Bioaugmentation Treatment of a PAH-Polluted Soil in a Slurry Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Soil Characteristics
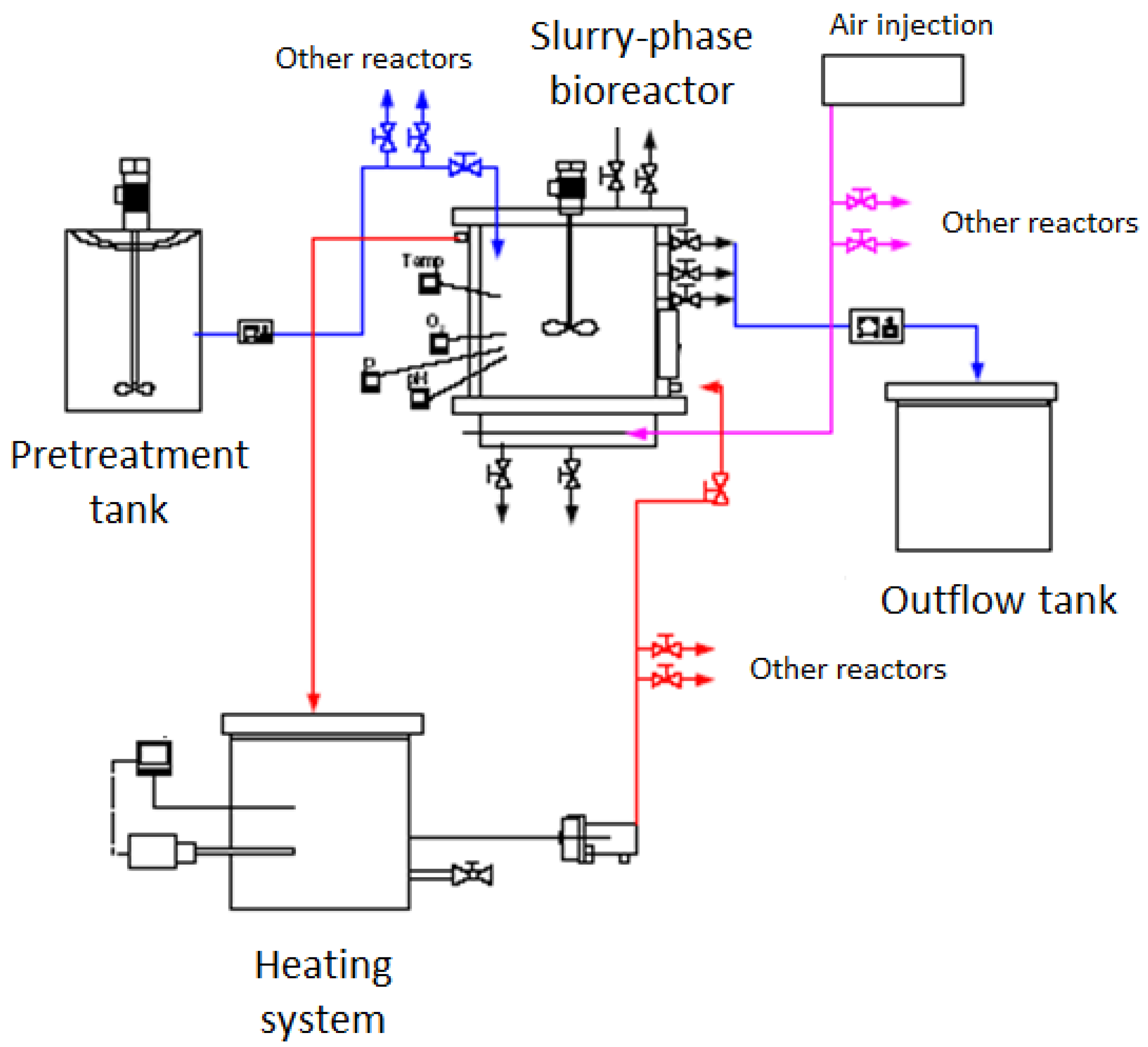
2.2. Slurry Bioreactor and Experimental Design
2.3. Analytical Methods
2.3.1. PAH Analyses
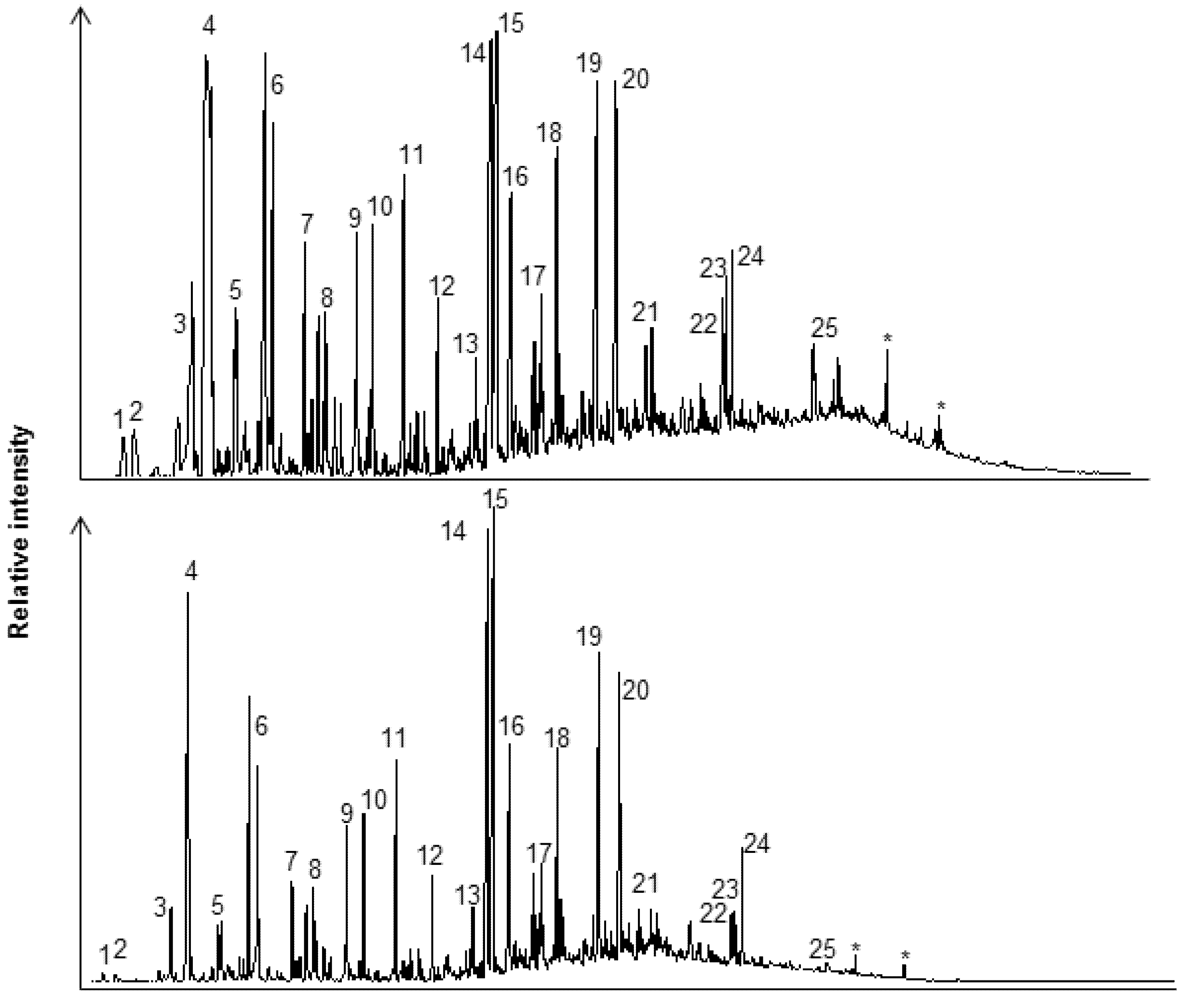
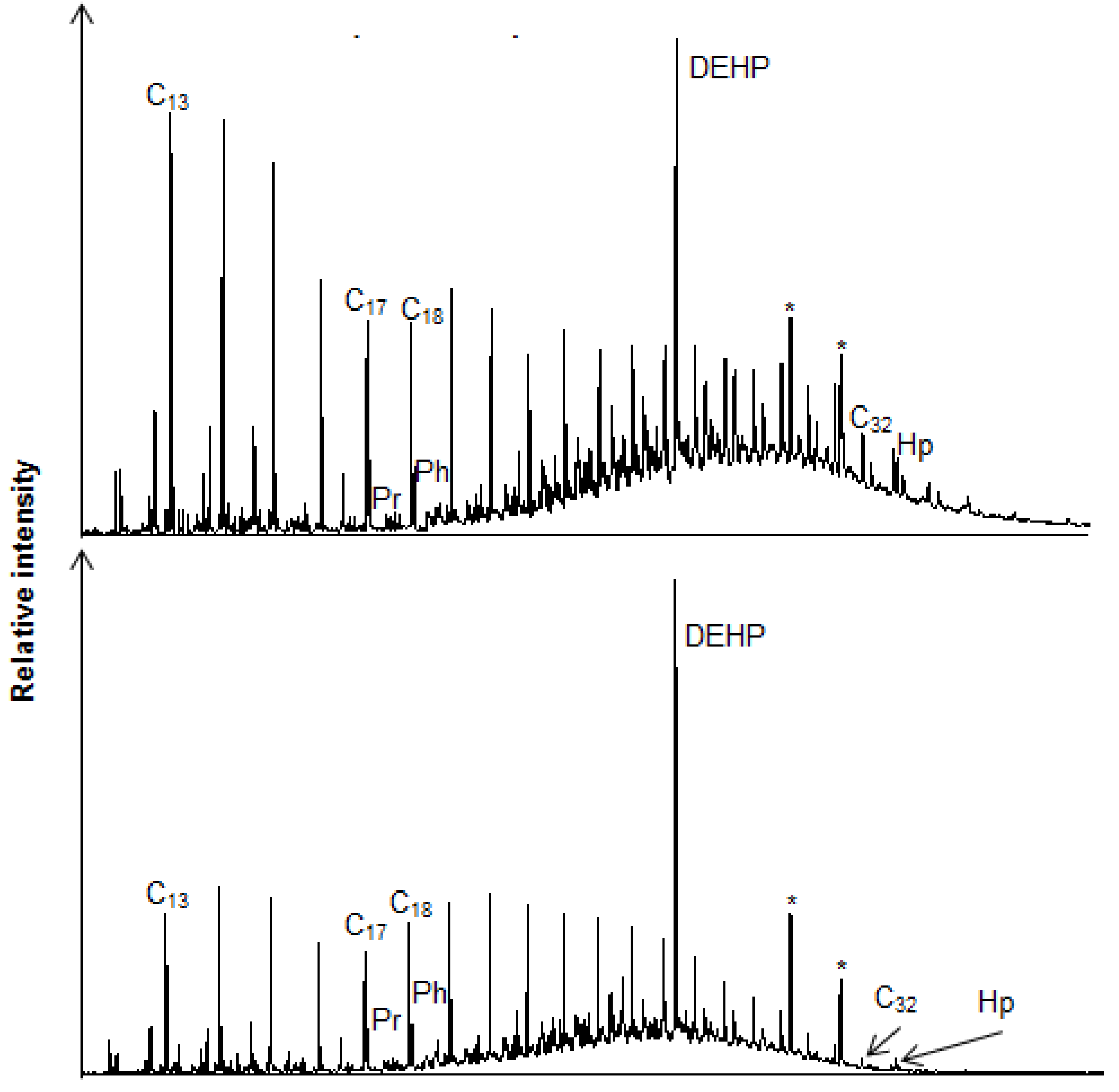
2.3.2. Qualitative GC-MS
2.4. Microbiological Methods
2.4.1. Plate Counting, Isolation, and Identification
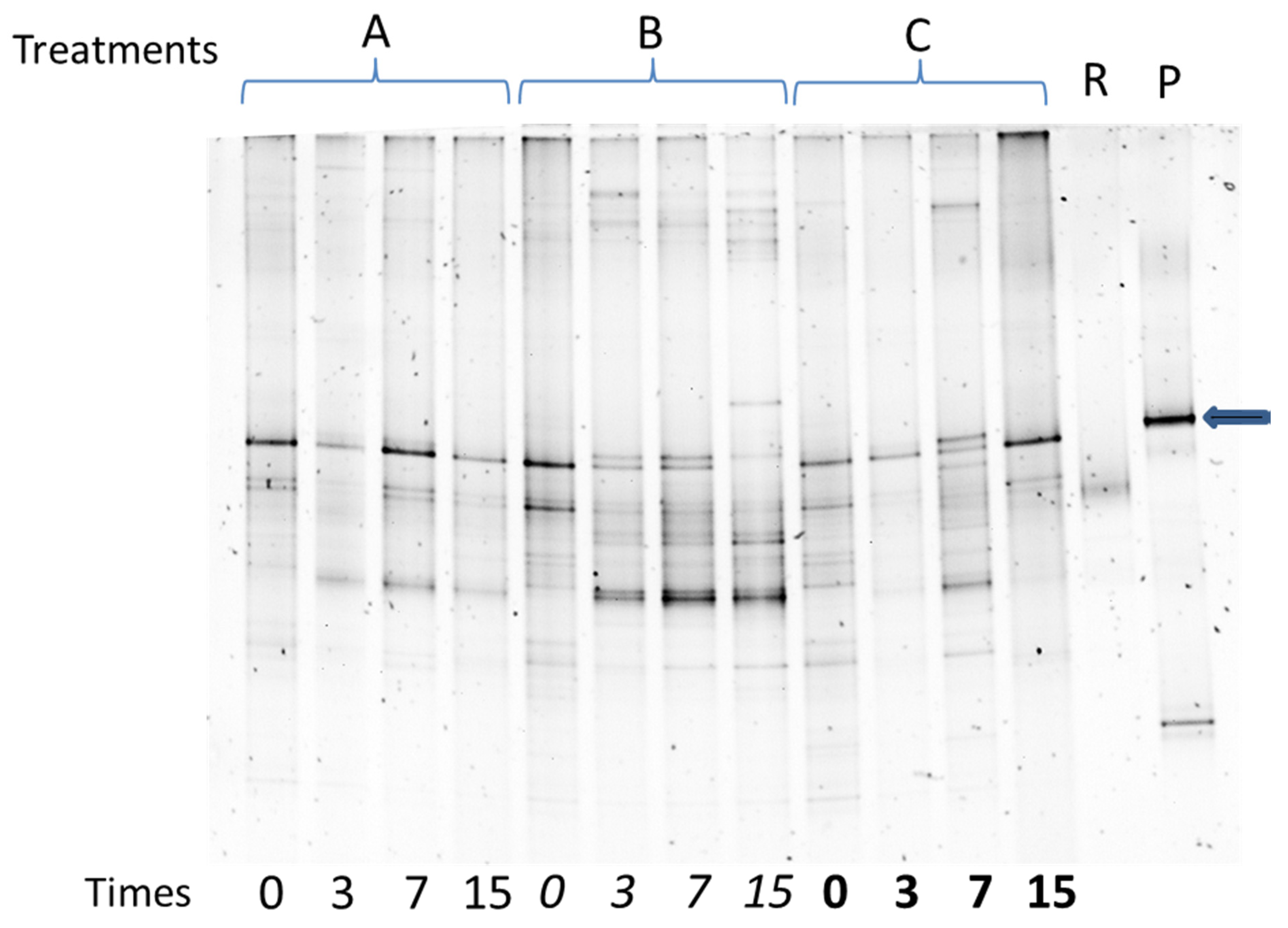
2.4.2. Denaturing Gradient Gel Electrophoresis (DGGE)
3. Results and Discussion
3.1. Soil Characterization
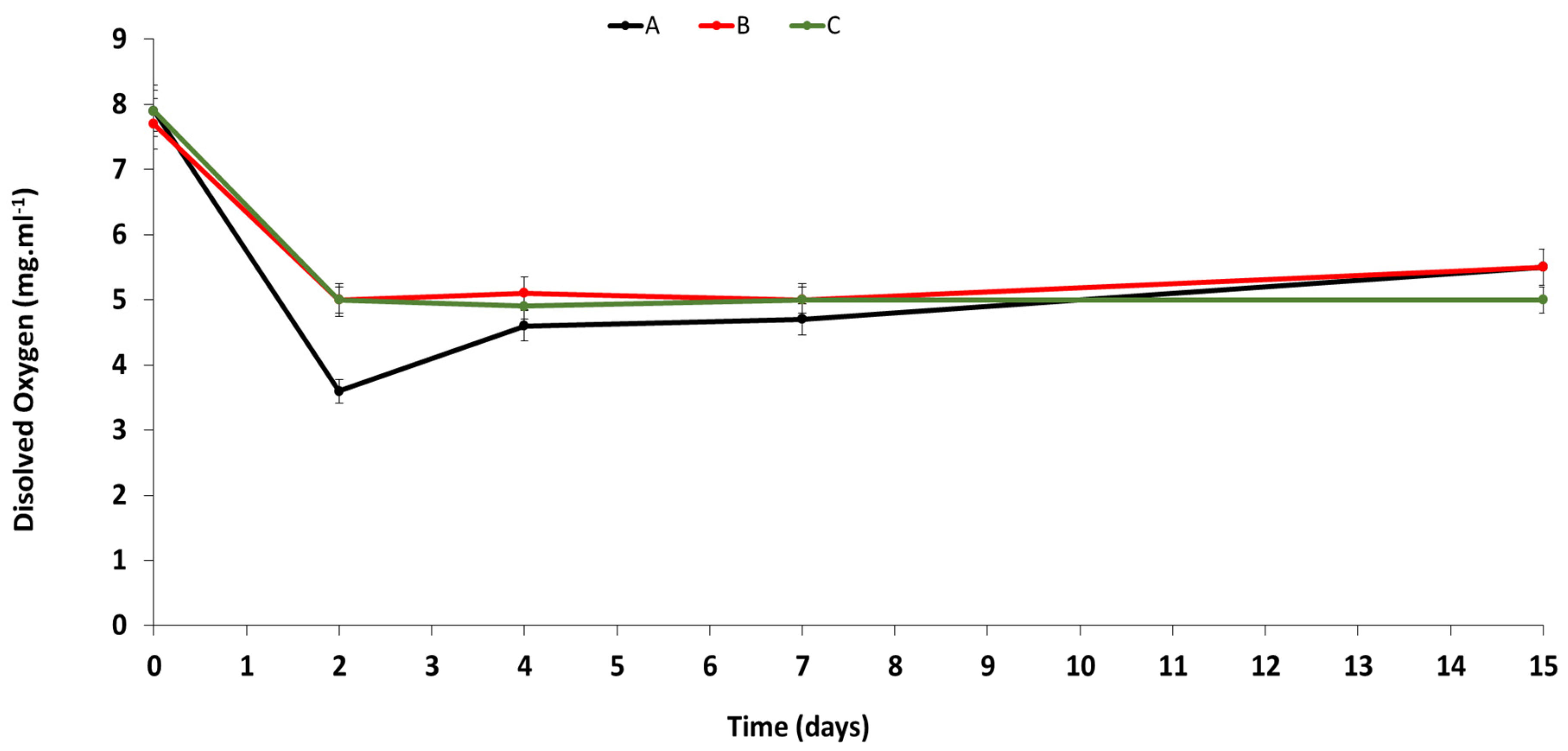
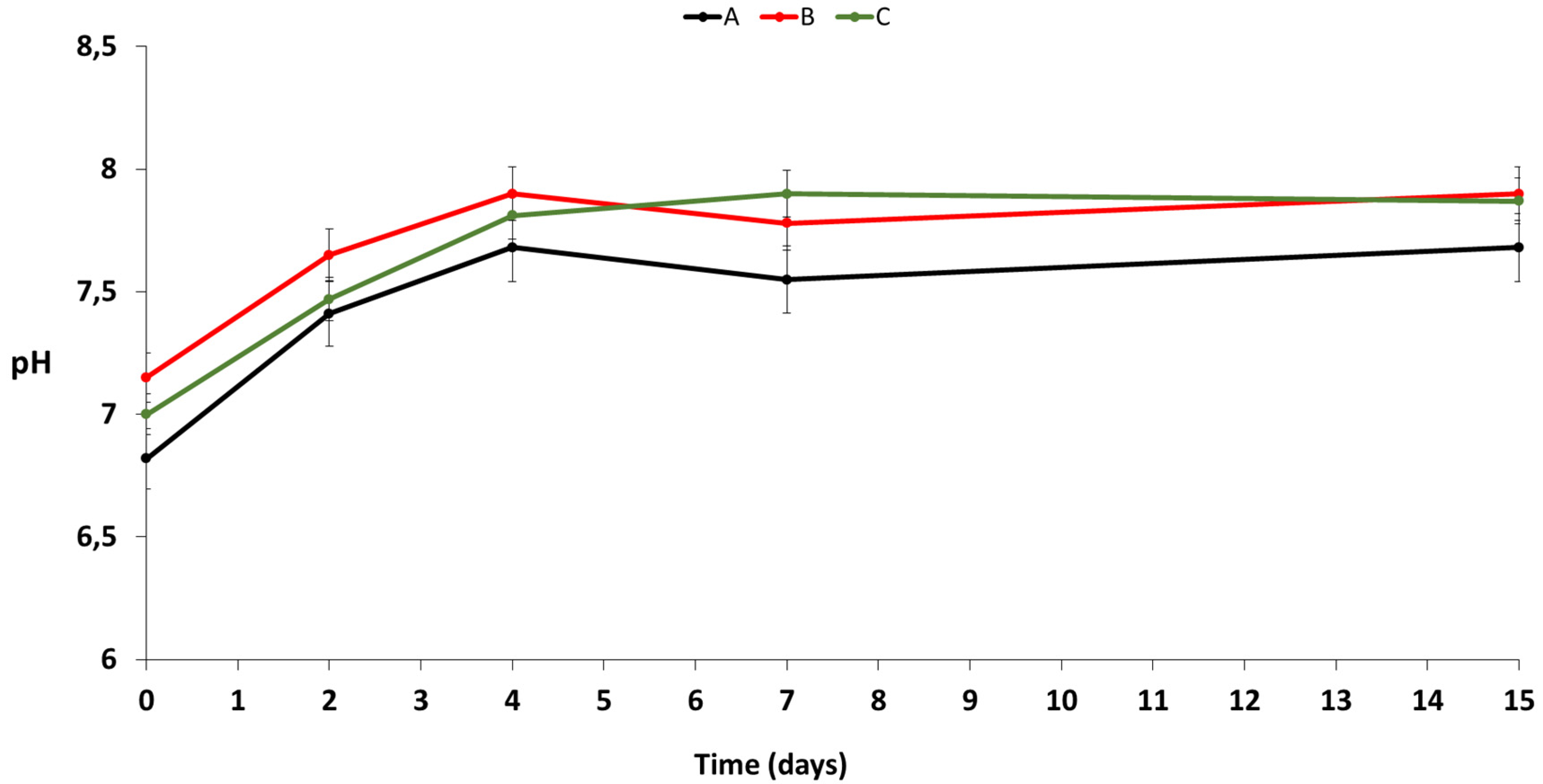
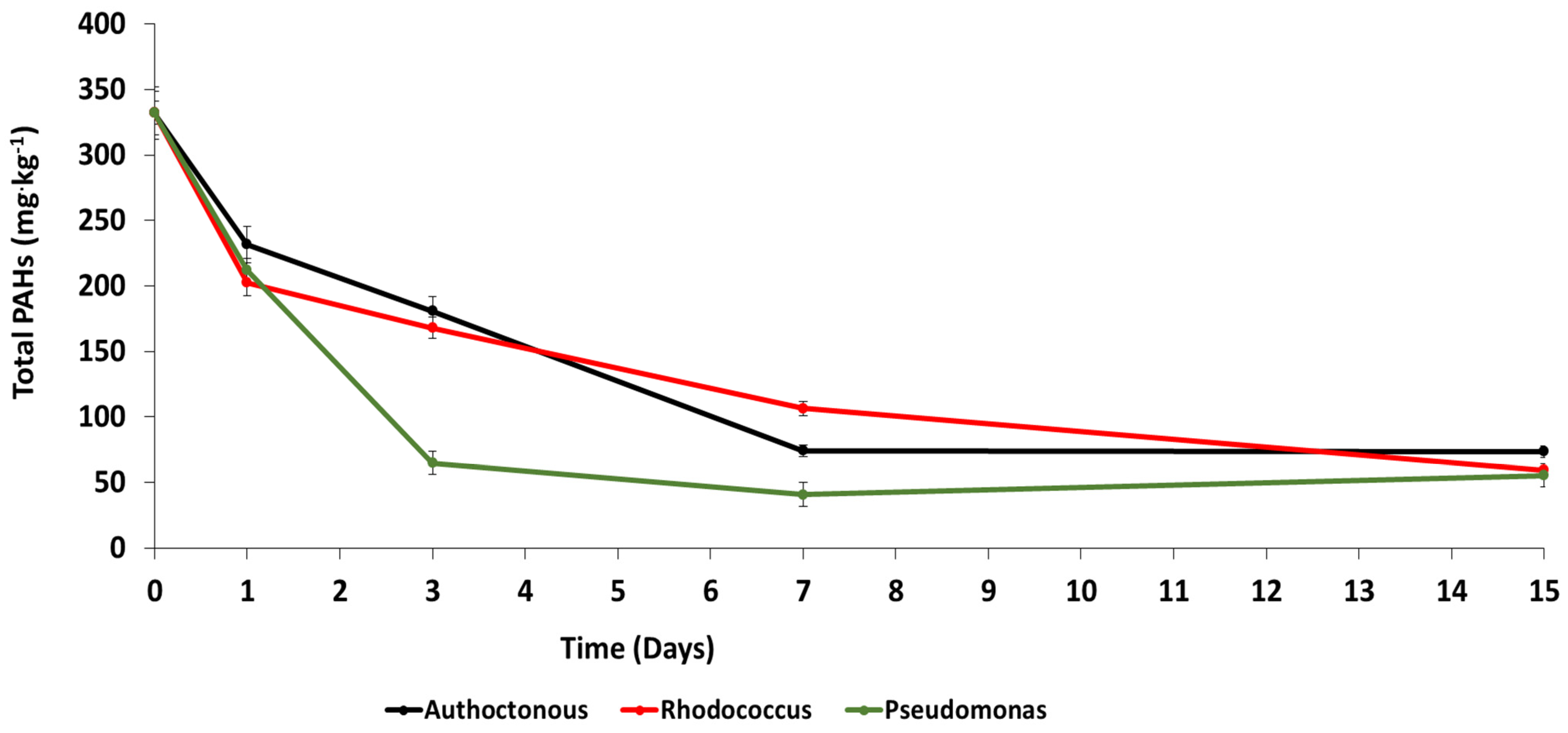
3.2. Chemical Analysis
3.3. Evolution of Bacterial Quantity and Diversity during the Bioslurry Treatments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mueller, J.; Chapman, P.; Pritchard, P. Creosote contaminated sites: Their potential for biodegradation. Environ. Sci. Technol. 1989, 23, 1197–1201. [Google Scholar] [CrossRef]
- Biaché, C.; Ouali, S.; Cébron, A.; Lorgeoux, C.; Colombano, S.; Fauré, P. Bioremediation of PAH-contamined soils: Consequences on formationand degradation of polar-polycyclic aromatic compounds andmicrobial community abundance. J. Hazard. Mater. 2017, 329, 1–10. [Google Scholar] [CrossRef]
- Cheung, P.Y.; Kinkle, B.K. Mycobacterium diversity and pyrene mineralization in petroleum-contaminated soils. Appl. Environ. Microbiol. 2001, 67, 2222–2229. [Google Scholar] [CrossRef]
- ATSDR. Toxicology profile for polyaromatic hydrocarbons. In ATSDR’s Toxicological Profiles on CD-ROM; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Sato, H.; Aoki, Y. Mutagenesis by environmental pollutants and bio-monitoring of environmental mutagens. Curr. Drug Metab. 2002, 3, 311–319. [Google Scholar] [CrossRef]
- Rodrigo, J.; Santos, R.; Bento, F.; Perarlba, M.; Selbach, P.; Sa, S.; Camargo, F. Anthacene biodegradation by Pseudomonas sp. Isolated from a petrochemical sludge landfarming site. Int. Biodeter. Biodegr. 2005, 56, 143–150. [Google Scholar]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Viesser, J.A.; Sugai-Guerios, M.H.; Malucelli, L.C.; Pincerati, M.R.; Karp, S.G.; Maranho, L.T. Petroleum-Tolerant Rhizospheric Bacteria: Isolation, Characterization and Bioremediation Potential. Sci. Rep. 2020, 10, 2060. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Bento, F.M.; Camargo, F.A.O.; Okeke, B.C.; Frankenberger, W.T. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour. Technol. 2005, 96, 1049–1055. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef]
- Whang, L.M.; Liu, P.W.G.; Ma, C.C.; Cheng, S.S. Application of Biosurfactants, Rhamnolipid, and Surfactin, for Enhanced Biodegradation of Diesel-Contaminated Water and Soil. J. Hazard. 2008, 151, 155–163. [Google Scholar] [CrossRef]
- Teng, Y.; Luo, Y.; Ping, L.; Zou, D.; Li, Z.; Christie, P. Effects of soil amendment with different carbon sources and other factors on the bioremediation of an aged PAH-contaminated soil. Biodegradation 2010, 21, 167–178. [Google Scholar] [CrossRef]
- Lin, T.C.; Pan, P.T.; Cheng, S.S. Ex situ bioremediation of oil-contaminated soil. J. Hazard. Mater. 2010, 176, 27–34. [Google Scholar] [CrossRef]
- Robles-González, I.V.; Fava, F.; Poggi-Varaldo, H.M. A review on slurry bioreactors for bioremediation of soils and sediments. Microb. Cell Fact. 2008, 7, 5. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Kim, J.D.; Shim, S.H.; Lee, C.G. Degradation of phenanthrene by bacterial strains isolated from soil in oil refinery fields in Korea. J. Microbiol. Biotechnol. 2005, 15, 337–345. [Google Scholar]
- Tirado-Torres, D.; Acevedo-Sandoval, O.; Rodríguez-Pastrana, B.R.; Gayosso-Canales, M. Phylogeny and polycyclic aromatic hydrocarbons degradation potential of bacteria isolated from crude oil-contaminated site. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2017, 52, 897–904. [Google Scholar] [CrossRef]
- Hou, N.; Zhang, N.; Jia, T.; Sun, Y.; Dai, Y.; Wang, Q.; Li, D.; Luo, Z.; Li, C. Biodegradation of phenanthrene by biodemulsifier-producing strain Achromobacter sp. LH-1 and the study on its metabolisms and fermentation kinetics. Ecotoxicol. Environ. Saf. 2018, 163, 205–214. [Google Scholar] [CrossRef]
- Peng, R.H.; Xiong, A.S.; Xue, Y.; Fu, X.Y.; Gao, F.; Zhao, W.; Tian, Y.S.; Yao, Q.H. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol. Rev. 2008, 32, 927–955. [Google Scholar] [CrossRef]
- Moscoso, F.; Teijiz, I.; Deive, F.J.; Sanromán, M.A. Efficient PAHs biodegradation by a bacterial consortium at flask and bioreactor scale. Bioresour. Technol. 2012, 119, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Jain, K.; Madamwar, D. Metabolism of pyrene through phthalic acid pathway by enriched bacterial consortium composed of Pseudomonas, Burkholderia, and Rhodococcus (PBR). Biotech 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, V.; Gianfreda, L. Bioremediation and monitoring of aromatic-polluted habitats. Appl. Microbiol. Biotechnol. 2007, 76, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Guazzaroni, M.E.; Herbst, F.; Tamames, J.; Lores, I.; Peláez, A.I.; López-Cortés, N.; Vieites, J.M.; Gallego, J.L.R.; Pieper, D.; Rosselló-Móra, R.; et al. Metaproteogenomic insights beyond bacterial response to naphthalene exposure and bio-stimulation. ISME J. 2013, 7, 122–136. [Google Scholar] [CrossRef]
- Ruffini, M.; Giorgetti, L.; Becarelli, S.; Siracusa, G.; Lorenzi, R.; Di Gregorio, S. Polycyclic aromatic hydrocarbon-contaminated soils: Bioaugmentation of autochthonous bacteria and toxicological assessment of the bioremediation process by means of Vicia faba L. Environ. Sci. Pollut. Res. Int. 2016, 23, 7930–7941. [Google Scholar] [CrossRef]
- Pelaez, A.I.; Lores, I.; Sotres, A.; Mendez-Garcia, C.; Fernandez-Velarde, C.; Santos, J.A.; Gallego, J.L.R.; Sanchez, J. Design and field-scale implementation of an “on site” bioremediation treatment in PAH-polluted soil. Environ. Pollut. 2013, 181, 190–199. [Google Scholar] [CrossRef]
- Alexander, M. Biodegradation and Bioremediation, 2nd ed.; Academic Press: San Diego, CA, USA, 1999; pp. 325–327. [Google Scholar]
- Montante, G.; Pinelli, D.; Magelli, F. Scale-up criteria for the solids distribution in slurry reactors strirred with multiple impellers. Chem. Eng. Sci. 2003, 58, 5363–5372. [Google Scholar] [CrossRef]
- Mueller, J.G.; Lantz, S.E.; Blattman, B.O.; Chapman, P.J. Bench-scale evaluation of alternative biological treatment processes for the remediation of pentachlorophenol and creosote contaminated materials: Slurry-phase bioremediation. Environ. Sci. Technol. 1991, 25, 1045–1055. [Google Scholar] [CrossRef]
- Christodoulatos, C.; Koutsospyros, A. Bioslurry reactors. In Biological Treatment of Hazardous Wastes; Lewandowsky, G.A., De Filippi, L.J., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1998; pp. 69–103. [Google Scholar]
- Mohan, V.S.; Shailaja, S.; Krishna, M.R.; Reddy, K.B.; Sarma, P.N. Bioslurry phase degradation of di-ethyl phthalate (DEP) contaminated soil in periodic discontinuous mode operation: Influence of bioaugmentation and substrate partition. Process Biochem. 2006, 41, 644–652. [Google Scholar] [CrossRef]
- He, Y.; Chi, J. Pilot-scale demonstration of phytoremediation of PAH-contaminated sediments by Hydrilla verticillata and Vallisneria spiralis. Environ. Technol. 2019, 40, 605–613. [Google Scholar] [CrossRef]
- Li, F.; Guo, S.; Wu, B.; Wang, S. Pilot-scale electro-bioremediation of heavily PAH-contaminated soil from an abandoned coking plant site. Chemosphere 2020, 244, 125467. [Google Scholar] [CrossRef]
- Evans, B.S.; Dudley, C.A.; Klasson, K.T. Sequential anaerobic-aerobic biodegradation of PCBs in soil slurry microcosms. Appl. Biochem. Biotechnol. 1996, 57, 885–894. [Google Scholar] [CrossRef]
- Machin-Ramirez, C.; Okoh, A.I.; Morales, D.; Mayolo-Deloisa, K.; Quintero, R.; Trejo-Hernández, M.R. Slurry-phase biodegradation of weathered oily sludge waste. Chemosphere 2008, 70, 737–744. [Google Scholar] [CrossRef]
- Nasseri, S.; Kalantary, R.R.; Nourieh, N.; Naddafi, K.; Mahvi, A.H.; Baradaran, N. Influence of bioaugmentation in biodegradation of PAHs-contaminated soil in bio-slurry phase reactor. J. Envrion. Health Sci. Eng. 2010, 7, 199–208. [Google Scholar]
- Chiavola, A.; Baciocchi, R.; Gavasci, R. Biological treatment of PAH-contaminated sediments in a Sequencing Batch Reactor. J. Hazard. Mater. 2010, 184, 97–104. [Google Scholar] [CrossRef][Green Version]
- Yu, C.C.; Chang, T.C.; Liao, C.S.; Chang, Y.T. A comparison of the microbial community and functional genes present in free-living and soil particle-attached bacteria from an aerobic bioslurry reactor treating high-molecular-weight PAHs. Sustainability 2019, 11, 1088. [Google Scholar] [CrossRef]
- Lara-Gonzalo, A.; Kruge, M.A.; Lores, I.; Gutiérrez, B.; Gallego, J.R. Pyrolysis GC-MS for the rapid environmental forensic screening of contaminated brownfield soil. Org. Geochem. 2015, 87, 9–20. [Google Scholar] [CrossRef]
- Gallego, J.L.R.; Sierra, C.; Permanyer, A.; Peláez, A.I.; Menéndez-Vega, D.; Sánchez, J. Full-scale remediation of a jet fuel-contaminated soil: Assessment of biodegradation, volatilization, and bioavailability. Water Air Soil Pollut. 2011, 217, 197–211. [Google Scholar] [CrossRef]
- Ikenaga, M.; Muraoka, Y.; Toyota, K.; Kimura, M. Community structure of the microbiota associated with nodal roots of rice plants along with the growth stages: Estimation by PCR-RFLP analysis. Biol. Fertil. Soils 2002, 36, 397–404. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Muyzer, G.; Teske, A.; Wirsen, C.O.; Jannasch, H.W. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Appl. Environ. Microbiol. 1995, 164, 165–172. [Google Scholar]
- Gallego, J.R.; Sierra, C.; Villa, R.; Peláez, A.I.; Sánchez, J. Weathering processes only partially limit the potential for bioremediation of hydrocarbon-contaminated soils. Org. Geochem. 2010, 41, 896–900. [Google Scholar] [CrossRef]
- Huesemann, M.H. Incomplete hydrocarbon biodegradation in contaminated soils: Limitations in bioavailability or inherent recalcitrance? Bioremediat. J. 1997, 1, 27–39. [Google Scholar] [CrossRef]
- Ortega, J.J.; Ball, W.P.; Schulin, R.; Semple, K.T.; Wick, L.Y. Bioavailability of pollutants and soil remediation. J. Environ. Qual. 2007, 36, 1383–1384. [Google Scholar] [CrossRef]
- Nocentini, M.; Pinelli, D.; Fava, F. Bioremediation of a soil contaminated by hydrocarbon mixtures: The residual concentration problem. Chemosphere 2000, 41, 1115–1123. [Google Scholar] [CrossRef]
- Rabodonirina, S.; Rasolomampianina, R.; Krier, F.; Drider, D.; Merhaby, D.; Net, S.; Ouddane, B. Degradation of fluorene and phenanthrene in PAHs-contaminated soil using pseudomonas and bacillus strains isolated from oil spill sites. J. Environ. Manag. 2019, 232, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.; Yao, X.; Tang, H.; Ji, F. Degradation of phenanthrene and fluoranthene in a slurry bioreactor using free and ca-alginate-immobilized sphingomonas pseudosanguinis and pseudomonas stutzeri bacteria. J. Environ. Manag. 2019, 249, 109388. [Google Scholar] [CrossRef]
- Morales, P.; Cáceres, M.; Scott, F.; Díaz-Robles, L.; Aroca, G.; Vergara-Fernández, A. Biodegradation of benzo[α]pyrene, toluene, and formaldehyde from the gas phase by a consortium of rhodococcus erythropolis and fusarium solani. Appl. Microbiol. Biotechnol. 2017, 101, 6765–6777. [Google Scholar] [CrossRef]
- Hu, X.; Qiao, Y.; Chen, L.; Du, J.; Fu, Y.; Wu, S.; Huang, L. Enhancement of solubilization and biodegradation of petroleum by biosurfactant from rhodococcus erythropolis HX-2. Geomicrobiol. J. 2020, 37, 159–169. [Google Scholar] [CrossRef]
- Gallego, J.R.; Fernández, J.R.; Díez-Sanz, F.; Ordoñez, S.; Sastre, H.; González-Rojas, E.; Peláez, A.I.; Sánchez, J. Bioremediation for shoreline cleanup: In situ vs. on-site treatments. Environ. Eng. Sci. 2007, 24, 493–504. [Google Scholar] [CrossRef]
- Ntougias, S.; Lapidus, A.; Han, J.; Mavromatis, K.; Pati, A.; Chen, A.; Klenk, H.; Woyke, T.; Fasseas, C.; Kyrpides, N.C.; et al. High quality draft genome sequence of Olivibacter sitiensis type strain (AW-6T), a diphenol degrader with genes involved in the catechol pathway. Stand. Genom. Sci. 2014, 9, 783. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Xu, H.; Guo, S. Isolation and characteristics of a microbial consortium for effectively degrading phenanthrene. Pet. Sci. 2007, 4, 68–75. [Google Scholar] [CrossRef]
- Huang, X.; Shi, J.; Cui, C.; Yin, H.; Zhang, R.; Ma, X.; Zhang, X. Biodegradation of phenanthrene by Rhizobium petrolearium SL-1. J. Appl. Microbiol. 2016, 121, 1616–1626. [Google Scholar] [CrossRef]
- Álvarez, M.S.; Rodríguez, A.; Sanromán, M.A.; Deive, F.J. Simultaneous biotreatment of Polycyclic Aromatic Hydrocarbons and dyes in a one-step bioreaction by an acclimated Pseudomonas strain. Bioresour. Technol. 2015, 198, 181–188. [Google Scholar] [CrossRef]
- Mrozika, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2009, 165, 363–375. [Google Scholar] [CrossRef]
| Reactors | Water (mL) | Soil (g) | Nutrients (g) | Surfactant (mL) | Augmentation | |
|---|---|---|---|---|---|---|
| NH4NO3 | NaH2PO4 | |||||
| A | 20,000 | 4000 | 20 | 4.8 | 8 | - |
| B | 20,000 | 4000 | 20 | 4.8 | 8 | Rhodococcus erythropolis |
| C | 20,000 | 4000 | 20 | 4.8 | 8 | Pseudomonas stutzeri |
| Texture | Sand (2 mm–63 μm) | 43% |
| Silt (63–2 μm) | 19% | |
| Clay (<2 μm) | 39% | |
| Chemical parameters | pH | 8.2 |
| Conductivity | 0.13 dS/m | |
| Organic matter | 0.20 % w/w | |
| Nitrogen | 0.03 % w/w | |
| C/N ratio | 3.9 | |
| Phosphorus | <2.6 mg/kg | |
| Carbonate content | 0.8 % w/w | |
| Microbiology | Total heterotrophs | 2.3 × 108 CFU/g soil |
| PAH content | mg/kg | 332.2 |
| Initial | 1 day | 3 days | 7 days | 15 days | % Reduction after 15 days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | ||
| two-ring PAHs | 145.7 | 104.1 | 72.2 | 80.2 | 76.1 | 67.9 | 23.0 | 26.7 | 37.5 | 12.6 | 25.1 | 15.6 | 20.7 | 82.8 | 89.3 | 85.8 |
| three-ring PAHs | 108.3 | 70.0 | 71.5 | 70.0 | 53.9 | 49.0 | 17.1 | 25.5 | 37.3 | 12.0 | 26.8 | 22.0 | 15.7 | 75.3 | 79.7 | 85.5 |
| four- to six-ring PAHs | 78.0 | 57.6 | 58.8 | 61.9 | 51.0 | 51.2 | 24.8 | 22.2 | 31.8 | 16.3 | 21.9 | 21.8 | 19.0 | 72.0 | 72.0 | 75.6 |
| Total PAHs | 332.2 | 231.7 | 202.5 | 212.2 | 180.9 | 168.1 | 64.9 | 74.4 | 106.5 | 40.9 | 73.7 | 59.4 | 55.5 | 77.8 | 82.1 | 83.3 |
| Phylogenetic Affiliation | Similarity % | Identified Bacteria | Experiments | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Actinobacteria | 98 | Microbacterium | |||
| Sphingobacteria | 100 | Olivibacter soli | |||
| Gammaproteobacteria | 98 | Pseudomonas chlororaphis | |||
| Betaproteobacteria | 98 | Diaphorobacter | |||
| Gammaproteobacteria | 99 | Pseudoxanthomonas sp. | |||
| Gammaproteobacteria | 99 | Pseudomonas sp. | |||
| Gammaproteobacteria | 99 | Pseudomonas stutzeri | |||
| Actinobacteria | 98 | Rhodococcus erythropolis | |||
| Alphaproteobacteria | 99 | Rhizobium sp. | |||
| Gammaproteobacteria | 99 | Pseudomonas alcaligenes | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forján, R.; Lores, I.; Sierra, C.; Baragaño, D.; Gallego, J.L.R.; Peláez, A.I. Bioaugmentation Treatment of a PAH-Polluted Soil in a Slurry Bioreactor. Appl. Sci. 2020, 10, 2837. https://doi.org/10.3390/app10082837
Forján R, Lores I, Sierra C, Baragaño D, Gallego JLR, Peláez AI. Bioaugmentation Treatment of a PAH-Polluted Soil in a Slurry Bioreactor. Applied Sciences. 2020; 10(8):2837. https://doi.org/10.3390/app10082837
Chicago/Turabian StyleForján, Rubén, Iván Lores, Carlos Sierra, Diego Baragaño, José Luis R. Gallego, and Ana Isabel Peláez. 2020. "Bioaugmentation Treatment of a PAH-Polluted Soil in a Slurry Bioreactor" Applied Sciences 10, no. 8: 2837. https://doi.org/10.3390/app10082837
APA StyleForján, R., Lores, I., Sierra, C., Baragaño, D., Gallego, J. L. R., & Peláez, A. I. (2020). Bioaugmentation Treatment of a PAH-Polluted Soil in a Slurry Bioreactor. Applied Sciences, 10(8), 2837. https://doi.org/10.3390/app10082837









