 830 ± 343 | |
| Vinylester | Peclyve 5 wt %-4.5 h | 3962 ± 243 | 628 ± 128 |
| Peclyve 10 wt %-6 h | 3554 ± 776 | 529 ± 65 |
| Matrix | Flax Yarn | σf(lc) [MPa] | IFSS [MPa] |
|---|---|---|---|
| Neat | 406 ± 18 | 19.3 ± 3.7 | |
| Epoxy | Peclyve 5 wt %, 4.5 h | 283 ± 10 | 18.1 ± 2.7 |
| Peclyve 10 wt %, 6 h | 203 ± 3 | 12.2 ± 1.1 | |
| Neat | 376 ± 15 | 13.9 ± 2.8 | |
| Vinylester | Peclyve 5 wt %, 4.5 h | 262 ± 2 | 10. 5 ± 1.4 |
| Peclyve 10 wt %, 6 h | 188 ± 9 | 7.8 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seghini, M.C.; Tirillò, J.; Bracciale, M.P.; Touchard, F.; Chocinski-Arnault, L.; Zuorro, A.; Lavecchia, R.; Sarasini, F. Surface Modification of Flax Yarns by Enzymatic Treatment and Their Interfacial Adhesion with Thermoset Matrices. Appl. Sci. 2020, 10, 2910. https://doi.org/10.3390/app10082910
Seghini MC, Tirillò J, Bracciale MP, Touchard F, Chocinski-Arnault L, Zuorro A, Lavecchia R, Sarasini F. Surface Modification of Flax Yarns by Enzymatic Treatment and Their Interfacial Adhesion with Thermoset Matrices. Applied Sciences. 2020; 10(8):2910. https://doi.org/10.3390/app10082910
Chicago/Turabian StyleSeghini, Maria Carolina, Jacopo Tirillò, Maria Paola Bracciale, Fabienne Touchard, Laurence Chocinski-Arnault, Antonio Zuorro, Roberto Lavecchia, and Fabrizio Sarasini. 2020. "Surface Modification of Flax Yarns by Enzymatic Treatment and Their Interfacial Adhesion with Thermoset Matrices" Applied Sciences 10, no. 8: 2910. https://doi.org/10.3390/app10082910
APA StyleSeghini, M. C., Tirillò, J., Bracciale, M. P., Touchard, F., Chocinski-Arnault, L., Zuorro, A., Lavecchia, R., & Sarasini, F. (2020). Surface Modification of Flax Yarns by Enzymatic Treatment and Their Interfacial Adhesion with Thermoset Matrices. Applied Sciences, 10(8), 2910. https://doi.org/10.3390/app10082910








