Synthesis of Molecularly Imprinted Polymer via Emulsion Polymerization for Application in Solanesol Separation
Abstract
Featured Application
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Instruments
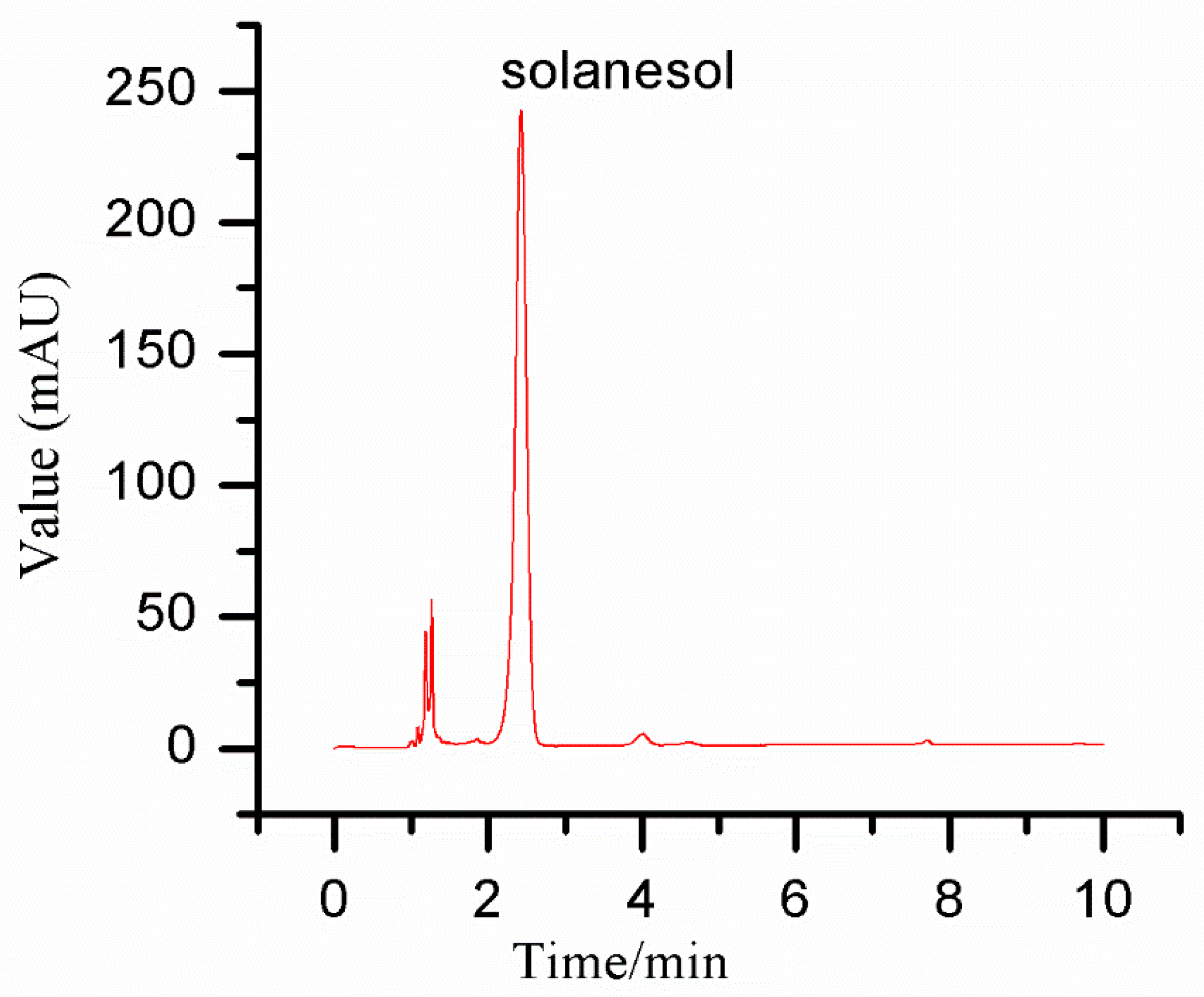
2.2. Solanesol Concentration Detection
2.3. Preparation of Standard Curve
2.4. Synthesis of SSO-MIPs
2.5. Static Adsorption Experiment
2.6. Kinetic Experiment
2.7. Adsorption Isotherm Detection
2.8. Experiment of Solanesol Purification by SSO-MIPs
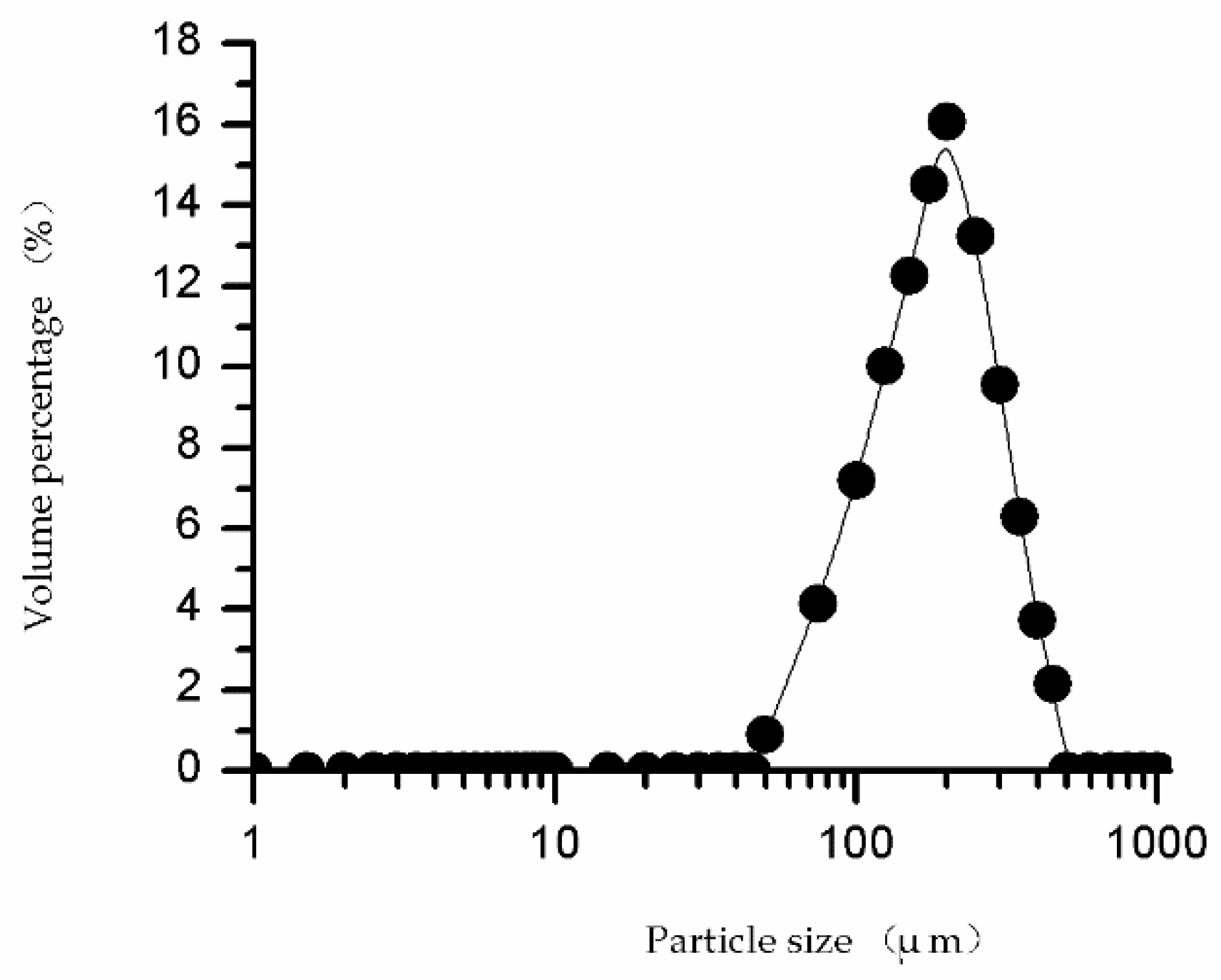
2.9. Particle Size and Pore Volume of SSO-MIPs Detection
3. Results and Discussions
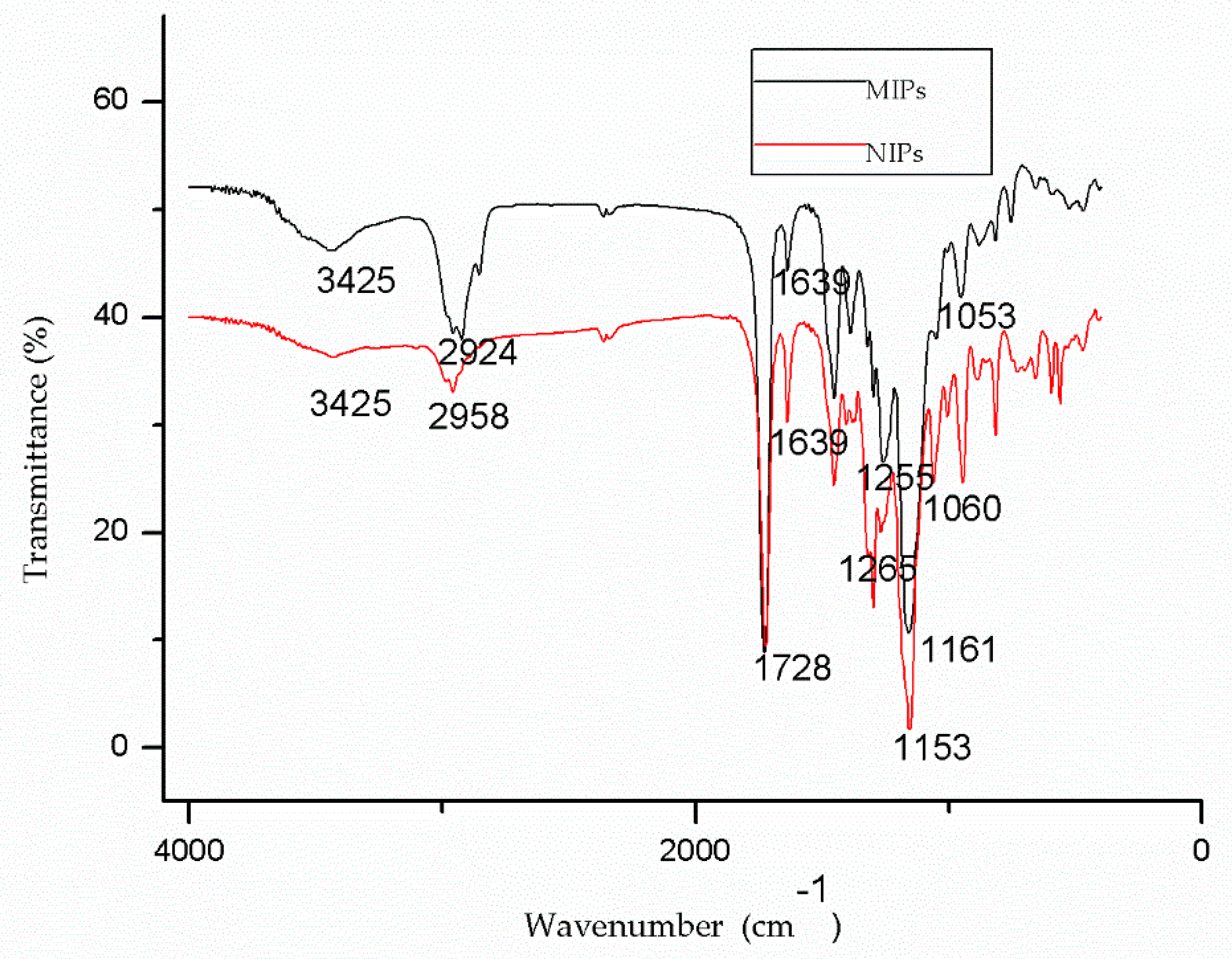
3.1. Characterization of SSO-MIPs and NIPs
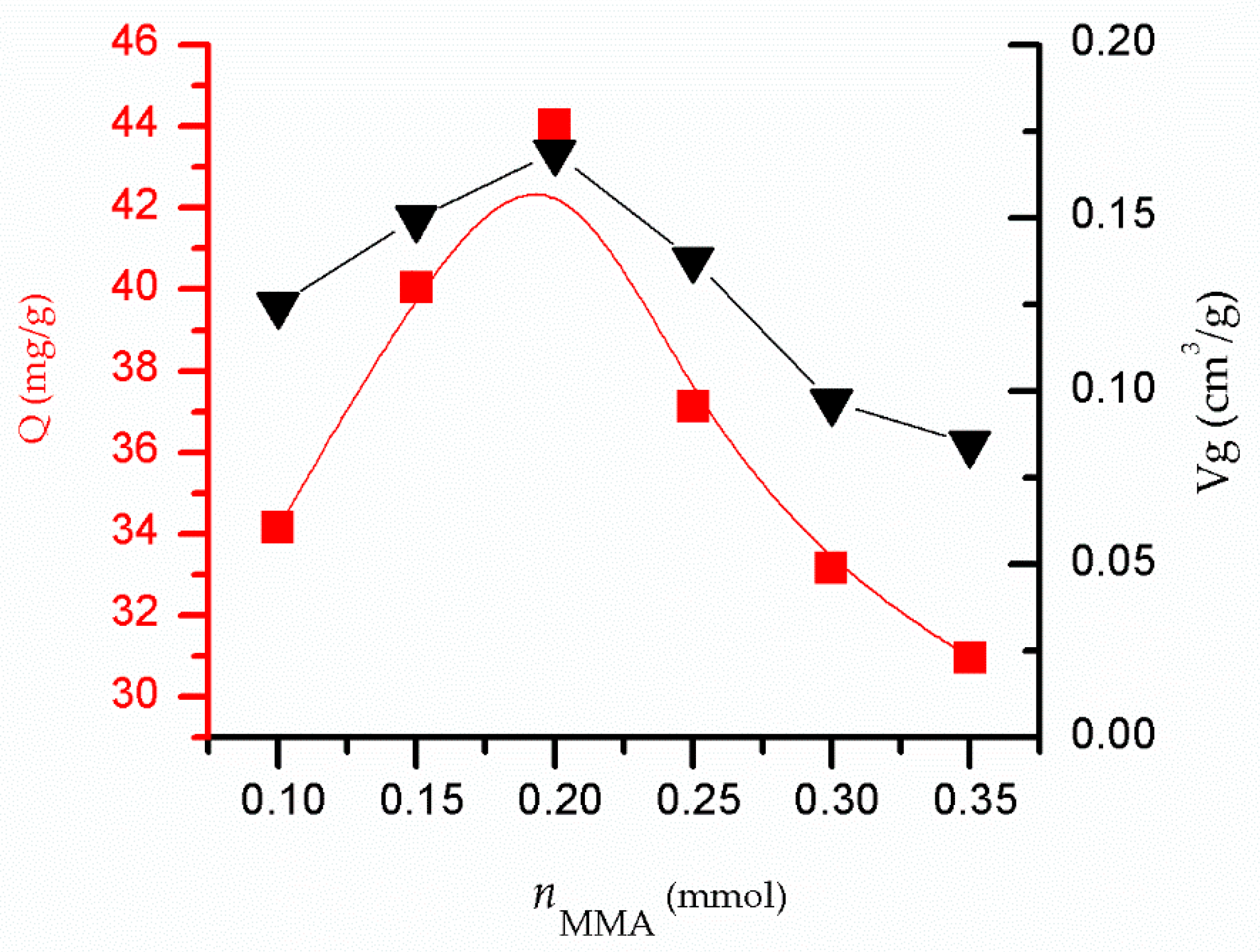
3.2. Influence of nMMA on the Adsorption Performance of MIPs
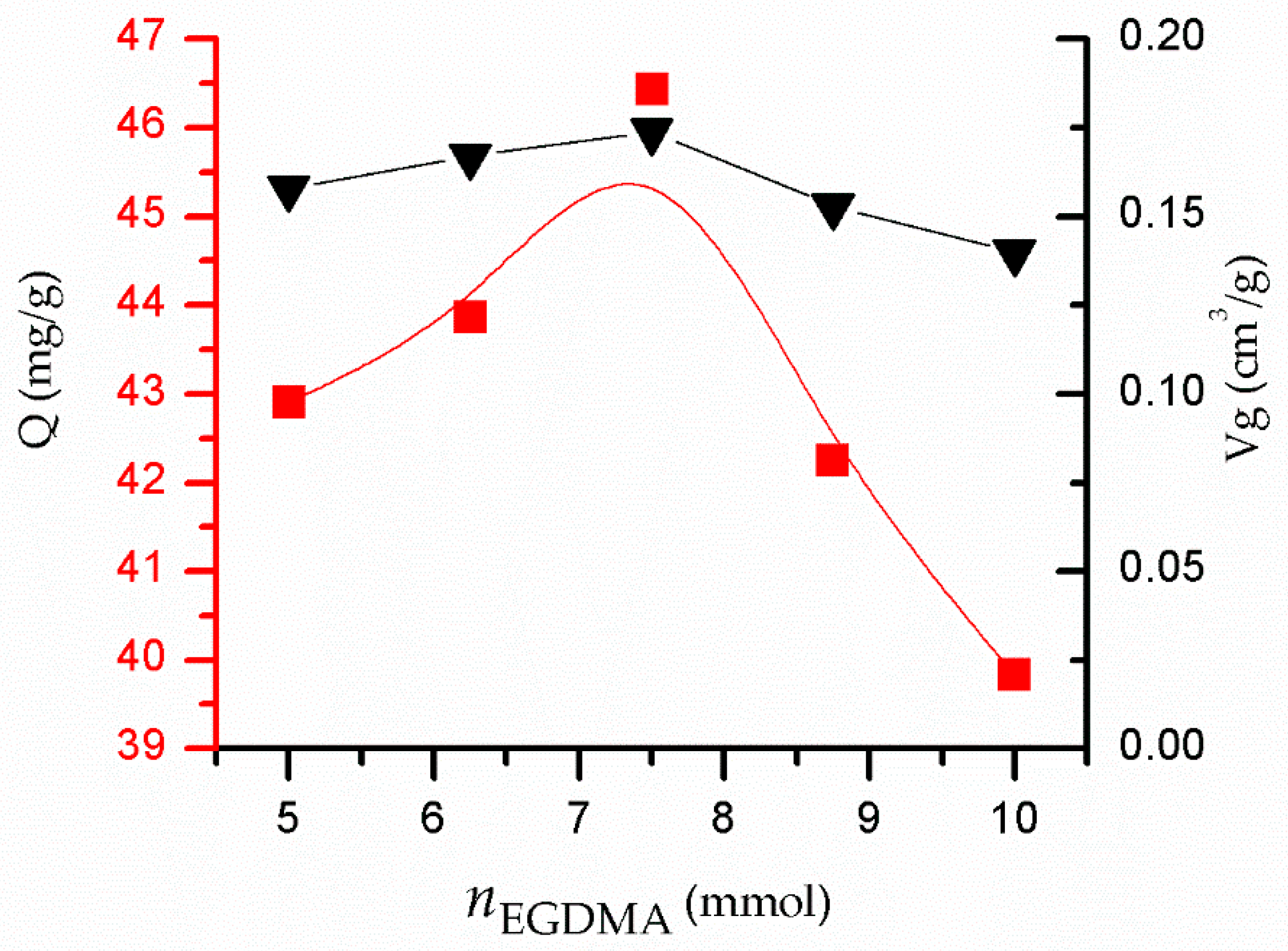
3.3. Influence of on the Adsorption Performance of MIPs
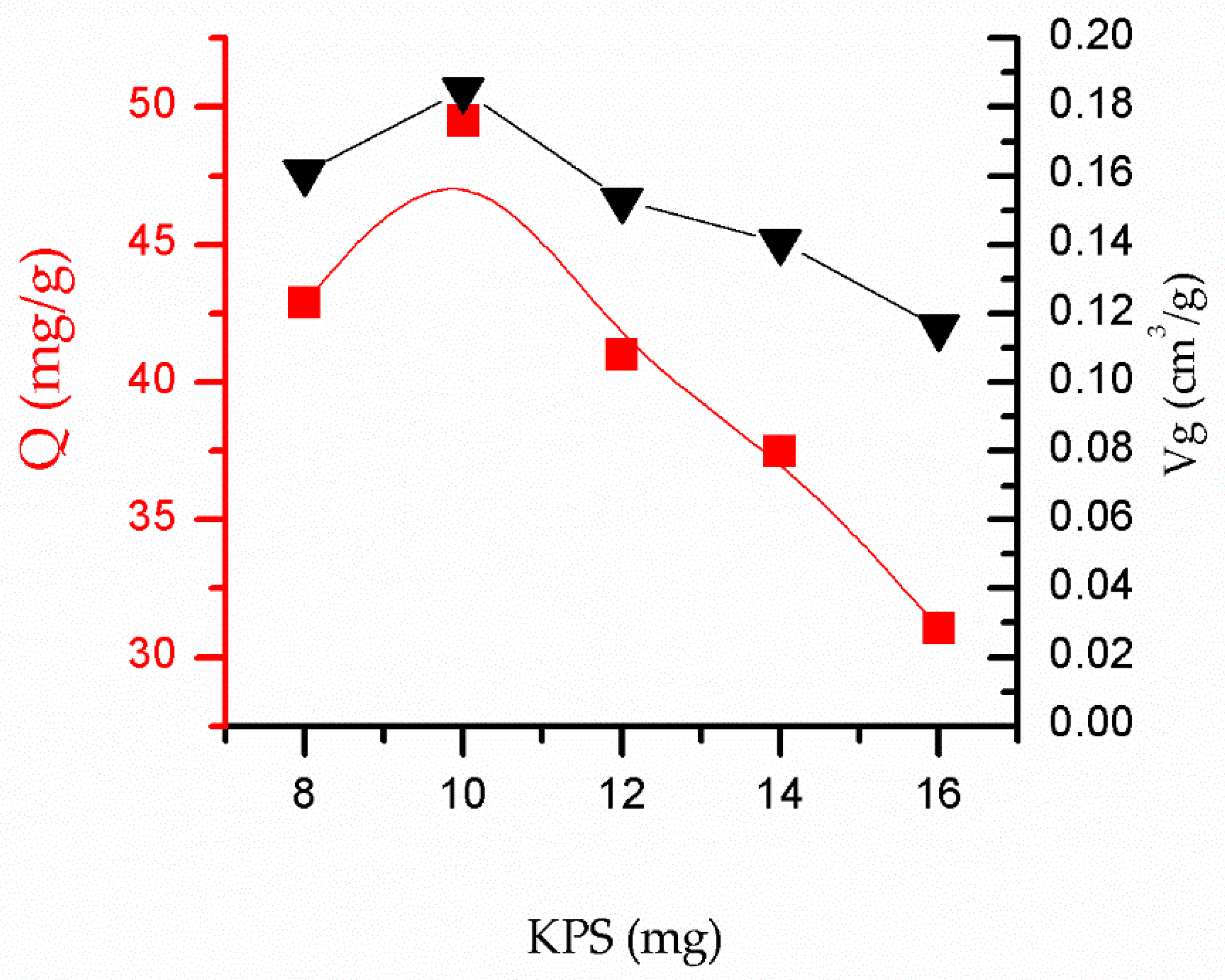
3.4. Influence of the Initiator Dosage on the Adsorption Performance of SSO-MIPs
3.5. Influence of the Synthesis Temperature on the Adsorption Performance of SSO-MIPs
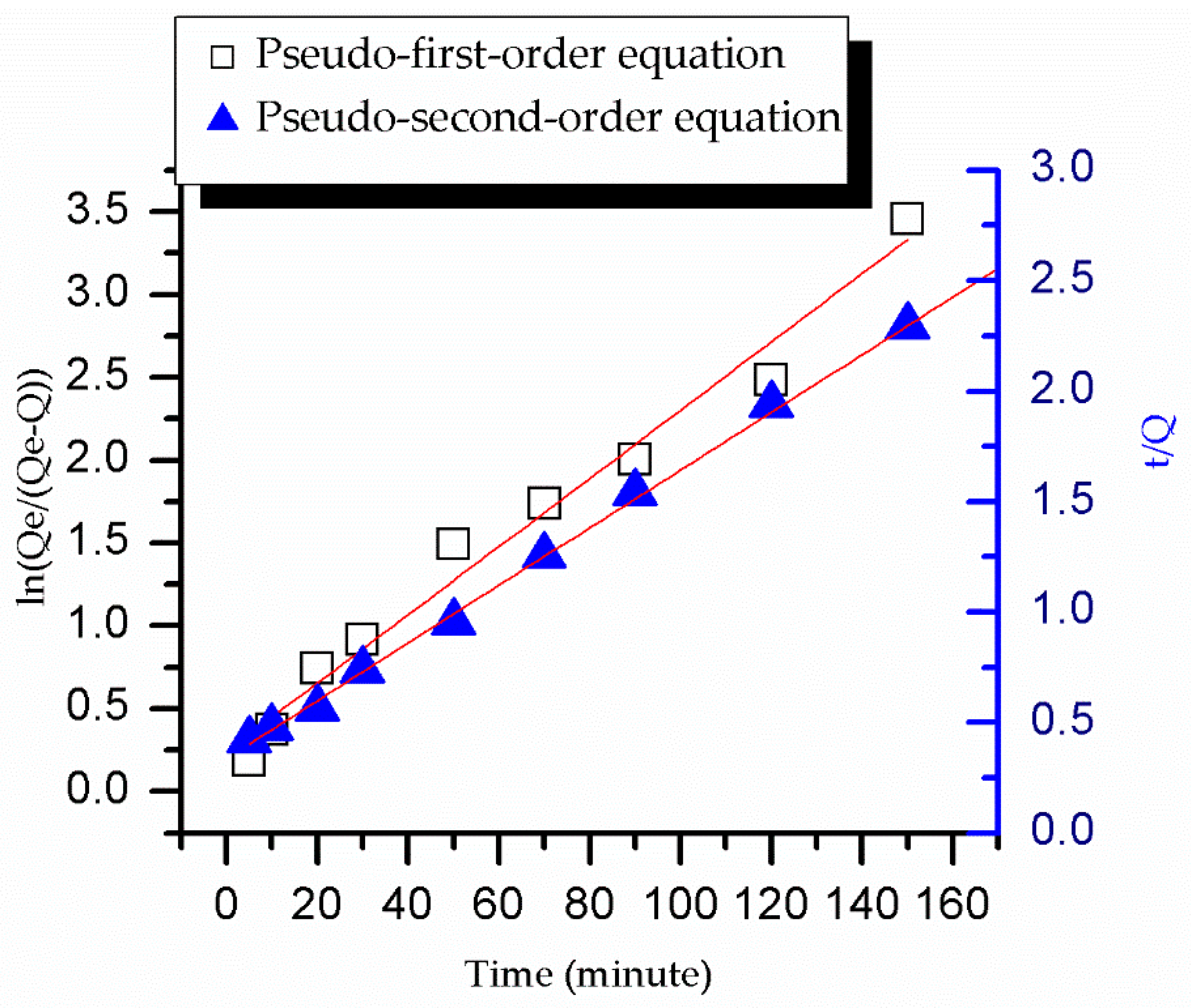
3.6. The Adsorption Kinetics of SSO-MIPs and NIPs
3.7. The Adsorption Isotherm of the SSO-MIPs
3.8. Purification Effect of SSO-MIPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramelow, U.S.; Pingili, S. Synthesis of ethylene glycol dimethacrylate-methyl methacrylate copolymers, determination of their reactivity ratios, and a study of dopant and temperature effects on their conductivities. Polymers 2010, 2, 265. [Google Scholar] [CrossRef]
- Zhao, X.L.; Cui, Y. Preparation of fluorescent molecularly imprinted polymers via pickering emulsion interfaces and the application for visual sensing analysis of listeria monocytogenes. Polymers 2019, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. A theory of the structure and process of formation of antibodies. J. Am. Chem. Soc. 2002, 62, 2643–2657. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.S. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; She, Y.X. Development of ELISA-Like fluorescence assay for melamine detection based on magnetic dummy molecularly imprinted polymers. Appl. Sci. 2018, 8, 560. [Google Scholar] [CrossRef]
- Martín-Esteban, A. Molecularly imprinted polymers: New molecular recognition materials for selective solid-phase extraction of organic compounds. Fresenius J. Anal. Chem. 2001, 370, 795–802. [Google Scholar] [CrossRef]
- Lee, W.C.; Cheng, C.H. Chromatographic characterization of molecularly imprinted polymers. Anal. Bioanal. Chem. 2008, 390, 1101–1109. [Google Scholar] [CrossRef]
- Ansell, R.J.; Kuah, J.K.L. Imprinted polymers for chiral resolution of (±)-ephedrine, 4: Packed column supercritical fluid chromatography using molecularly imprinted chiral stationary phases. J. Chromatogr. A 2012, 1264, 117–123. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.K. Molecularly imprinted polymer electrochemical sensor based on Fe3O4/MnO2 doped graphene composites for determination of17β-estradiol in aquatic environment. Chin. J. Anal. Chem. 2018, 46, 1047–1054. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, X. preparation and adsorption characteristics of lysozyme-imprinted polymer membrane. Chin. J. Chromatogr. 2019, 37, 392–397. [Google Scholar] [CrossRef]
- Liu, H.; Qiao, L. Electro-deposited poly-luminol molecularly imprinted polymer coating on carboxyl graphene for stir bar sorptive extraction of estrogens in milk. J. Chromatogr. B 2016, 1027, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Xu, K.Y. Sensitive and selective detection of new red colorant based on surface-enhanced raman spectroscopy using molecularly imprinted hydrogels. Appl. Sci. 2019, 9, 2672. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, D. Molecularly imprinted polymeric stir bar: Preparation and application for the determination of naftopidil in plasma and urine samples. J. Sep. Sci. 2016, 39, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Feith, M. Evaluation of the impact of imprinted polymer particles on morphology and motility of breast cancer cells by using digital holographic cytometry. Appl. Sci. 2020, 10, 750. [Google Scholar] [CrossRef]
- Díaz-álvarez, M.; Turiel, E. Molecularly imprinted polymer monolith containing magnetic nanoparticles for the stir-bar sorptive extraction of triazines from environmental soil samples. J. Chromatogr. A 2016, 1469, 1–7. [Google Scholar] [CrossRef]
- Barasc, M. Molecularly Imprinted Polymers for the Detection of Caffeine in Water. Master’s Thesis, University of New Hampshire, Durham, NH, USA, 2007. [Google Scholar]
- Uezu, K.; Nakamura, H. Metal ion-imprinted polymer prepared by the combination of surface template polymerization with postirradiation by γ-rays. Macromolecules 1997, 30, 3888–3891. [Google Scholar] [CrossRef]
- Cao, T.Y.; Liu, Q.P. Synthesis Principle, Performance and Application of Polymer Emulsion; China Chemical Industry Press: Beijing, China, 2007; pp. 7–9. [Google Scholar]
- Yan, N.; Liu, Y. Solanesol biosynthesis in plants. Molecules 2017, 22, 510. [Google Scholar] [CrossRef]
- Ma, X.Q.; Meng, Z.H. Solanesol extraction from tobacco leaves by Flash chromatography based on molecularly imprinted polymers. J. Chromatogr. B 2016, 1020, 1–5. [Google Scholar] [CrossRef]
- Long, J.P.; Chen, Z.B. Preparation and adsorption property of solanesol molecular imprinted polymers. Des. Monomers Polym. 2015, 18, 641–649. [Google Scholar] [CrossRef]
- Liu, X.J. Preparation and Adsorption Performance Research of Solenesol Molecularly Imprinted Polymers. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2011. [Google Scholar]
- Hameed, B.H.; Daud, F.B.M. Adsorption studies of basic dye on activated carbon derived from agricultural waste: Hevea brasiliensis seed coat. Chem. Eng. J. 2008, 139, 48–55. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Z. Adsorption kinetics and isotherm of vanadium with melamine. Water Sci. Technol. 2017, 75, 2316–2321. [Google Scholar] [CrossRef]
- Matsui, J.; Miyoshi, Y. A molecularly imprinted synthetic polymer receptor selective for atrazine. Anal. Chem. 1995, 67, 4404–4408. [Google Scholar] [CrossRef]
- Zhao, N.; Hu, X.L. Preparation of erythromycin-imprinted polymeric microspheres by emulsion polymerization and their adsorption properties. Acta Phys. Chim. Sin. 2014, 30, 121–128. [Google Scholar] [CrossRef]
- Pu, D.; Hu, X.L. Synthesis of glycylglycine imprinted polymer microspheres via emulsion polymerization. Chin. J. Process Eng. 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Li, Q. Synthesis of salicylic acid imprinted polymer microspheres via emulsion polymerization. J. Chem. Ind. Eng. (China) 2008, 6, 216–220. [Google Scholar]
- Fu, H.Q.; Huang, H. Initiator and effect on emulsion polymerization. Synth. Mater. Aging Appl. 2004, 33, 39–42. [Google Scholar] [CrossRef]







| Experiment | MMA (mmol) | EGDMA (mmol) | KPS (mg) | Temperature (°C) | SDS:H2O (mg: mL) |
|---|---|---|---|---|---|
| 1 | 0.1~0.35 | 6.25 | 8 | 65 | 40:15 |
| 2 | 0.2 | 5~10 | 8 | 65 | 40:15 |
| 3 | 0.2 | 7.5 | 8~16 | 65 | 40:15 |
| 4 | 0.2 | 7.5 | 10 | 65~85 | 40:15 |
| 5 | 0.2 | 7.5 | 10 | 70 | 40:15 |
| 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Liu, J.; Liu, M.; Han, X.; Peng, Y.; Tian, X.; Liu, J.; Zhang, S. Synthesis of Molecularly Imprinted Polymer via Emulsion Polymerization for Application in Solanesol Separation. Appl. Sci. 2020, 10, 2868. https://doi.org/10.3390/app10082868
Zhao G, Liu J, Liu M, Han X, Peng Y, Tian X, Liu J, Zhang S. Synthesis of Molecularly Imprinted Polymer via Emulsion Polymerization for Application in Solanesol Separation. Applied Sciences. 2020; 10(8):2868. https://doi.org/10.3390/app10082868
Chicago/Turabian StyleZhao, Guojie, Jing Liu, Minghong Liu, Xiaobin Han, Yulong Peng, Xiatian Tian, Jialei Liu, and Shaofeng Zhang. 2020. "Synthesis of Molecularly Imprinted Polymer via Emulsion Polymerization for Application in Solanesol Separation" Applied Sciences 10, no. 8: 2868. https://doi.org/10.3390/app10082868
APA StyleZhao, G., Liu, J., Liu, M., Han, X., Peng, Y., Tian, X., Liu, J., & Zhang, S. (2020). Synthesis of Molecularly Imprinted Polymer via Emulsion Polymerization for Application in Solanesol Separation. Applied Sciences, 10(8), 2868. https://doi.org/10.3390/app10082868






