Pressure Applied during Deep Friction Massage: Characterization and Relationship with Time of Onset of Analgesia
Abstract
1. Introduction
2. Materials and Methods
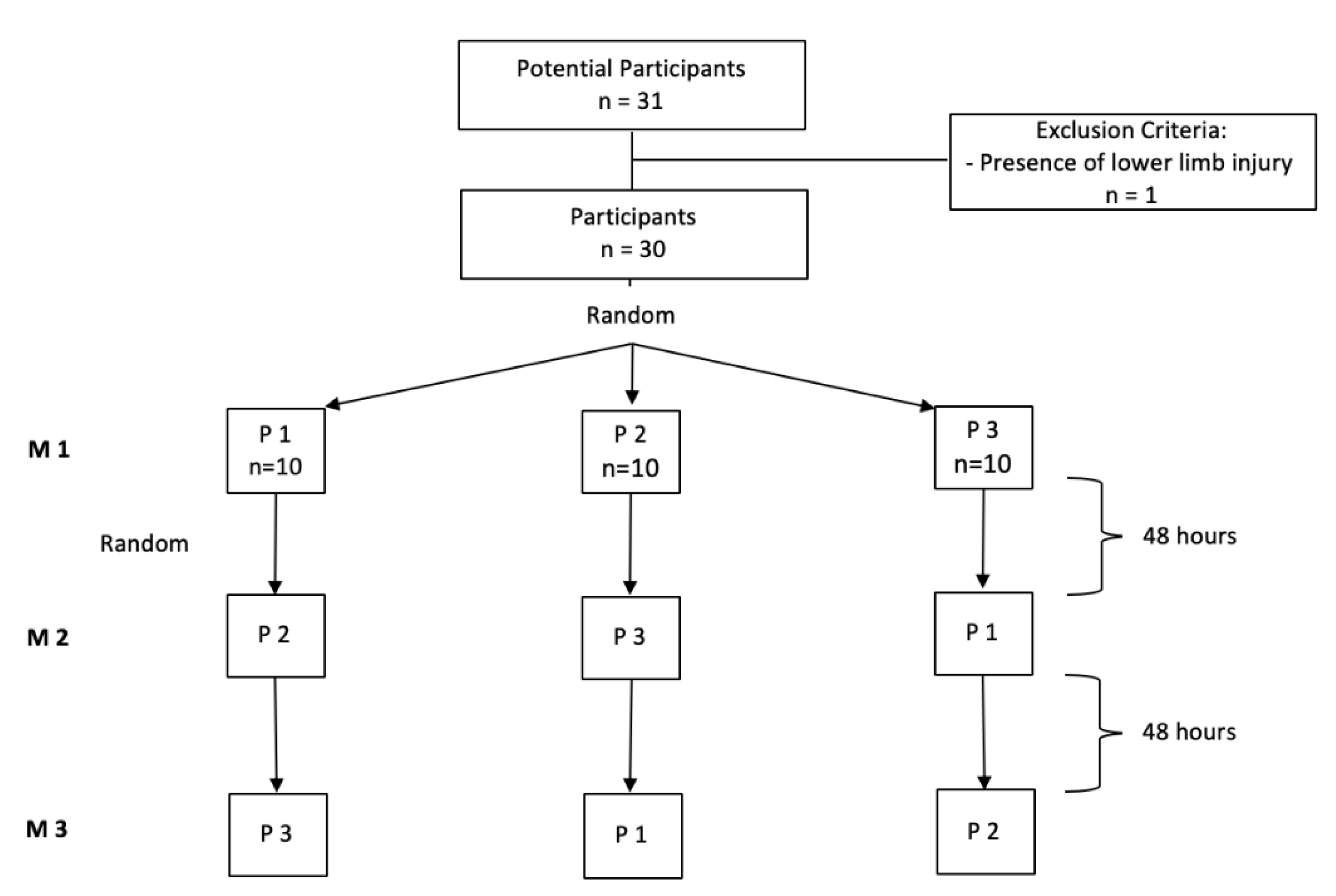
2.1. Study Design and Subjects
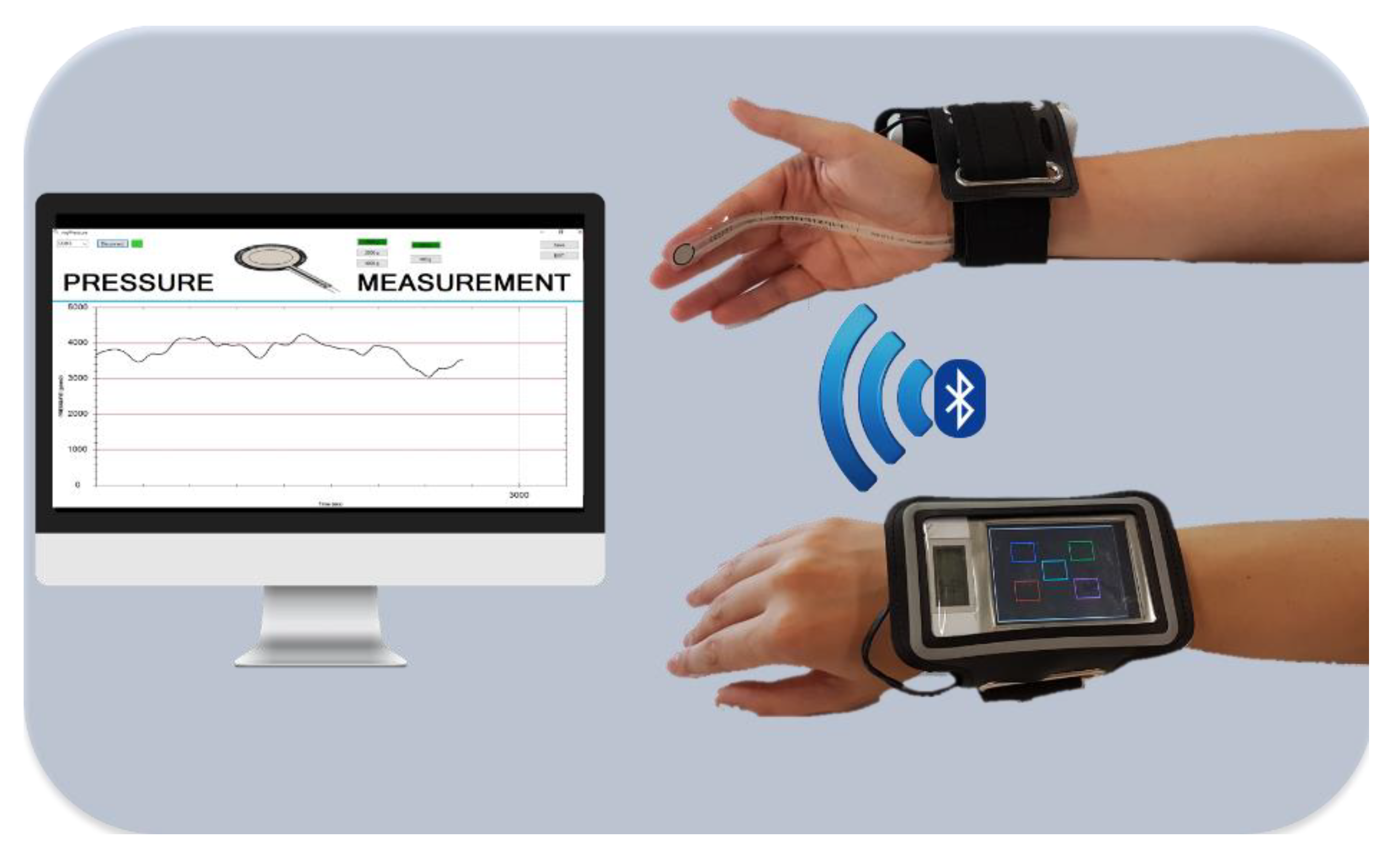
2.2. Assessment and Monitoring of the Pressure Intensity during DFM
2.3. Study 1
2.4. Study 2
2.5. Statistical Analysis
3. Results
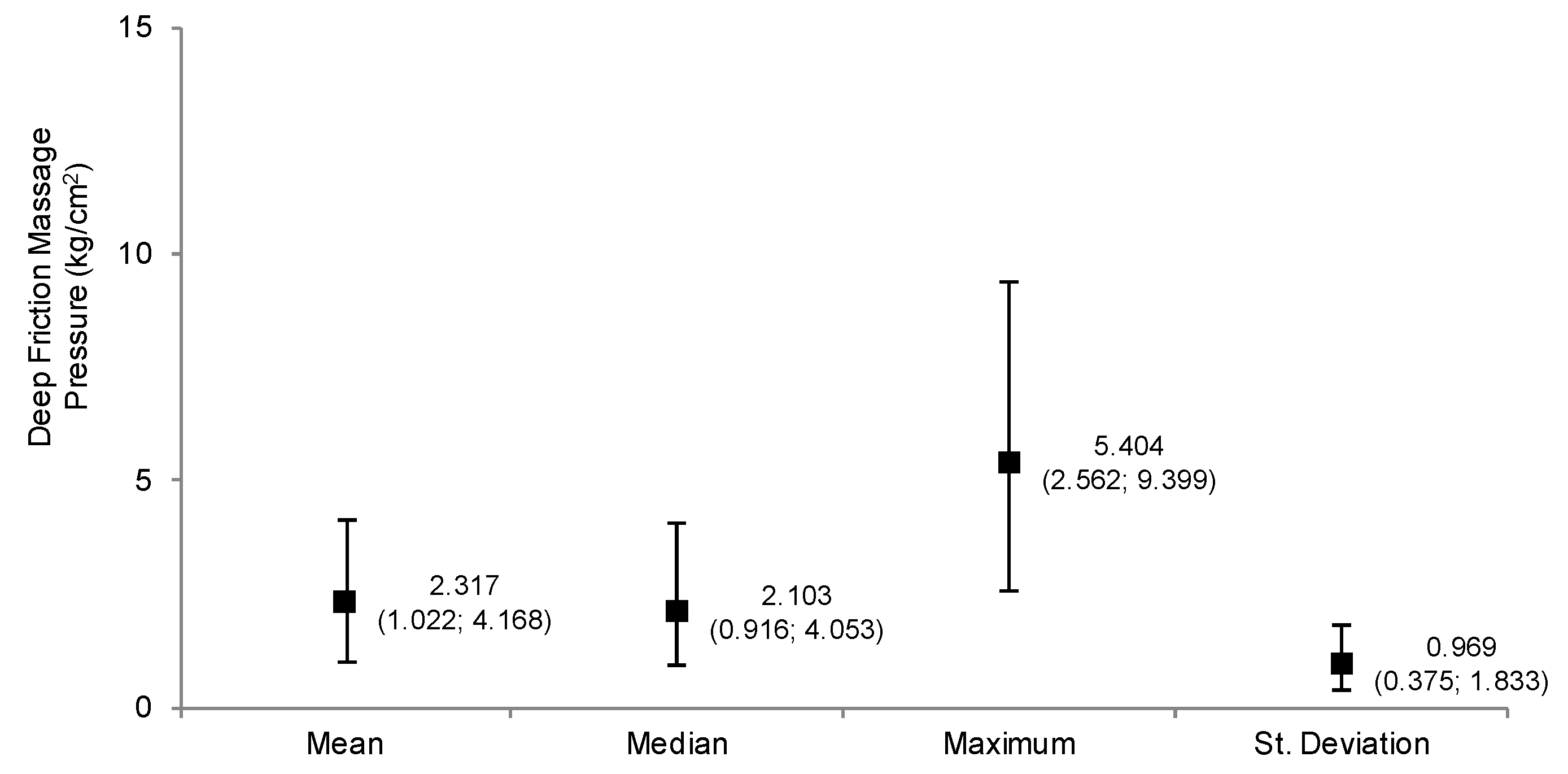
3.1. Characterization of the Pressures Applied during DFM (Study 1)
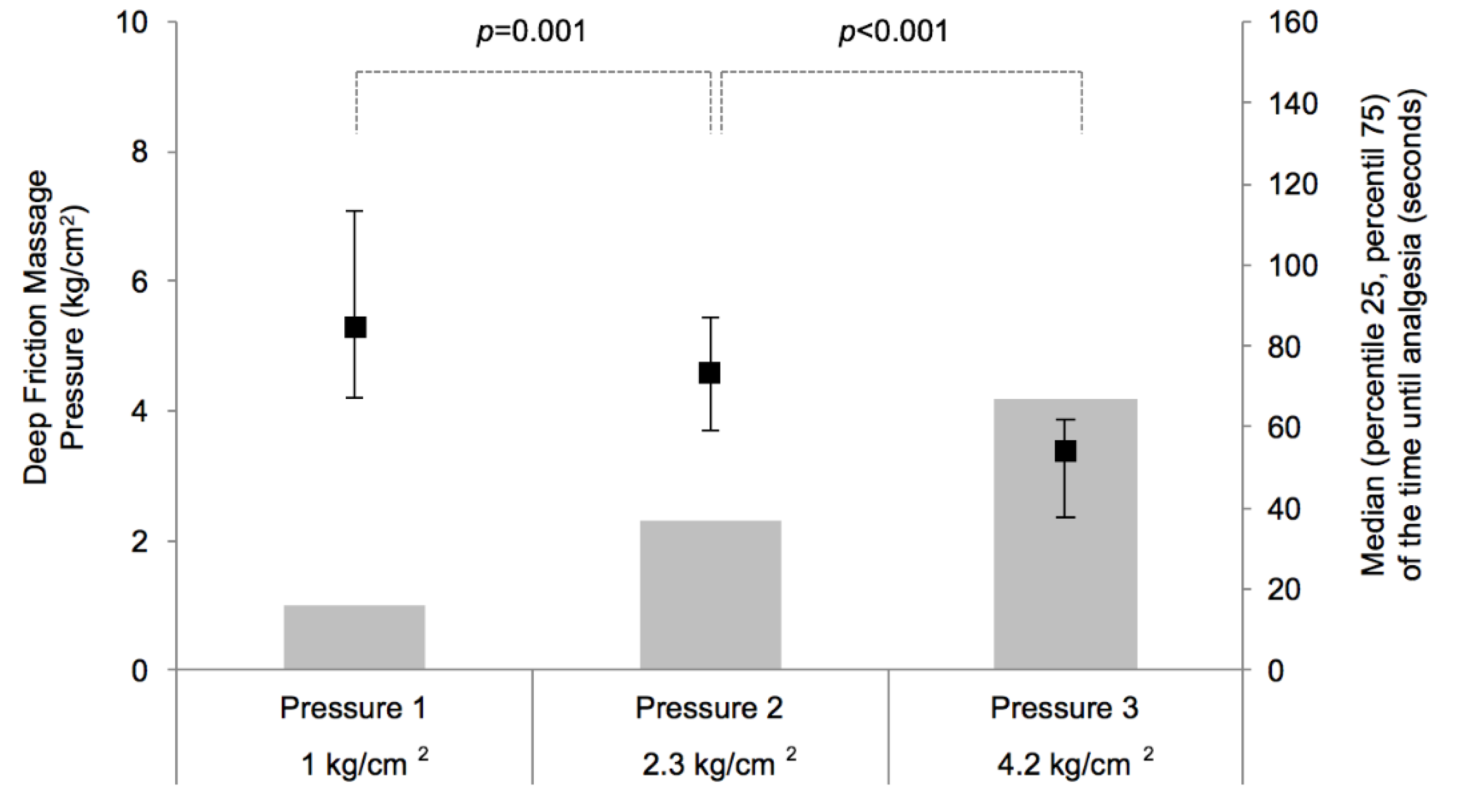
3.2. Effects of Different DFM Pressures on the Time to the Onsite of Analgesia (Study 2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brosseau, L.; Casimiro, L.; Milne, S.; Welch, V.; Shea, B.; Tugwell, P.; Wells, G.A. Deep Transverse Friction Massage for Treating Tendinitis. Cochrane Database Syst. Rev. 2002, 4, CD003528. [Google Scholar]
- Childress, M.A.; Beutler, A. Management of Chronic Tendon Injuries. Am. Fam. Phys. 2013, 87, 486–490. [Google Scholar]
- Cook, J.L.; Purdam, C.R. Is Tendon Pathology a Continuum? A Pathology Model to Explain the Clinical Presentation of Load-Induced Tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, R. Deep Transverse Friction: Its Analgesic Effect. Int. J. Sports Med. 1984, 5, 35–36. [Google Scholar] [CrossRef]
- Goats, G.C. Massage--the Scientific Basis of an Ancient Art: Part 2. Physiological and Therapeutic Effects. Br. J. Sports Med. 1994, 28, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, J.D. The Effect of Therapeutic Modalities on Tendinopathy. In Tendon Injuries: Basic Science and Clinical Medicine; Maffulli, N., Wayne, P.R., Leadbetter, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 9. [Google Scholar]
- Loppini, M.; Maffulli, N. Conservative Management of Tendinopathy: An Evidence-Based Approach. MuscleLigaments Tendons J. 2011, 1, 134–137. [Google Scholar]
- Maffulli, N.; Longo, U.G.; Denaro, V. Novel Approaches for the Management of Tendinopathy. J. Bone Jt. Surg. Am. 2010, 92, 2604–2613. [Google Scholar]
- Rees, J.D.; Maffulli, N.; Cook, J. Management of Tendinopathy. Am. J. Sports Med. 2009, 37, 1855–1867. [Google Scholar] [CrossRef]
- Stasinopoulos, D.; Johnson, M.I. Cyriax Physiotherapy for Tennis Elbow/Lateral Epicondylitis. Br. J. Sports Med. 2004, 38, 675–677. [Google Scholar] [CrossRef]
- Atkins, E.; Kerr, J.; Goodlad, E. A Practical Approach to Orthopaedic Medicine: Assessment, Diagnosis, Treatment, 3rd ed.; Churchill Livingstone: Edinburgh, UK, 2010. [Google Scholar]
- Chamberlain, G.J. Cyriax’s Friction Massage: A Review. J. Orthop. Sports Phys. Ther. 1982, 4, 16–22. [Google Scholar] [CrossRef]
- Gregory, M.A.; Deane, M.N.; Mars, M. Ultrastructural Changes in Untraumatised Rabbit Skeletal Muscle Treated with Deep Transverse Friction. Physiotherapy 2003, 89, 408–416. [Google Scholar] [CrossRef]
- Hassan, S.M.; Hafez, A.R.; Seif, H.E.; Kachanathu, S.J. The Effect of Deep Friction Massage Versus Stretching of Wrist Extensor Muscles in the Treatment of Patients with Tennis Elbow. Open J. Ther. Rehabil. 2016, 4, 48–54. [Google Scholar] [CrossRef]
- Viswas, R.; Ramachandran, R.; Anantkumar, P.K. Comparison of Effectiveness of Supervised Exercise Program and Cyriax Physiotherapy in Patients with Tennis Elbow (Lateral Epicondylitis): A Randomized Clinical Trial. Sci. World J. 2012, 2012, 939645. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain: A Comprehensive Model. Man. Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Meritxell, L.-T.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus Pathways in Pain Modulation. Neuroscience 2016, 338, 93–113. [Google Scholar]
- Pud, D.; Granovsky, Y.; Yarnitsky, D. The Methodology of Experimentally Induced Diffuse Noxious Inhibitory Control (Dnic)-Like Effect in Humans. Pain 2009, 144, 16–19. [Google Scholar] [CrossRef]
- Andrew, V.; Ryan, D.; Bruhns, P. The Role of Descending Modulation in Manual Therapy and Its Analgesic Implications: A Narrative Review. Pain Res. Treat. 2015, 2015, 1–11. [Google Scholar]
- Cyriax, J.H.; Cyriax, P.J. Cyriax’s Illustrated Manual of Orthopaedic Medicine, 2nd; Butterworth-Heinemann: Oxford, UK, 1993. [Google Scholar]
- Loew, L.M.; Brosseau, L.; Tugwell, P.; Wells, G.A.; Welch, V.; Shea, B.; Poitras, S.; De Angelis, G.; Rahman, P. Deep Transverse Friction Massage for Treating Lateral Elbow or Lateral Knee Tendinitis. Cochrane Database Syst. Rev. 2014, 11, CD003528. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.J.; Ganion, L.R.; Gehlsen, G.M.; Verhoestra, B.; Roepke, J.E.; Sevier, T.L. Rat Tendon Morphologic and Functional Changes Resulting from Soft Tissue Mobilization. Med. Sci. Sports Exerc. 1997, 29, 313–319. [Google Scholar] [CrossRef]
- Gehlsen, G.M.; LGanion, R.; Helfst, R. Fibroblast Responses to Variation in Soft Tissue Mobilization Pressure. Med. Sci. Sports Exerc. 1999, 31, 531–535. [Google Scholar] [CrossRef]
- Sterns Mary, L. Studies on the Development of Connective Tissue in Transparent Chambers in the Rabbit’s Ear. II. Am. J. Anat. 1940, 67, 55–97. [Google Scholar] [CrossRef]
- Paula, C.; Simões, D.; Paço, M.; Pinho, F.; Duarte, J.A.; Ribeiro, F. Cyriax’s Deep Friction Massage Application Parameters: Evidence from a Cross-Sectional Study with Physiotherapists. Musculoskelet. Sci. Pract. 2017, 14, 92–97. [Google Scholar]
- Joseph, M.F.; Taft, K.; Moskwa, M.; Denegar, C.R. Deep Friction Massage to Treat Tendinopathy: A Systematic Review of a Classic Treatment in the Face of a New Paradigm of Understanding. J. Sport Rehabil. 2012, 21, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.J.; Kage, V.; Anap, D. Effectiveness of Cyriax Physiotherapy in Subjects with Tennis Elbow. J. Nov. Physiother. 2013, 3. [Google Scholar] [CrossRef]
- Blackwood, J.; Ghazi, F. Can the Addition of Transverse Friction Massage to an Exercise Programme in Treatment of Infrapatellar Tendinopathy Reduce Pain and Improve Function? A Pilot Study. Int. Musculoskelet. Med. 2012, 34, 108–114. [Google Scholar] [CrossRef]
- Goats, G.C. Massage--the Scientific Basis of an Ancient Art: Part 1. The Techniques. Br. J. Sports Med. 1994, 28, 149–152. [Google Scholar] [CrossRef]
- Yarnitsky, D. Conditioned Pain Modulation (the Diffuse Noxious Inhibitory Control-Like Effect): Its Relevance for Acute and Chronic Pain States. Curr. Opin. Anesthesiol. 2010, 23, 611–615. [Google Scholar] [CrossRef]
- Wright, A.; Sluka, K.A. Nonpharmacological Treatments for Musculoskeletal Pain. Clin. J. Pain 2001, 17, 33–46. [Google Scholar] [CrossRef]
- Antti, P. The Noradrenergic Pain Regulation System: A Potential Target for Pain Therapy. Eur. J. Pharmacol. 2013, 716, 2–7. [Google Scholar]
| Final Sample | ||
|---|---|---|
| n | (%) | |
| Sex | ||
| Female | 29 | 72.5 |
| Male | 11 | 27.5 |
| Academic degree | ||
| Physiotherapy degree | 34 | 85.0 |
| Master degree | 6 | 15.0 |
| Workplace | ||
| Public hospital | 2 | 5.0 |
| Private hospital | 1 | 2.5 |
| Private practice, national health service | 28 | 70.0 |
| Private practice, others | 9 | 22.5 |
| Mean (SD) | ||
| Age (years) | 30.5 (6.10) | |
| Weight (kg) | 63.6 (9.90) | |
| Height (cm) | 168.7 (7.24) | |
| BMI (kg/m2) | 22.3 (3.02) | |
| Median (percentile 25 (P25); percentile 75 (P75)) | ||
| Clinical experience (years) | 7.0 (1.00; 10.00) | |
| Work intensity (hour per week) | 40.0 (30.00; 40.00) | |
| Number of patients treated (N per hour) | 4.0 (3.00; 4.75) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, P.; Simões, D.; Paço, M.; Pinho, F.; Duarte, J.A.; Ribeiro, F. Pressure Applied during Deep Friction Massage: Characterization and Relationship with Time of Onset of Analgesia. Appl. Sci. 2020, 10, 2705. https://doi.org/10.3390/app10082705
Chaves P, Simões D, Paço M, Pinho F, Duarte JA, Ribeiro F. Pressure Applied during Deep Friction Massage: Characterization and Relationship with Time of Onset of Analgesia. Applied Sciences. 2020; 10(8):2705. https://doi.org/10.3390/app10082705
Chicago/Turabian StyleChaves, Paula, Daniela Simões, Maria Paço, Francisco Pinho, José Alberto Duarte, and Fernando Ribeiro. 2020. "Pressure Applied during Deep Friction Massage: Characterization and Relationship with Time of Onset of Analgesia" Applied Sciences 10, no. 8: 2705. https://doi.org/10.3390/app10082705
APA StyleChaves, P., Simões, D., Paço, M., Pinho, F., Duarte, J. A., & Ribeiro, F. (2020). Pressure Applied during Deep Friction Massage: Characterization and Relationship with Time of Onset of Analgesia. Applied Sciences, 10(8), 2705. https://doi.org/10.3390/app10082705






