The Effect of Moisture Content on the Tar Characteristic of Wood Pellet Feedstock in a Downdraft Gasifier
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Pellet Feedstock
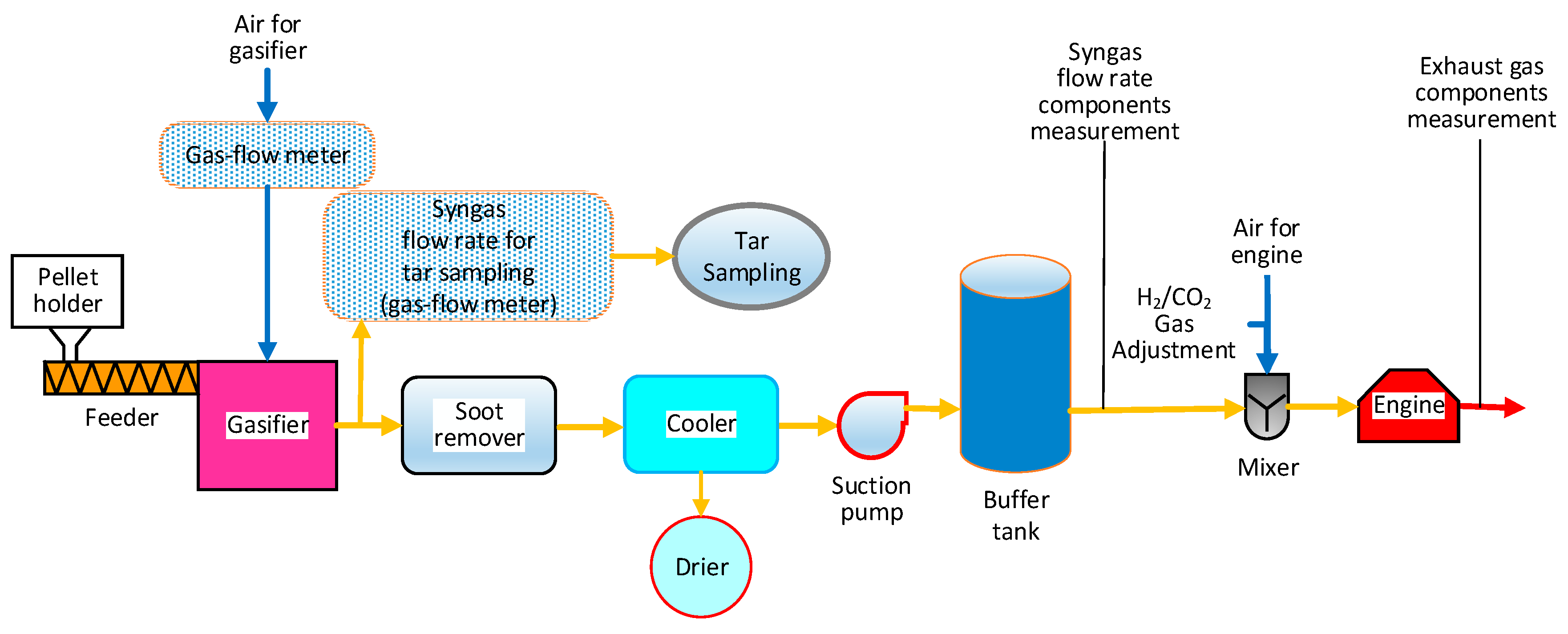
2.2. Downdraft Gasifier System
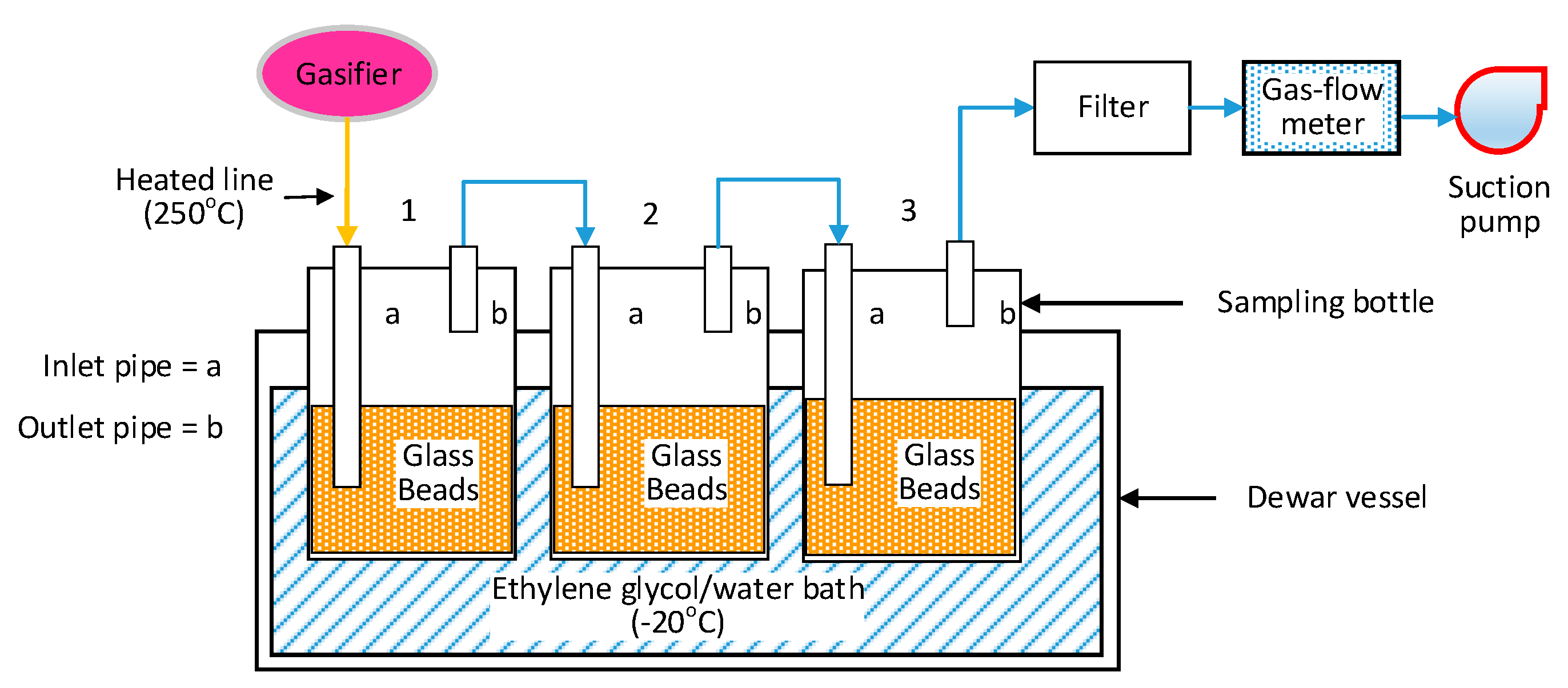
2.3. Tar Sampling
2.4. Test Procedure and Analysis of Tar Sampling
3. Results and Discussion
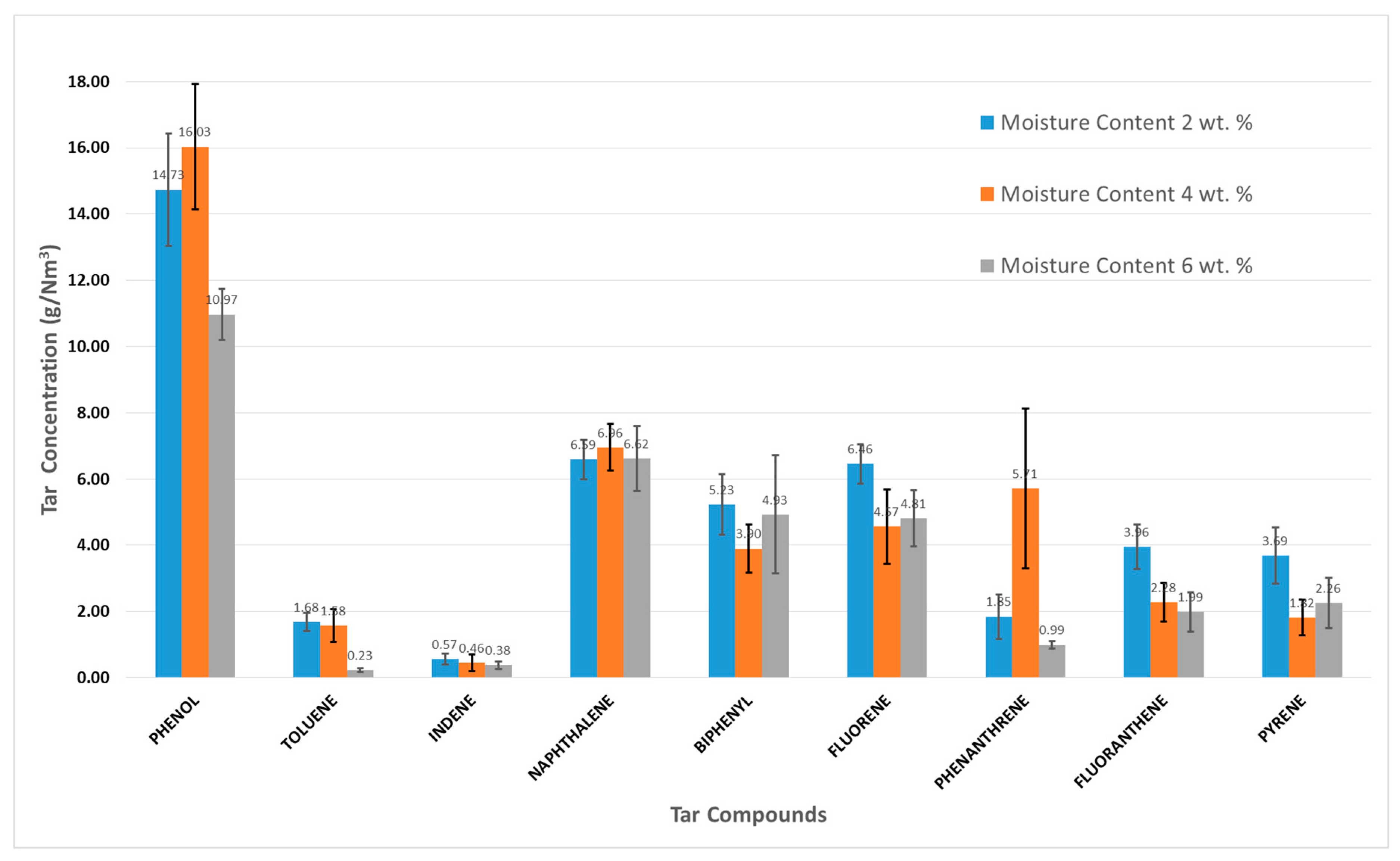
3.1. Impact of Moisture Content on Tar Formation
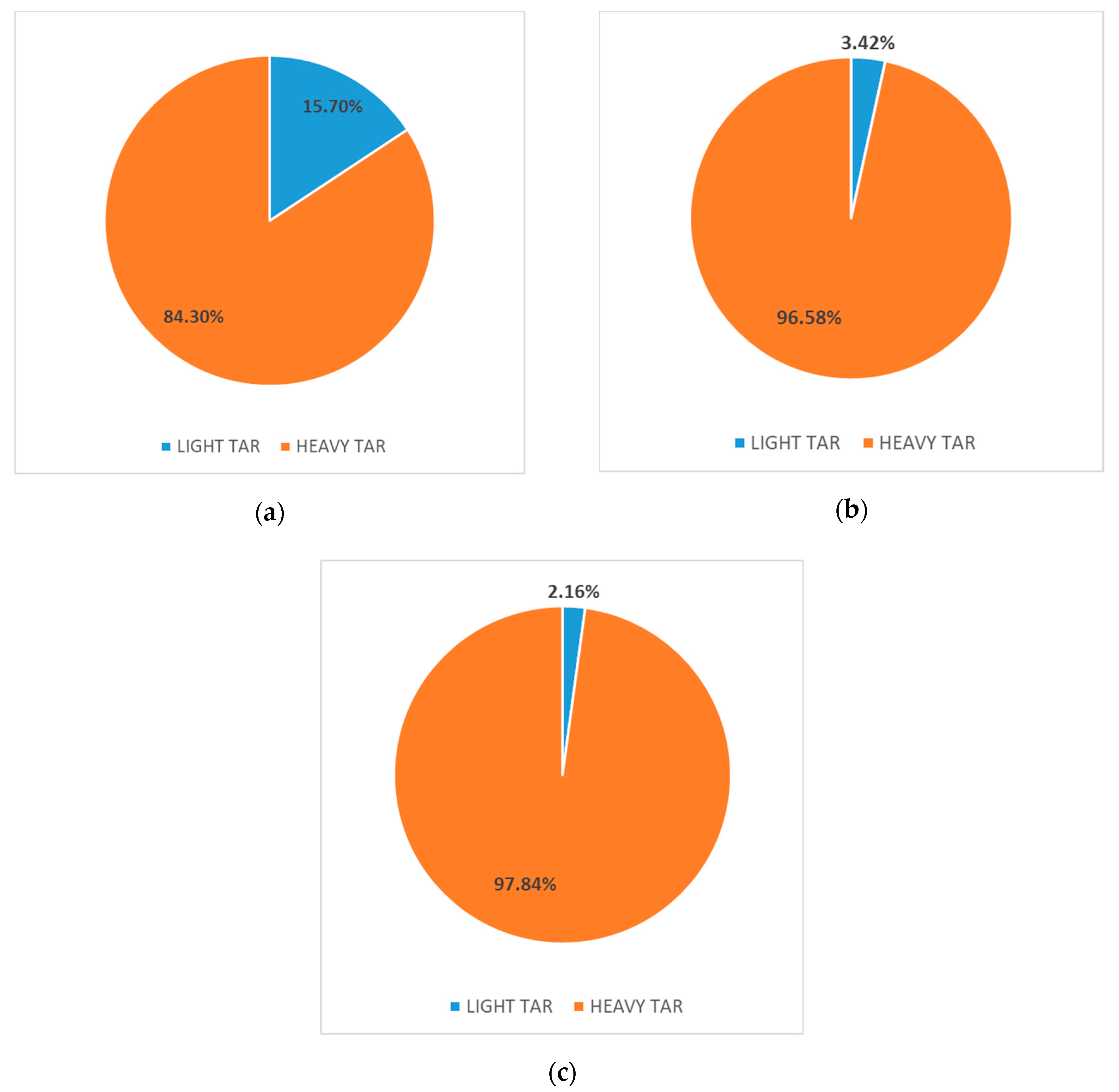
3.2. Impact of Moisture Content on Tar Classification
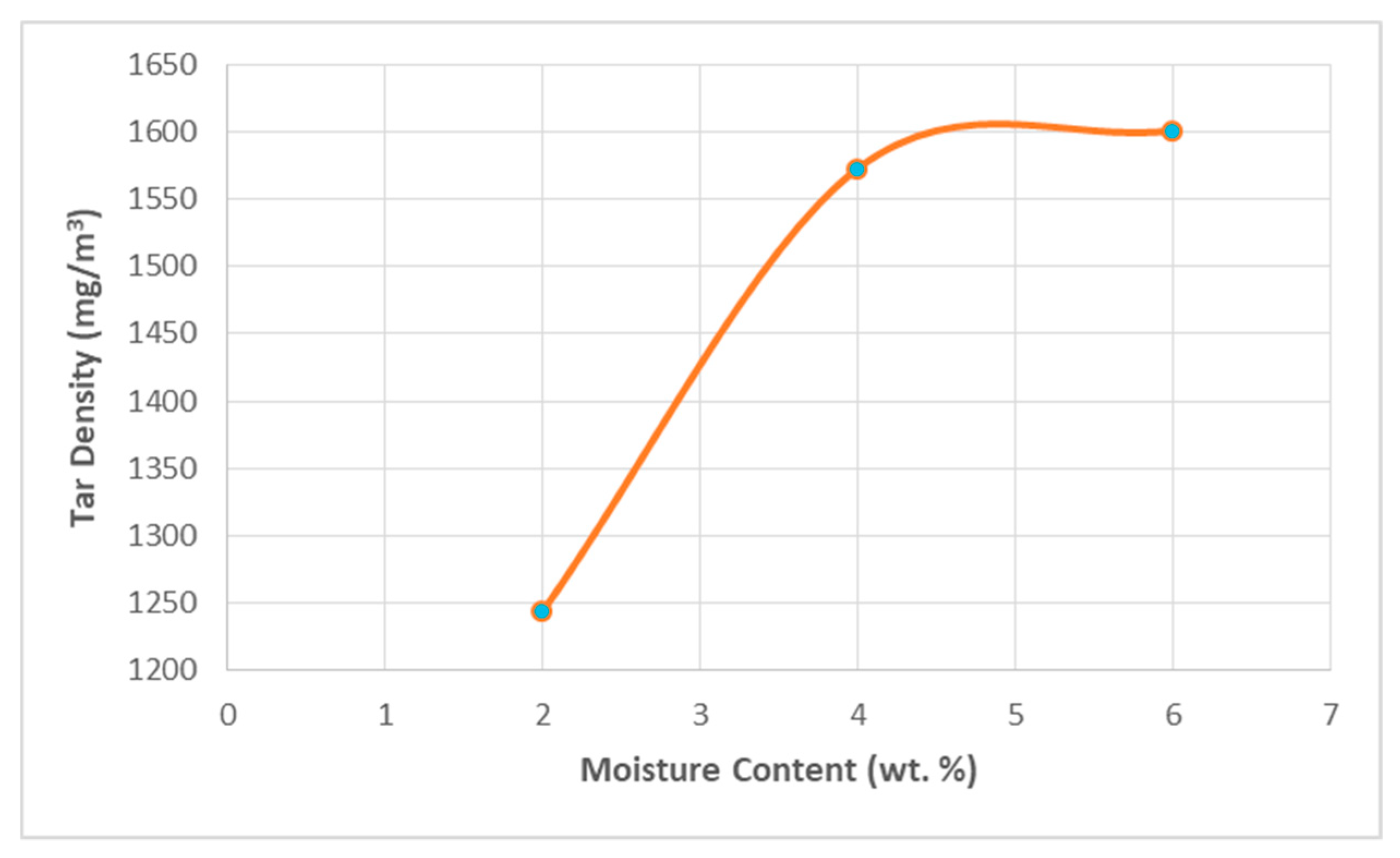
3.3. Impact of Moisture Content on Tar Density
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oveisi, E.; Sokhansanj, S.; Lau, A.; Lim, J.; Bi, X.; Preto, F.; Mui, C. Characterization of recycled wood chips, syngas yield, and tar formation in an industrial updraft gasifier. Environments 2018, 5, 84. [Google Scholar] [CrossRef]
- Rivas, A.M.J.; Yuan, W.; Boyette, M.D. The effect of biomass physical properties on top-lit updraft gasification of woodchips. Energies 2016, 9, 283. [Google Scholar] [CrossRef]
- Oveisi, E.; Sokhansanj, S.; Lau, A.; Lim, C.J.; Bi, X.; Ebadian, M.; Preto, F.; Mui, C.; Gill, R. In-depot upgrading the quality of fuel chips for a commercial gasification plant. Biomass Bioenergy 2018, 108, 138–145. [Google Scholar] [CrossRef]
- Bridgwater, A.V. The technical and economic feasibility of biomass gasification for power generation. Fuel 1994, 74, 631–653. [Google Scholar] [CrossRef]
- Monteiro, E.; Sotton, J.; Bellenoue, M.; Moreira, N.A.; Malheiro, S. Experimental study of syngas combustion at engine-like conditions in a rapid compression machine. Exp. Therm. Fluid Sci. 2011, 35, 1473–1479. [Google Scholar] [CrossRef]
- Naryanto, R.F.; Enomoto, H.; Anh, V.C.; Chunti, C.; Noda, R. Effect of tar formation on biomass downdraft gasification reactor of wood pellet with variation of moisture content. In Proceedings of the 14th Biomass Science Conference, Hiroshima, Japan, 16–18 January 2019; pp. 39–40. [Google Scholar]
- Naryanto, R.F.; Enomoto, H.; Hieda, N.; Teraoka, Y.; Cunti, C.; Noda, R. The influence of wood pellet feedstock water content on tar component in biomass system using downdraft gasifer. J. Jpn. Inst. Energy 2019, 98, 115–118. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasi cation processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Maniatis, K.; Beenackers, A.A.C.M. Tar protocols. IEA bioenergy gasification task. Biomass Bioenergy 2000, 18, 1–4. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- El-Rub, Z.A.; Bramer, E.A.; Brem, G. Review of catalysts for tar elimination in biomass gasification processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Rivas, A.M.J.; Yuan, W.; Wang, D.; Wang, D.; Kumar, A. The effect of gasification conditions on the surface properties of biochar produced in a top-lit updraft gasifier. Appl. Sci. 2020, 10, 688. [Google Scholar] [CrossRef]
- Ohara, H.; Matsuzawa, K. Method and apparatus for repairing tar JP 2009-40885. Available online: https://www.j-platpat.inpit.go.jp/c1800/PU/JP-2007-207391/F1A915D9A45E9E7DAFCE569F7E33F799406F7C84AFAD38B7774536F153ED103F/10/ja (accessed on 30 August 2018).
- Hanaoka, T.; Inoue, S.; Uno, S.; Ogi, T.; Minowa, T. Effect of woody biomass components on air-steam gasification. Biomass Bioenergy 2005, 28, 69–76. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J. Heating values of wood pellets from different species. Biomass Bioenergy 2011, 35, 2634–2639. [Google Scholar] [CrossRef]
- Bronson, B. The Effects of Feedstock Pre-Treatment on the Fluidized Bed Gasification of Biomass; Faculty of Engineering, University of Ottawa: Ottawa, ON, Canada, 2014. [Google Scholar]
- Kiel, J.H.A.; van Paasen, S.V.B.; Neeft, J.P.A.; Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G.; Meijer, R.; Berends, R.H.; Temmink, H.M.G.; Brem, G.; et al. Primary Measures to Reduce Tar Formation in Fluidised-Bed Biomass Gasifiers; ECN Biomass, Energy Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2004; p. 108. [Google Scholar]
- Cong, A.V.; Enomoto, H.; Naryanto, R.F.; Fukadu, K.; Zong, Z.; Chunti, C.; Noda, R. Major tar compounds in raw producer gas and deposits from a small downdraft gasifier: Analysis and comparison. BioResources 2020, 15, 1773–1790. [Google Scholar]
- Yang, Y.B.; Sharifi, V.N.; Swithenbank, J. Swithenbank. Effect of air flow rate and fuel moisture on the burning behaviours of biomass and simulated municipal solid wastes in packed beds. Fuel 2004, 83, 1553–1562. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Rapagna, S.; Provendier, H.; Petitc, C.; Kiennemannc, A.; Foscoloa, P.U. Development of catalysts suitable for hydrogen or syn-gas production from biomass gasi cation. Biomass Bioenergy 2002, 22, 377–388. [Google Scholar] [CrossRef]
- Ross, D.; Noda, R.; Horio, M.; Kosminski, A.; Ashman, P.; Mullinger, P. Axial gas profiles in a bubbling fluidised bed biomass gasifier. Fuel 2007, 86, 1417–1429. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Ollero, P.; Leckner, B. Optimization of char and tar conversion in fluidized bed biomass gasifiers. Fuel 2013, 103, 42–52. [Google Scholar] [CrossRef]
- Gautam, G.; Adhikari, S.; Thangalazhy-Gopakumar, S.; Brodbeck, C.; Bhavnani, S.; Taylor, S. Tar analysis in syngas derived from pelletized biomass in a commercial stratified downdraft gasifier. BioResources 2011, 6, 4653–4661. [Google Scholar]
- Zhang, Y.; Kajitani, S.; Ashizawa, M.; Miura, K. Peculiarities of rapid pyrolysis of biomass covering medium- and high-temperature ranges. Energy Fuels 2006, 20, 2705–2712. [Google Scholar] [CrossRef]
- Kamp, W.V.D.; Wild, P.D.; Zielke, U.; Suomalainen, M.; Knoef, H.; Good, J.; Liliedahl, T.; Unger, C.; Whitehouse, M.; Neeft, J.; et al. Tar measurement standard for sampling and analysis of tars and particles in biomass gasification product gas. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005; pp. 1–8. [Google Scholar]
- Vakalis, S.; Patuzzi, F.; Moustakas, K.; Sotiropoulos, A.; Malamis, D.; Baratieri, M. Analysis of tar compounds and quantification of naphthalene from thermal treatment of household biowaste. J. Environ. Manag. 2018, 216, 153–159. [Google Scholar] [CrossRef] [PubMed]


| Property of Wood Pellet | |
|---|---|
| Length (mm) | 12 |
| Diameter (mm) | 6 |
| Bulk density (kg/m3) | 790 |
| Ultimate Analysis (wt. %, Dry Ash-Free) JIS M8813. | |
|---|---|
| O (balance) | 43.37 |
| N | 0.09 |
| S | 0.09 |
| H | 6.43 |
| C (dry, ash-free) | 50.02 |
| Proximate Analysis (wt. %, Dry Basis) JIS M8812 | |
|---|---|
| Volatile matter (dry base) | 81.82 |
| Ash | 0.53 |
| Fixed carbon | 17.65 |
| Low heating value (LHV) | 15.37 MJ/kg—dry |
| Stoichiometry | Standard Heat of Reaction (kJ/mol) | Name | Number |
|---|---|---|---|
| Biomass → char + tar + H2O + light gas (CO + CO2 + H2 +CH4 + C2 + N2 + …) | Endothermic | Biomass de-volatilization | R1 |
| Char combustion | |||
| C + 1/2O2 → CO | −111 | Partial combustion | R2 |
| C + O2 →CO2 | −394 | Complete combustion | R3 |
| Char gasification | |||
| C + CO2 →2CO | +173 | Boudouard reaction | R4 |
| C + H2O → CO +H2 | +131 | Steam gasification | R5 |
| C +2H2 →CH4 | −75 | Hydrogen gasification | R6 |
| Homogeneous volatile oxidation | |||
| CO + 1/2O2 →CO2 | −283 | Carbon monoxide oxidation | R7 |
| H2 + 1/2O2 →H2O | −242 | Hydrogen oxidation | R8 |
| CH4 + 2O2 →CO2 + 2H2O | −283 | Methane oxidation | R9 |
| CO + H2O → CO2 + H2 | −41 | Water gas-shift reaction | R10 |
| CO +3H2 → CH4 + H2O | −206 | Methanation | R11 |
| Tar reaction (tar assumed CnHm) | |||
| CnHm + (n/2)O2 → nCO + (m/2)H2 | Endothermic (except R12) (200–300) | Partial oxidation | R12 |
| CnHm + nH2O → (m/2 + n)H2 +nCO | Steam reforming | R13 | |
| CnHm + nCO2 → 2nCO + (m/2)H2 | Dry reforming | R14 | |
| CnHm +(2n-m/2)H2 → nCH4 | Hydrogenation | R15 | |
| CnHm → (m/4)CH4 + (n-m/4)C | Thermal cracking | R16 |
| Compound | Chemical Formula | Molecular Weight(g/mol) | Boiling Point (°C) |
|---|---|---|---|
| Phenol | C6H5OH | 94.11 | 181.7 |
| Toluene | C7H8 | 92.14 | 110.6 |
| Indene | C9H8 | 116.16 | 181.6 |
| Naphthalene | C10H8 | 128.17 | 218 |
| Biphenyl | C12H10 | 154.21 | 255 |
| Fluorene | C13H10 | 166.22 | 295 |
| Phenanthrene | C14H10 | 178.23 | 336 |
| Fluoranthene | C16H10 | 202,26 | 375 |
| Pyrene | C16H10 | 202.25 | 404 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naryanto, R.F.; Enomoto, H.; Vo Cong, A.; Fukadu, K.; Zong, Z.; Delimayanti, M.K.; Chunti, C.; Noda, R. The Effect of Moisture Content on the Tar Characteristic of Wood Pellet Feedstock in a Downdraft Gasifier. Appl. Sci. 2020, 10, 2760. https://doi.org/10.3390/app10082760
Naryanto RF, Enomoto H, Vo Cong A, Fukadu K, Zong Z, Delimayanti MK, Chunti C, Noda R. The Effect of Moisture Content on the Tar Characteristic of Wood Pellet Feedstock in a Downdraft Gasifier. Applied Sciences. 2020; 10(8):2760. https://doi.org/10.3390/app10082760
Chicago/Turabian StyleNaryanto, Rizqi Fitri, Hiroshi Enomoto, Anh Vo Cong, Kazuki Fukadu, Zheng Zong, Mera Kartika Delimayanti, Chuntima Chunti, and Reiji Noda. 2020. "The Effect of Moisture Content on the Tar Characteristic of Wood Pellet Feedstock in a Downdraft Gasifier" Applied Sciences 10, no. 8: 2760. https://doi.org/10.3390/app10082760
APA StyleNaryanto, R. F., Enomoto, H., Vo Cong, A., Fukadu, K., Zong, Z., Delimayanti, M. K., Chunti, C., & Noda, R. (2020). The Effect of Moisture Content on the Tar Characteristic of Wood Pellet Feedstock in a Downdraft Gasifier. Applied Sciences, 10(8), 2760. https://doi.org/10.3390/app10082760






