Evaluating Earthworms’ Potential for Remediating Soils Contaminated with Olive Mill Waste Sediments
Abstract
1. Introduction
2. Materials and Methods
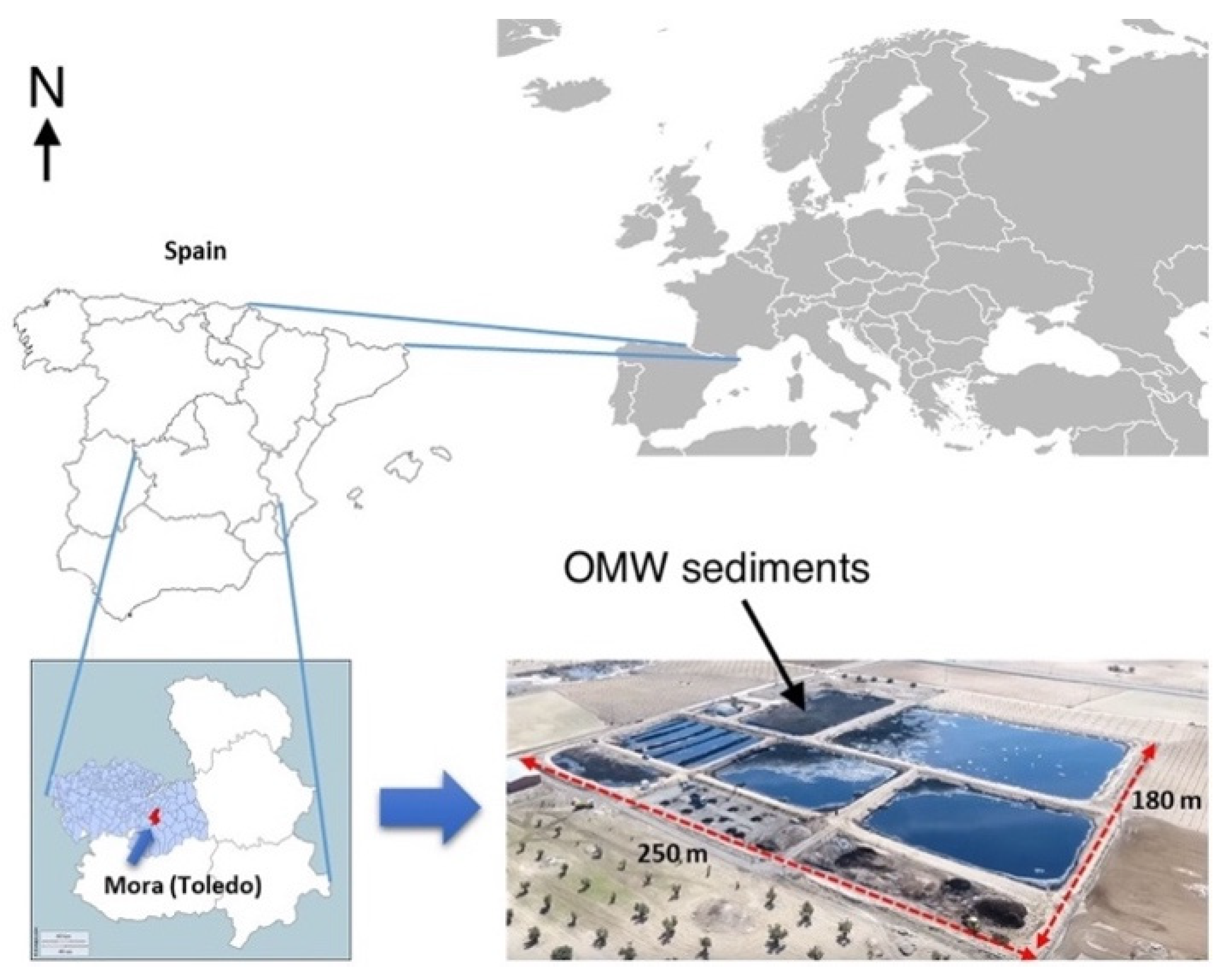
2.1. Study Area, OMW Sediments, Soil and Earthworms
2.2. Experimental Design
2.3. Sample Preparation for (Bio)Chemical Analysis
2.4. Earthworm Biomarkers
2.5. Soil and Cast Enzyme Activities
2.6. Physicochemical Analysis of OMW-Amended Soil
2.7. Data Analysis
3. Results and Discussion
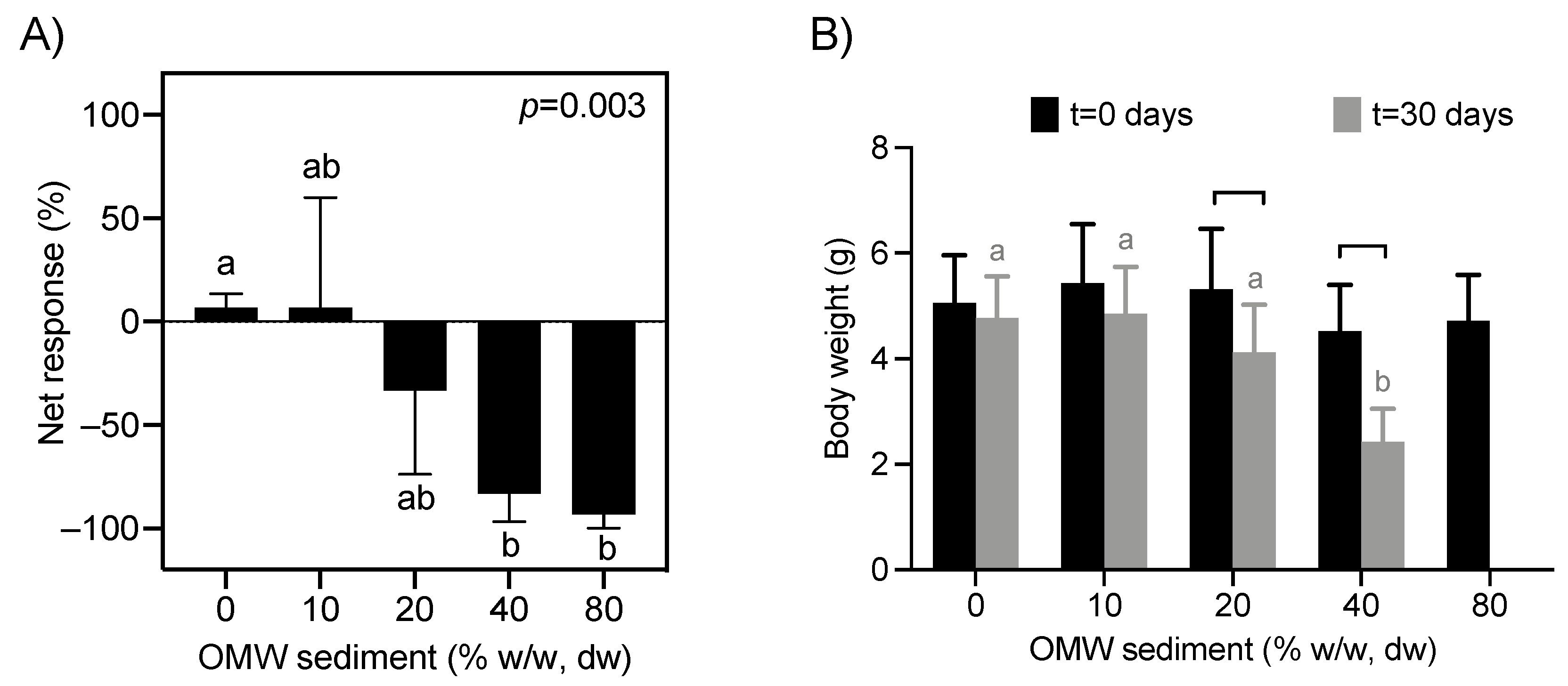
3.1. Avoidance Behaviour Response Test
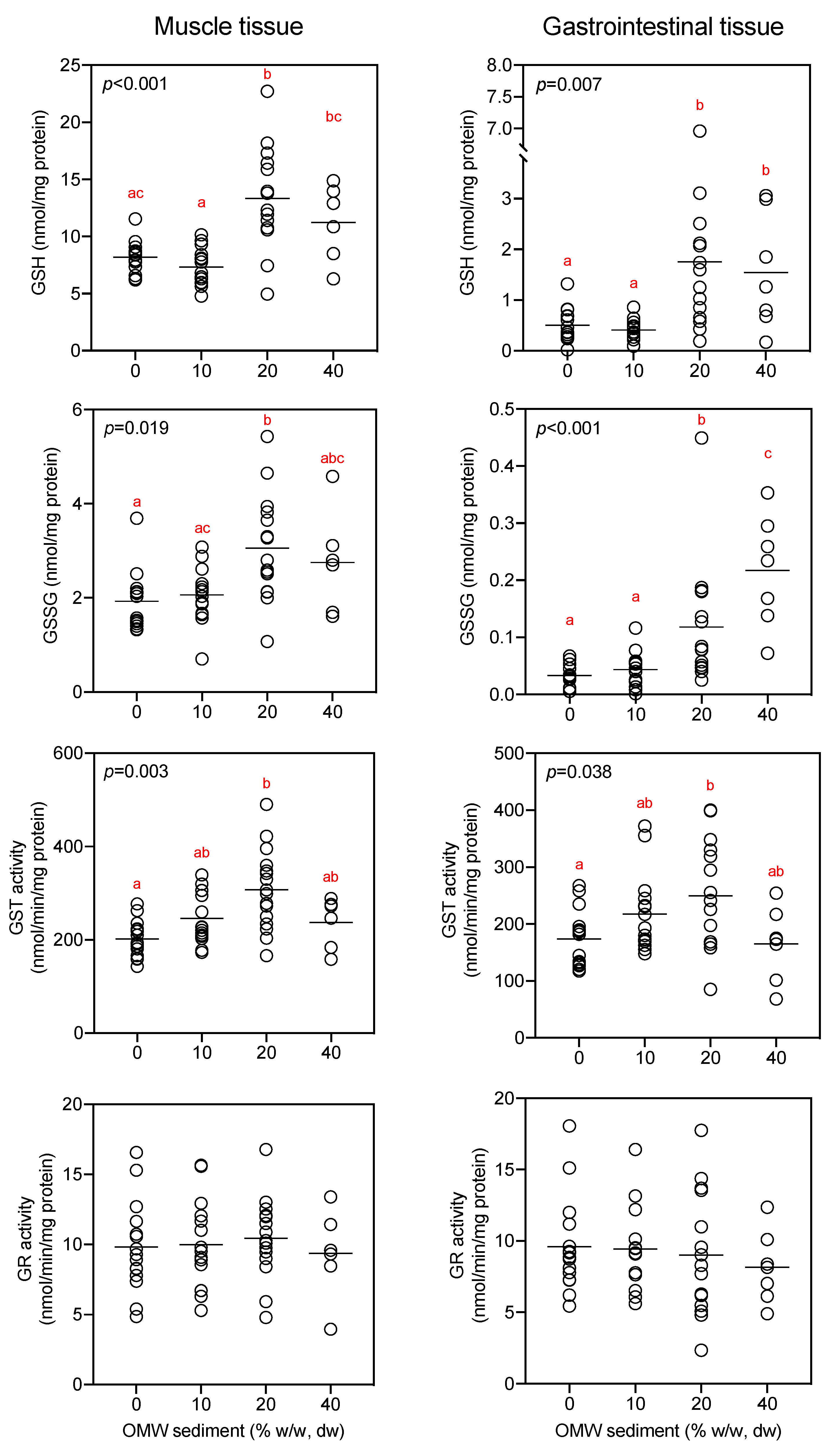
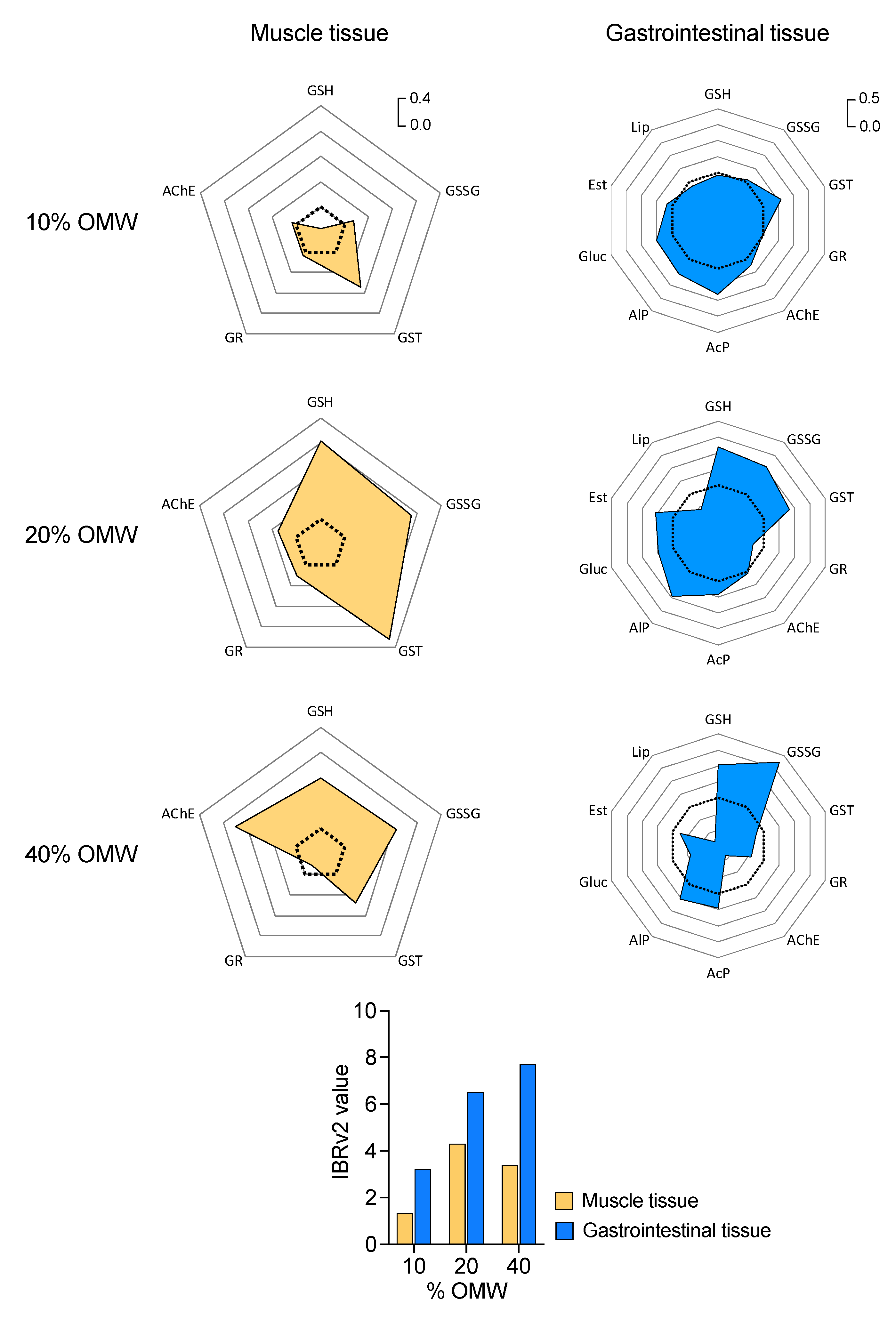
3.2. Microcosm: Impact of OMW on Earthworm Biomarkers
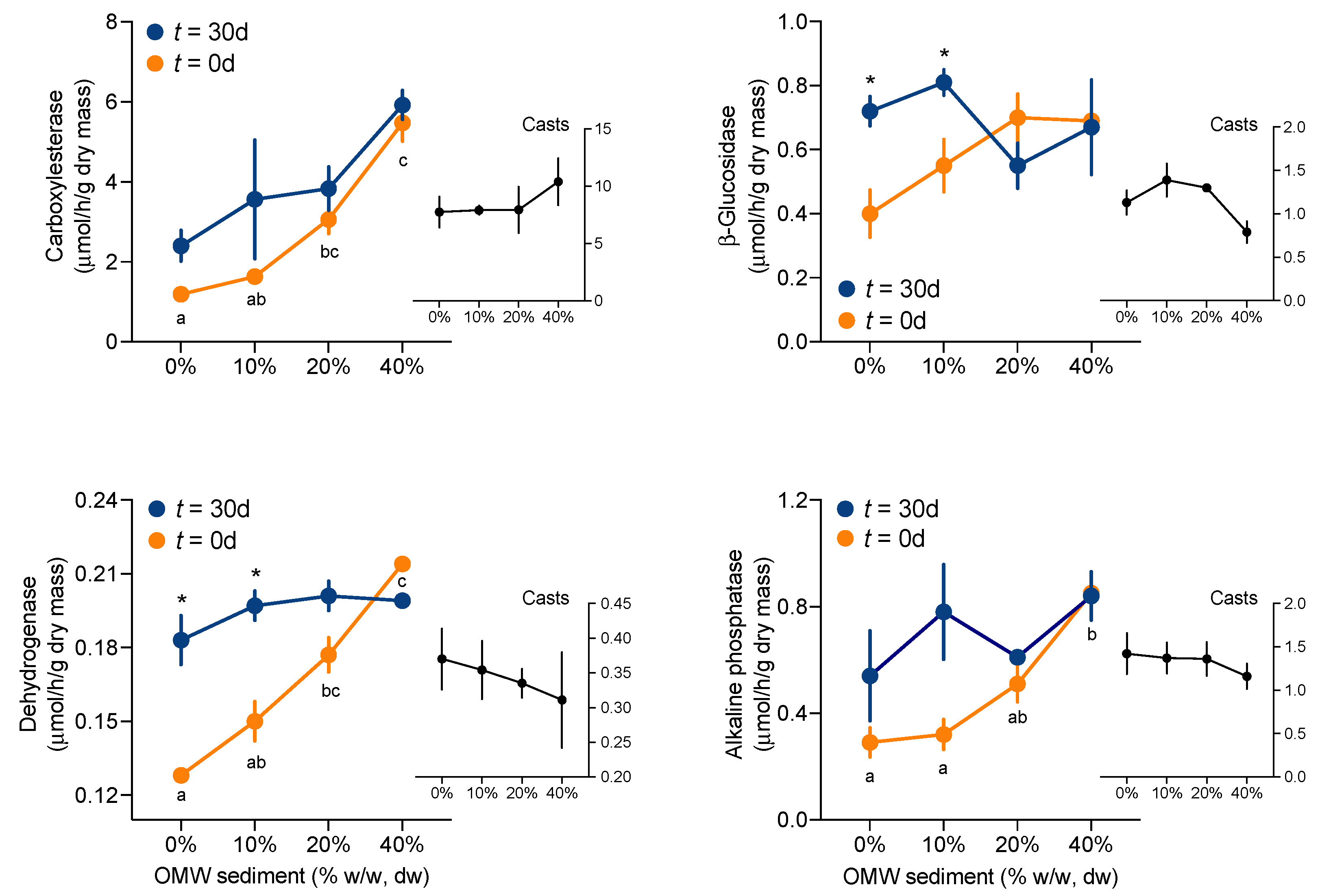
3.3. Microcosm: Impact of OMW on Soil Enzyme Activities
3.4. Microcosm: Impact of Earthworms on Soil Physicochemical Variables
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kavvadias, V.; Doula, M.K.; Komnitsas, K.; Liakopoulou, N. Disposal of olive oil mill wastes in evaporation ponds: Effects on soil properties. J. Hazard. Mater. 2010, 182, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Kavvadias, V.; Elaiopoulos, K.; Theocharopoulos, S.; Soupios, P. Fate of Potential Contaminants Due to Disposal of Olive Mill Wastewaters in Unprotected Evaporation Ponds. Bull. Environ. Contam. Toxicol. 2017, 98, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Proc. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Senol, A.; Hasdemir, İ.M.; Hasdemir, B.; Kurdaş, İ. Adsorptive removal of biophenols from olive mill wastewaters (OMW) by activated carbon: Mass transfer, equilibrium and kinetic studies. Asia-Pac. J. Chem. Eng. 2017, 12, 128–146. [Google Scholar] [CrossRef]
- Hentati, O.; Oliveira, V.; Sena, C.; Bouji, M.S.M.; Wali, A.; Ksibi, M. Soil contamination with olive mill wastes negatively affects microbial communities, invertebrates and plants. Ecotoxicology 2016, 25, 1500–1513. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Belaqziz, M.; Wichern, M.; Lübken, M. Phytotoxicity assessment of olive mill wastewater treated by different technologies: Effect on seed germination of maize and tomato. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Malev, O.; Pflieger, M.; Lebedev, A.T.; Mazur, D.M.; Kužić, A.; Čož-Rakovac, R.; Trebše, P. Toxicity evaluation of olive oil mill wastewater and its polar fraction using multiple whole-organism bioassays. Sci. Total Environ. 2019, 686, 903–914. [Google Scholar] [CrossRef]
- Smeti, E.; Kalogianni, E.; Karaouzas, I.; Laschou, S.; Tornés, E.; De Castro-Català, N.; Anastasopoulou, E.; Koutsodimou, M.; Andriopoulou, A.; Vardakas, L.; et al. Effects of olive mill wastewater discharge on benthic biota in Mediterranean streams. Environ. Pollut. 2019, 254, 113057. [Google Scholar] [CrossRef]
- Aharonov-Nadborny, R.; Tsechansky, L.; Raviv, M.; Graber, E.R. Mechanisms governing the leaching of soil metals as a result of disposal of olive mill wastewater on agricultural soils. Sci. Total Environ. 2018, 630, 1115–1123. [Google Scholar] [CrossRef]
- Zema, D.A.; Esteban Lucas-Borja, M.; Andiloro, S.; Tamburino, V.; Zimbone, S.M. Short-term effects of olive mill wastewater application on the hydrological and physico-chemical properties of a loamy soil. Agric. Water Manag. 2019, 221, 312–321. [Google Scholar] [CrossRef]
- Meftah, O.; Guergueb, Z.; Braham, M.; Sayadi, S.; Mekki, A. Long term effects of olive mill wastewaters application on soil properties and phenolic compounds migration under arid climate. Agric. Water Manag. 2019, 212, 119–125. [Google Scholar] [CrossRef]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Sáez, J.A.; Moral, R.; Moreno, J. Bioremediation of Olive Mill Wastewater sediments in evaporation ponds through in situ composting assisted by bioaugmentation. Sci. Total Environ. 2019, 135537. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Campos, J.; Dendooven, L.; Alvarez-Bernal, D.; Contreras-Ramos, S.M. Potential of earthworms to accelerate removal of organic contaminants from soil: A review. Appl. Soil Ecol. 2014, 79, 10–25. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Reference Base for Soil Resources 2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Lowe, C.N.; Butt, K.R. Culture techniques for soil dwelling earthworms: A review. Pedobiologia 2005, 49, 401–413. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Ríos, J.M.; Attademo, A.M.; Malcevschi, A.; Andrade Cares, X. Assessing biochar impact on earthworms: Implications for soil quality promotion. J. Hazard. Mater. 2019, 366, 582–591. [Google Scholar] [CrossRef]
- ISO. Soil Quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour-Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei); ISO: Geneva, Switzerland, 2007. [Google Scholar]
- De Silva, P.M.C.S.; van Gestel, C.A.M. Comparative sensitivity of Eisenia andrei and Perionyx excavatus in earthworm avoidance tests using two soil types in the tropics. Chemosphere 2009, 77, 1609–1613. [Google Scholar] [CrossRef]
- Deng, S.; Popova, I.E.; Dick, L.; Dick, R. Bench scale and microplate format assay of soil enzyme activities using spectroscopic and fluorometric approaches. Appl. Soil Ecol. 2013, 64, 84–90. [Google Scholar] [CrossRef]
- Wheelock, C.E.; Eder, K.J.; Werner, I.; Huang, H.; Jones, P.D.; Brammell, B.F.; Elskus, A.A.; Hammock, B.D. Individual variability in esterase activity and CYP1A levels in Chinook salmon (Oncorhynchus tshawytscha) exposed to esfenvalerate and chlorpyrifos. Aquat. Toxicol. 2005, 74, 172–192. [Google Scholar] [CrossRef]
- Eyer, P.; Worek, F.; Kiderlen, D.; Sinko, G.; Stuglin, A.; Simeon-Rudolf, V.; Reiner, E. Molar absorption coefficients for the reduced Ellman reagent: Reassessment. Anal. Biochem. 2003, 312, 224–227. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Ramos-Martinez, J.I.; Bartolomé, T.R.; Pernas, R.V. Purification and properties of glutathione reductase from hepatopancreas of Mytilus edulis L. Comp. Biochem. Physiol. 1983, 75B, 689–692. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Ito, K.; Miura, C.; Miura, T. Examination of digestive enzyme distribution in gut tract and functions of intestinal caecum, in megascolecid earthworms (Oligochaeta: Megascolecidae) in Japan. Zool. Sci. 2013, 30, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Rathi, P.; Gupta, R. Simplified para-nitrophenyl palmitate assay for lipases and esterases. Anal. Biochem. 2002, 311, 98–99. [Google Scholar] [CrossRef]
- Thompson, H.M. Esterases as markers of exposure to organophosphates and carbamates. Ecotoxicology 1999, 8, 369–384. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Ríos, J.M.; Attademo, A.M. Response of digestive enzymes and esterases of ecotoxicological concern in earthworms exposed to chlorpyrifos-treated soils. Ecotoxicology 2018, 27, 890–899. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Sandoval, M.; Pierart, A. Short-term response of soil enzyme activities in a chlorpyrifos-treated mesocosm: Use of enzyme-based indexes. Ecol. Indic. 2017, 73, 525–535. [Google Scholar] [CrossRef]
- Lessard, I.; Sauvé, S.; Deschênes, L. Toxicity response of a new enzyme-based functional diversity methodology for Zn-contaminated field-collected soils. Soil Biol. Biochem. 2014, 71, 87–94. [Google Scholar] [CrossRef]
- Shaw, L.J.; Burns, R.G. Enzyme Activity Profiles and Soil Quality; CABI: Oxford, UK, 2006; pp. 158–182. [Google Scholar]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Moreno-Caselles, J.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Bernal, M.P. Co-composting of distillery and winery wastes with sewage sludge. Water Sci. Technol. 2007, 56, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Kitson, R.E.; Mellon, M.G. Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Ind. Eng. Chem. Anal. Ed. 1944, 16, 379–383. [Google Scholar] [CrossRef]
- Beltrán, F.J.; García-Araya, J.F.; Álvarez, P.M. Wine Distillery Wastewater Degradation. 1. Oxidative Treatment Using Ozone and Its Effect on the Wastewater Biodegradability. J. Agric. Food Chem. 1999, 47, 3911–3918. [Google Scholar] [CrossRef]
- Sanchez, W.; Burgeot, T.; Porcher, J.M. A novel Integrated Biomarker Response calculation based on reference deviation concept. Environ. Sci. Pollut. Res. Int. 2013, 20, 2721–2725. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Achazi, R.; Römbke, J.; Warnecke, D. Avoidance test with Eisenia fetida as indicator for the habitat function of soils: Results of a laboratory comparison test. J. Soils Sediments 2003, 3, 7–12. [Google Scholar] [CrossRef]
- Delgadillo, V.; Verdejo, J.; Mondaca, P.; Verdugo, G.; Gaete, H.; Hodson, M.E.; Neaman, A. Proposed modification to avoidance test with Eisenia fetida to assess metal toxicity in agricultural soils affected by mining activities. Ecotoxicol. Environ. Saf. 2017, 140, 230–234. [Google Scholar] [CrossRef]
- De Sousa, A.P.; de Andréa, M.M. Earthworm (Eisenia andrei) avoidance of soils treated with cypermethrin. Sensors (Basel) 2011, 11, 11056–11063. [Google Scholar] [CrossRef]
- Saggioro, E.M.; do Espírito Santo, D.G.; Sales Júnior, S.F.; Hauser-Davis, R.A.; Correia, F.V. Lethal and sublethal effects of acetamiprid on Eisenia andrei: Behavior, reproduction, cytotoxicity and oxidative stress. Ecotoxicol. Environ. Saf. 2019, 183, 109572. [Google Scholar] [CrossRef]
- Cao, Q.; Steinman, A.D.; Yao, L.; Xie, L. Toxicological and biochemical responses of the earthworm Eisenia fetida to cyanobacteria toxins. Sci. Rep. 2017, 7, 15954. [Google Scholar] [CrossRef]
- Alves, P.R.L.; Bandeira, F.O.; Giraldi, M.; Presotto, R.; Segat, J.C.; Cardoso, E.J.B.N.; Baretta, D. Ecotoxicological assessment of Fluazuron: Effects on Folsomia candida and Eisenia andrei. Environ. Sci. Pollut. Res. Int. 2019, 26, 5842–5850. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Katsiamides, A.; Abbass, M.; Sturzenbaum, S.R.; Thorpe, K.L.; Hodson, M.E. Polyester-derived microfibre impacts on the soil-dwelling earthworm Lumbricus terrestris. Environ. Pollut. 2019, 251, 453–459. [Google Scholar] [CrossRef]
- Elliston, T.; Oliver, I.W. Ecotoxicological assessments of biochar additions to soil employing earthworm species Eisenia fetida and Lumbricus terrestris. Environ. Sci. Pollut. Res. Int. 2019. [Google Scholar] [CrossRef]
- Li, D.; Hockaday, W.C.; Masiello, C.A.; Alvarez, P.J.J. Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol. Biochem. 2011, 43, 1732–1737. [Google Scholar] [CrossRef]
- Brami, C.; Glover, A.R.; Butt, K.R.; Lowe, C.N. Avoidance, biomass and survival response of soil dwelling (endogeic) earthworms to OECD artificial soil: Potential implications for earthworm ecotoxicology. Ecotoxicology 2017, 26, 576–579. [Google Scholar] [CrossRef]
- Pelosi, C.; Toutous, L.; Chiron, F.; Dubs, F.; Hedde, M.; Muratet, A.; Ponge, J.-F.; Salmon, S.; Makowski, D. Reduction of pesticide use can increase earthworm populations in wheat crops in a European temperate region. Agric. Ecosyst. Environ. 2013, 181, 223–230. [Google Scholar] [CrossRef]
- Jager, T.; Fleuren, R.H.L.J.; Hogendoorn, E.A.; de Korte, G. Elucidating the routes of exposure for organic chemicals in the earthworm, Eisenia andrei (Oligochaeta). Environ. Sci. Technol. 2003, 37, 3399–3404. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Ann. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- LaCourse, E.J.; Hernandez-Viadel, M.; Jefferies, J.R.; Svendsen, C.; Spurgeon, D.J.; Barrett, J.; Morgan, A.J.; Kille, P.; Brophy, P.M. Glutathione transferase (GST) as a candidate molecular-based biomarker for soil toxin exposure in the earthworm Lumbricus rubellus. Environ. Pollut. 2009, 157, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Bonnail, E.; Buruaem, L.M.; Araujo, G.S.; Abessa, D.M.; DelValls, T.Á. Multiple biomarker responses in Corbicula fluminea exposed to copper in laboratory toxicity tests. Arch. Environ. Contam. Toxicol. 2016, 71, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.E.; Costa, P.G.; Lunardelli, B.; de Oliveira, L.F.; Cabrera, L.C.; Risso, W.E.; Primel, E.G.; Meletti, P.C.; Fillmann, G.; Martinez, C.B. Multiple biomarker responses in Prochilodus lineatus subjected to short-term in situ exposure to streams from agricultural areas in Southern Brazil. Sci. Total Environ. 2016, 542, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, M.A.; Fisk, M.C.; Yavitt, J.B.; Fahey, T.J.; Balser, T.C. Exotic earthworms alter soil microbial community composition and function. Soil Biol. Biochem. 2013, 67, 263–270. [Google Scholar] [CrossRef]
- Stromberger, M.E.; Keith, A.M.; Schmidt, O. Distinct microbial and faunal communities and translocated carbon in Lumbricus terrestris drilospheres. Soil Biol. Biochem. 2012, 46, 155–162. [Google Scholar] [CrossRef]
- Hoang, D.T.T.; Razavi, B.S.; Kuzyakov, Y.; Blagodatskaya, E. Earthworm burrows: Kinetics and spatial distribution of enzymes of C-, N- and P-cycles. Soil Biol. Biochem. 2016, 99, 94–103. [Google Scholar] [CrossRef]
- Yang, P.; van Elsas, J.D. Mechanisms and ecological implications of the movement of bacteria in soil. Appl. Soil Ecol. 2018, 129, 112–120. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.E.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]

| Parameter | OMW Sediments | Soil |
|---|---|---|
| pH | 9.38 ± 0.02 | 8.76 ± 0.06 |
| Electrical conductivity (dS/cm) | 1.40 ± 0.06 | 0.08 ± 0.01 |
| Organic matter (%) | 3.06 ± 0.09 | 0.36 ± 0.01 |
| Total organic carbon (%) | 3.08 ± 0.05 | 0.27 ± 0.02 |
| Total nitrogen (%) | 0.26 ± 0.05 | 0.03 ± 0.01 |
| Total soluble polyphenols (mg/kg) | 873 ± 55 | 85 ± 6.5 |
| CEC (meq/100 g OM) | 244 ± 31 | 381 ± 17 |
| Total P (g/kg) | 3.2 ± 0.09 | 0.067 ± 0.005 |
| K (g/kg) | 11.9 ± 0.7 | 3.9 ± 0.35 |
| Na (g/kg) | 1.9 ± 0.01 | 0.6 ± 0.05 |
| PO4−3 (mg/kg) | 32 ± 0.6 | 8 ± 0.4 |
| SO4−2 (mg/kg) | 0.8 ± 0.09 | 0.4 ± 0.03 |
| OMW Dose (%, w/w) | |||||
|---|---|---|---|---|---|
| Digestive Enzymes 1 | 0 | 10 | 20 | 40 | p-Value 2 |
| Gastrointestinal tissue | |||||
| Esterase | 74.2 ± 38.5 | 76.8 ± 36.4 | 92.3 ± 30.7 | 68.0 ± 46.7 | 0.407 |
| Lipase | 3.01 ± 0.85 a | 2.83 ± 0.80 ab | 2.56 ± 1.20 ab | 1.84 ± 0.80 b | 0.038 |
| Glucosidase | 5.76 ± 1.06 ab | 6.78 ± 1.78 a | 7.10 ± 3.45 ab | 4.90 ± 1.22 b | 0.039 |
| Acid phosphatase | 6.30 ± 1.16 a | 8.14 ± 1.87 b | 7.82 ± 4.15 ab | 7.26 ± 1.84 ab | 0.028 |
| Alkaline phosphatase | 6.87 ± 1.24 a | 8.58 ± 2.50 ab | 10.5 ± 5.64 b | 9.00 ± 4.67 ab | 0.034 |
| Gastrointestinal content | |||||
| Glucosidase | 1.09 ± 0.39 a | 0.85 ± 0.25 ab | 0.77 ± 0.25 b | –3 | 0.049 |
| Acid phosphatase | 1.55 ± 0.70 | 1.65 ± 0.63 | 1.21 ± 0.47 | – | 0.086 |
| Alkaline phosphatase | 1.50 ± 0.46 a | 1.34 ± 0.51 ab | 1.01 ± 0.49 b | – | 0.029 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Hernandez, J.C.; Sáez, J.A.; Vico, A.; Moreno, J.; Moral, R. Evaluating Earthworms’ Potential for Remediating Soils Contaminated with Olive Mill Waste Sediments. Appl. Sci. 2020, 10, 2624. https://doi.org/10.3390/app10072624
Sanchez-Hernandez JC, Sáez JA, Vico A, Moreno J, Moral R. Evaluating Earthworms’ Potential for Remediating Soils Contaminated with Olive Mill Waste Sediments. Applied Sciences. 2020; 10(7):2624. https://doi.org/10.3390/app10072624
Chicago/Turabian StyleSanchez-Hernandez, Juan C., Jose A. Sáez, Alberto Vico, Joaquín Moreno, and Raúl Moral. 2020. "Evaluating Earthworms’ Potential for Remediating Soils Contaminated with Olive Mill Waste Sediments" Applied Sciences 10, no. 7: 2624. https://doi.org/10.3390/app10072624
APA StyleSanchez-Hernandez, J. C., Sáez, J. A., Vico, A., Moreno, J., & Moral, R. (2020). Evaluating Earthworms’ Potential for Remediating Soils Contaminated with Olive Mill Waste Sediments. Applied Sciences, 10(7), 2624. https://doi.org/10.3390/app10072624






