An Alternative Approach to Cataract Surgery Using BSS Temperature of 2.7 °C
Abstract
1. Introduction
2. Materials and Methods
- Bottle kept in the operating room for at least three hours
- Bottle kept in a thermal “coat” made of water and ice.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thylefors, B. The World Health Organization’s programme for the prevention of blindness. Int. Ophthalmol. 1990, 14, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Glynn, R.J.; Ajani, U.A. Smoking cessation and risk of age-related cataract in men. JAMA 2000, 284, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Doors, M.; Berendschot, T.T.J.M.; Touwslager, W.; Webers, C.A.; Nuijts, R.M.M.A. Phacopower modulation and the risk for postoperative corneal decompensation: A randomized clinical trial. JAMA Ophthalmol. 2013, 131, 1443–1450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lundberg, B.; Jonsson, M.; Behndig, A. Postoperative corneal swelling correlates strongly to corneal endothelial cell loss after phacoemulsification cataract surgery. Am. J. Ophthalmol. 2005, 139, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Spalton, D.J. Changes in anterior chamber flare and cells following cataract surgery. Br. J. Ophthalmol. 1994, 78, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.S. Cystoid macular edema after cataract surgery. J. Cataract Refract. Surg. 2018, 44, 1536. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.V.; Boianovsky, C.; Saraiva, T.J.; Godoy, R.B.; de Lake, J. Safety and efficacy of intracameral moxifloxacin injection for prophylaxis of endophthalmitis after phacoemulsification. Arq. Bras. Oftalmol. 2017, 80, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Lim, D.H.; Yang, C.M.; Chung, E.S.; Chung, T.Y. Comparison of Intraocular Lens Power Calculation Methods Following Myopic Laser Refractive Surgery: New Options Using a Rotating Scheimpflug Camera. Korean J. Ophthalmol. 2018, 32, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Zhao, J.; Xu, M.; Yu, Z. Isolated Capsulorhexis Flap Technique in Femtosecond Laser-Assisted Cataract Surgery to Protect the Corneal Endothelial Cells. J. Investig. Surg. 2017, 32, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Stănilă, D.M.; Florea, A.M.; Stănilă, A.; Panga, A.A. Endothelial cells loss to the hyperopic pacients during phacoemulsification. Rom. J. Ophthalmol. 2017, 61, 256. [Google Scholar]
- Pajic, B.; Cvejic, Z.; Pajic-Eggspuehler, B. Cataract Surgery Performed by High Frequency LDV Z8 Femtosecond Laser: Safety, Efficacy, and Its Physical Properties. Sensors 2017, 17, 1429. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Jacob, S. Current and effective advantages of femtophacoemulsification. Curr. Opin. Ophthalmol. 2017, 28, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, A.J.; Asimellis, G. Standard manual capsulorhexis/Ultrasound phacoemulsification compared to femtosecond laser-assisted capsulorhexis and lens fragmentation in clear cornea small incision cataract surgery. Eye Vis. 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Barth, U.; Çubuk, H.; Koch, H.R. Effect of irrigating solution and irrigation temperature on the cornea and pupil during phacoemulsification. J. Cataract Refract. Surg. 2000, 26, 392–397. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- R2Evo 3DMarkerLab. Available online: http://www.3dmarketlab.it (accessed on 20 January 2020).
- Handheld Meter pH315i Operating Manual. Available online: http://www.globalw.com/downloads/WQ/PH3150i.pdf (accessed on 20 January 2020).
- Chylack, L.T.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S. The Lens Opacities Classification System III—Longitudinal Study of cataract study Group. Arch. Ophtalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Whitestar Signature PRO—Johnson & Johnson Vision. Available online: https://www.dc-ophthalmology.com/products/193 (accessed on 20 January 2020).
- SL990-Series Slitlamps Operating Manual. Available online: http://www.iproweb.fr/test/0%20-%20MATOS%20GUILLAUME/CSO%20-%20SL990%20TYPE%205X%20D%20LAMPE%20A%20FENTE/SL990.pdf (accessed on 20 January 2020).
- Takahashi, H. Corneal Endothelium and Phacoemulsification. Cornea 2016, 35, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- R Software Open Source. Available online: https://rextester.com/l/r_online_compiler (accessed on 8 January 2020).
- Walkow, T.; Anders, N.; Klebe, S. Endothelial cell loss after phacoemulsification: Relation to preoperative and intraoperative parameters. J. Cataract Refract. Surg. 2000, 26, 727–732. [Google Scholar] [CrossRef]



| LOCS III Classification | Grade of Opacities | % of Patients |
|---|---|---|
| Nuclear Opalescence (NO) and Nuclear Color (NC) | ≥3 | 75% |
| Cortical Cataract (C) | ≥3 | 20% |
| Posterior Subcapsular Cataract (P) | ≥3 | 5% |
| Group A | Group B | |
|---|---|---|
| Number of patients | 110 | 104 |
| Males | 67 | 63 |
| Females | 43 | 41 |
| Mean age | 70 ± 8.5 | 68 ± 8.2 |
| OD | 58 | 48 |
| OS | 52 | 54 |
| Preoperative status lens Phakic Pseudophakic | 88 22 | 87 17 |
| Surgery Condition | Solution Temperature in the Bottle (°C) | Temperature Output from the Handpiece (°C) | Time |
|---|---|---|---|
| 1 | 18.0 ± 0.1 | 19.0 ± 0.1 | 4 min |
| 2 | 2.7 ± 0.1 | 7.4 ± 0.1 | 4 min |
| Surgery Condition | Average Temperature Variation (°C) over the Time | Maximum Temperature Variation (°C) over the Time | Time |
|---|---|---|---|
| 1 | +2.5 (from 19 °C to 21.5 °C) | +3.1 (from 19 °C to 22.1 °C) | 1 min |
| 2 | +0.4 (from 7.4 °C to 7.8 °C) | +1.0 (from 7.8 °C to 8.4 °C) | 1 min |
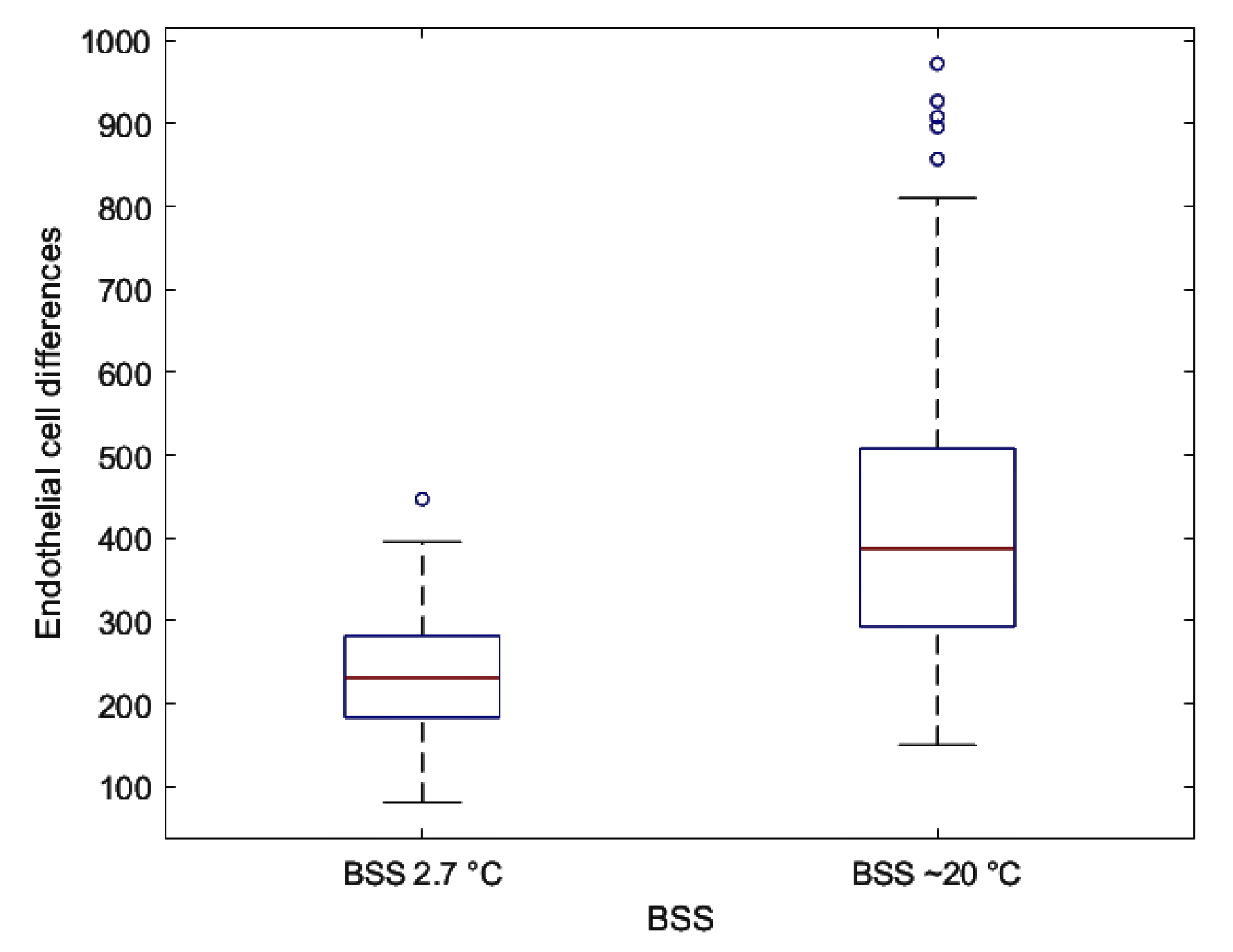
| Treatment | Endothelial Cells at 2.7 °C Mean ± SD | Endothelial Cells at ~20 °C Mean ± SD | p-Value (Student’s Unpaired t-Test) |
|---|---|---|---|
| PRE | 2354 ± 308.93 | 2295 ± 234.46 | 0.12 |
| POST | 2113 ± 283.61 | 1877 ± 276.54 | <0.001 § |
| p-value (Student’s paired t-test) | <0.001 § | <0.001 § | |
| Differences | 240.87 ± 73.09 | 418.63 ± 185.79 | <0.001 § |
| Treatment | AVG | Patients Number | Mean ± SD | p | |
|---|---|---|---|---|---|
| PRE | 0–9 | 45 | 2316.2 ± 255.37 | 0.7 | Group A (BSS ~20 °C) |
| 10–19 | 49 | 2275.45 ± 221.15 | |||
| 20–29 | 16 | 2297.15 ± 221.47 | |||
| POST | 0–9 | 45 | 2050.31 ± 240.56 | <0.001 § | |
| 10–19 | 49 | 1830.79 ± 210.55 | |||
| 20–29 | 16 | 1528.62 ± 132.56 | |||
| PRE | 0–9 | 23 | 2312.08 ± 313.02 | 0.58 | Group B (BSS 2.7 °C) |
| 10–19 | 53 | 2368.87 ± 333.57 | |||
| 20–29 | 23 | 2392.35 ± 261.01 | |||
| >30 | 5 | 2215.2 ± 227.77 | |||
| POST | 0–9 | 23 | 2139.65 ± 290.43 | 0.13 | |
| 10–19 | 53 | 2141.3 ± 295.09 | |||
| 20–29 | 23 | 2081.96 ± 242.66 | |||
| >30 | 5 | 1838.4 ± 191.84 |
| AVG | Treatment | Group A Mean ± SD | Group B Mean ± SD | p-Value (Student’s Unpaired t-Test) |
|---|---|---|---|---|
| 0–9 | PRE | 2316.2 ± 255.37 | 2312.08 ± 313.02 | 0.95 |
| POST | 2050.31 ± 240.56 § | 2139.65 ± 290.43 § | 0.18 | |
| 10–19 | PRE | 2275.45 ± 221.15 | 2368.87 ± 333.57 | 0.1 |
| POST | 1830.79 ± 210.55 § | 2141.3 ± 295.09 § | <0.001 §§ | |
| 20–29 | PRE | 2297.15 ± 221.47 | 2392.35 ± 261.01 | 0.24 |
| POST | 1528.62 ± 132.56 § | 2081.96 ± 242.66 § | <0.001 §§ | |
| >30 | PRE | - | 2215.2 ± 227.77 | - |
| POST | - | 1838.4 ± 191.84 § | - |
| AVG | Group A Mean ± SD | Group B Mean ± SD | p-Value (Student’s Unpaired t-Test) |
|---|---|---|---|
| 0–9 | 268.19 ± 69.14 | 172.44 ± 29.22 | <0.001 § |
| 10–19 | 446.17 ± 79.39 | 227.57 ± 52.79 | <0.001 § |
| 20–29 | 768.5 ± 119.74 | 310.39 ± 50.89 | <0.001 § |
| >30 | - | 376.8 ± 47.44 | - |
| p-value (ANOVA) | <0.001§ | <0.001§ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meduri, A.; Aragona, P.; Testagrossa, B.; Scolaro, S.; Gurgone, S.; Bonanno, L.; Caridi, F.; Acri, G. An Alternative Approach to Cataract Surgery Using BSS Temperature of 2.7 °C. Appl. Sci. 2020, 10, 2682. https://doi.org/10.3390/app10082682
Meduri A, Aragona P, Testagrossa B, Scolaro S, Gurgone S, Bonanno L, Caridi F, Acri G. An Alternative Approach to Cataract Surgery Using BSS Temperature of 2.7 °C. Applied Sciences. 2020; 10(8):2682. https://doi.org/10.3390/app10082682
Chicago/Turabian StyleMeduri, Alessandro, Pasquale Aragona, Barbara Testagrossa, Sergio Scolaro, Sergio Gurgone, Lilla Bonanno, Francesco Caridi, and Giuseppe Acri. 2020. "An Alternative Approach to Cataract Surgery Using BSS Temperature of 2.7 °C" Applied Sciences 10, no. 8: 2682. https://doi.org/10.3390/app10082682
APA StyleMeduri, A., Aragona, P., Testagrossa, B., Scolaro, S., Gurgone, S., Bonanno, L., Caridi, F., & Acri, G. (2020). An Alternative Approach to Cataract Surgery Using BSS Temperature of 2.7 °C. Applied Sciences, 10(8), 2682. https://doi.org/10.3390/app10082682








