Pine Resin Derivatives as Sustainable Additives to Improve the Mechanical and Thermal Properties of Injected Moulded Thermoplastic Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
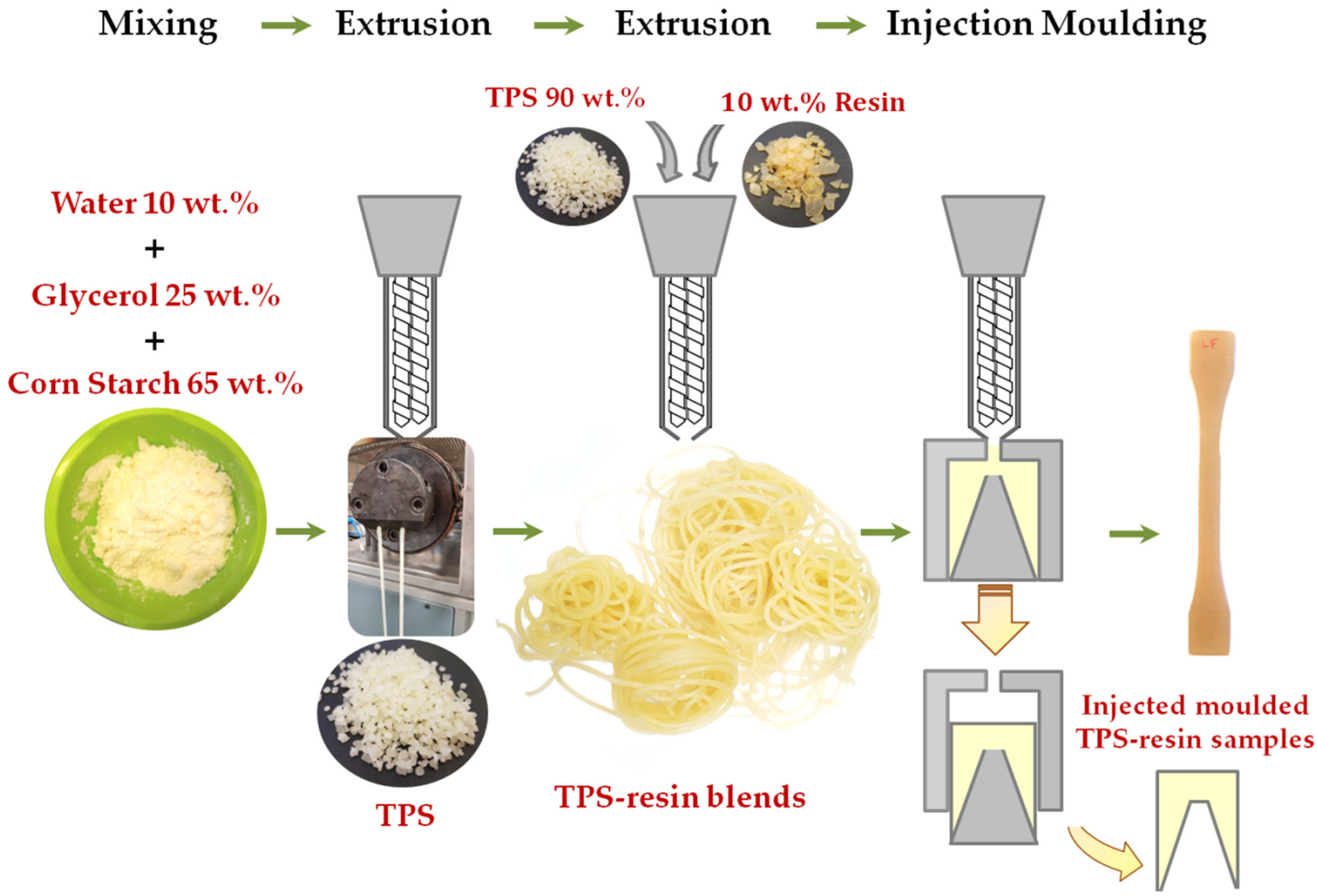
2.2.1. TPS-Resin Blends Preparation
2.2.2. Mechanical Characterization
2.2.3. Thermal Characterization
2.2.4. Structural Characterization
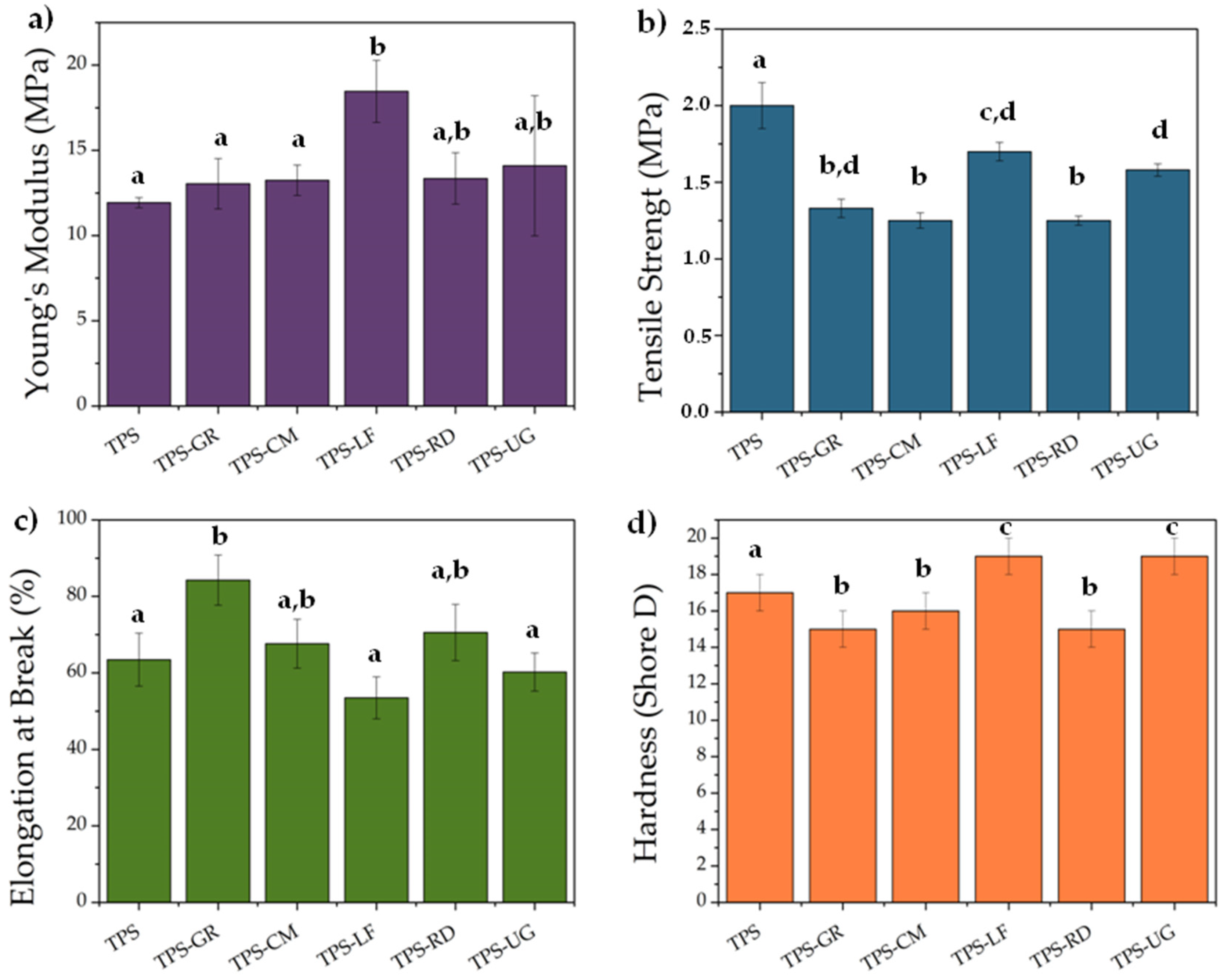
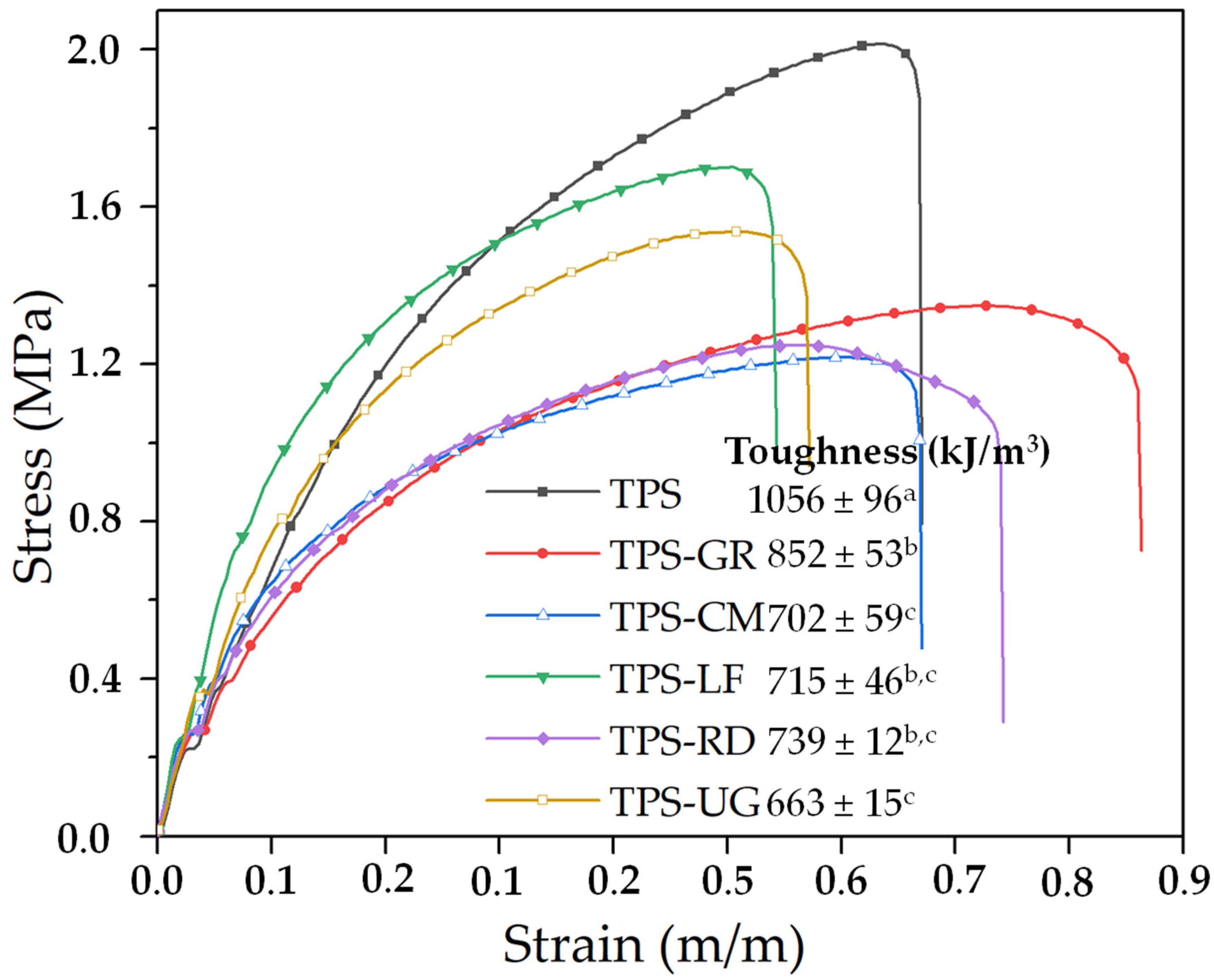
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plastics Europe—The Facts 2018. An Analysis of European Plastics Production, Demand and Waste Data. 2018. Available online: https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf (accessed on 7 April 2020).
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Averous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Aldas, M.; Ferri, J.; Lopez-Martinez, J.; Samper, M.; Arrieta, M. Effect of pine resin derivatives on the structural, thermal, and mechanical properties of Mater-Bi type bioplastic. J. Appl. Polym. Sci. 2020, 137, 48236. [Google Scholar] [CrossRef]
- Aldas, M.; Paladines, A.; Valle, V.; Pazmiño, M.; Quiroz, F. Effect of the prodegradant-additive plastics incorporated on the polyethylene recycling. Int. J. Polym. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Simon, J.; Müller, H.; Koch, R.; Müller, V. Thermoplastic and biodegradable polymers of cellulose. Polym. Degrad. Stab. 1998, 59, 107–115. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Burgos, N.; Peltzer, M.A.; López, J.; Peponi, L. Nanocellulose-Based Polymeric Blends for Food Packaging Applications. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; Elsevier: Oxford, UK, 2016; pp. 205–252. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Cui, C.; Argyropoulos, D.S. Toward thermoplastic lignin polymers. Part 1. Selective masking of phenolic hydroxyl groups in kraft lignins via methylation and oxypropylation chemistries. Ind. Eng. Chem. Res. 2012, 51, 16713–16720. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Cai, J.; Xie, W.; Long, T.E.; Turner, S.R.; Lyons, A.; Gross, R.A. Polymers from fatty acids: Poly (ω-hydroxyl tetradecanoic acid) synthesis and physico-mechanical studies. Biomacromolecules 2011, 12, 3291–3298. [Google Scholar] [CrossRef]
- Galbis, J.A.; García-Martín, M.d.G.; de Paz, M.V.; Galbis, E. Synthetic polymers from sugar-based monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef]
- Lu, D.; Xiao, C.; Xu, S. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Ciardelli, F.; Bertoldo, M.; Bronco, S.; Passaglia, E. The obtainment of bioplastics. In Polymers from Fossil and Renewable Resources; Springer: Cham, Switzerland, 2019; pp. 107–132. [Google Scholar]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Thermoplastic starch foamed composites reinforced with cellulose nanofibers: Thermal and mechanical properties. Carbohydr. Polym. 2018, 197, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Sessini, V.; Arrieta, M.P.; Fernández-Torres, A.; Peponi, L. Humidity-activated shape memory effect on plasticized starch-based biomaterials. Carbohydr. Polym. 2018, 179, 93–99. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic starch− waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef]
- Jiugao, Y.; Ning, W.; Xiaofei, M. The effects of citric acid on the properties of thermoplastic starch plasticized by glycerol. Starch-Stärke 2005, 57, 494–504. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.d.C.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Torres-Giner, S.; Ferrandiz-Bou, S.; Quiles-Carrillo, L.; Montanes, N. Development of sustainable and cost-competitive injection-molded pieces of partially bio-based polyethylene terephthalate through the valorization of cotton textile waste. Int. J. Mol. Sci. 2019, 20, 1378. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, D. European Parliamentary Research Service. 2018. Available online: http://www.europarl.europa.eu/RegData/etudes/ATAG/2018/625163/EPRS_ATA_ATA(2018)625163_EN.pdf (accessed on 31 March 2020).
- Sarasini, F.; Puglia, D.; Fortunati, E.; Kenny, J.; Santulli, C. Effect of fiber surface treatments on thermo-mechanical behavior of poly (lactic acid)/phormium tenax composites. J. Polym. Environ. 2013, 21, 881–891. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Samper, M.D.; Bertomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J. Interference of biodegradable plastics in the polypropylene recycling process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef]
- Dolores, S.M.; Patricia, A.M.; Santiago, F.; Juan, L. Influence of biodegradable materials in the recycled polystyrene. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Samper-Madrigal, M.D.; Fenollar, O.; Dominici, F.; Balart, R.; Kenny, J.M. The effect of sepiolite on the compatibilization of polyethylene–thermoplastic starch blends for environmentally friendly films. J. Mater. Sci. 2014, 50, 863–872. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Raquez, J.-M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/Thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]
- Ferri, J.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Fenollar, O.; Balart, R. Poly (lactic acid) formulations with improved toughness by physical blending with thermoplastic starch. J. Appl. Polym. Sci. 2018, 135, 45751. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez-Rivera, M.A.; Sanfuentes, E.A.; Peña-Farfal, C. Antioxidant and antifungal effects of eugenol incorporated in bionanocomposites of poly (3-hydroxybutyrate)-thermoplastic starch. LWT 2018, 98, 260–267. [Google Scholar] [CrossRef]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2016, 31, 111–126. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Martín, J.A.; López, R.; Sanz, A.; Gil, L. Effect of four tapping methods on anatomical traits and resin yield in Maritime pine (Pinus pinaster Ait). Ind. Crop. Prod. 2016, 86, 143–154. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. Dimerization of abietic acid for the design of renewable polymers by ADMET. Eur. Polym. J. 2015, 67, 409–417. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Jiménez-López, M.; Aldas, M.; López, J. Combined effect of linseed oil and gum rosin as natural additives for PVC. Ind. Crop. Prod. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Kumooka, Y. Analysis of rosin and modified rosin esters in adhesives by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Forensic Sci. Int. 2008, 176, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Aldas, M.; Rayón, E.; López-Martínez, J.; Arrieta, M.P. A deeper microscopic study of the interaction between gum rosin derivatives and a Mater-Bi type bioplastic. Polymers 2020, 12, 226. [Google Scholar] [CrossRef]
- Oniszczuk, T.; Janssen, L. Influence of addition of fiber on the mechanical properties of TPS moldings. In Thermoplastic Starch: A Green Material for Various Industries; Wiley-VCH Verlag GmbH & Co.: Winheim, Germany, 2009; pp. 197–208. [Google Scholar]
- International Standards Organization. ISO 527-1:2012—Plastics—Determination of tensile properties—Part 1: General principles. 2012. [Google Scholar]
- International Standards Organization. ISO 868:2003—Plastics and ebonite—Determination of indentation hardness by means of a durometer (Shore hardness). 2003. [Google Scholar]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB packaging for the storage of food products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Barbosa, S.E.; Kenny, J.M. Processing of short fiber reinforced polypropylene. II: Statistical study of the effects of processing conditions on the impact strength. Polym. Eng. Sci. 1999, 39, 1880–1890. [Google Scholar] [CrossRef]
- Olivato, J.; Grossmann, M.; Bilck, A.; Yamashita, F. Effect of organic acids as additives on the performance of thermoplastic starch/polyester blown films. Carbohydr. Polym. 2012, 90, 159–164. [Google Scholar] [CrossRef]
- Narayanan, M.; Loganathan, S.; Valapa, R.B.; Thomas, S.; Varghese, T.O. UV protective poly(lactic acid)/rosin films for sustainable packaging. Int. J. Biol. Macromol. 2017, 99, 37–45. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Ferrándiz, S. New materials for 3D-printing based on polycaprolactone with gum rosin and beeswax as additives. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Bergström, J. 2—Experimental Characterization Techniques. In Mechanics of Solid Polymers; Bergström, J., Ed.; William Andrew Publishing: San Diego, CA, USA, 2015; pp. 19–114. [Google Scholar] [CrossRef]
- Wattanakornsiri, A.; Pachana, K.; Kaewpirom, S.; Traina, M.; Migliaresi, C. Preparation and properties of green composites based on tapioca starch and differently recycled paper cellulose fibers. J. Polym. Environ. 2012, 20, 801–809. [Google Scholar] [CrossRef]
- Forssell, P.M.; Mikkilä, J.M.; Moates, G.K.; Parker, R. Phase and glass transition behaviour of concentrated barley starch-glycerol-water mixtures, a model for thermoplastic starch. Carbohydr. Polym. 1997, 34, 275–282. [Google Scholar] [CrossRef]
- Karlberg, A.-T. Colophony: Rosin in unmodified and modified form. In Kanerva’s Occupational. Dermatology, 2nd ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 467–479. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Sun, X.; Zhang, J.; He, J. Thermal degradation studies of cyclic olefin copolymers. Polym. Degrad. Stab. 2003, 81, 197–205. [Google Scholar] [CrossRef]
- Teixeira, E.d.M.; Pasquini, D.; Curvelo, A.A.; Corradini, E.; Belgacem, M.N.; Dufresne, A. Cassava bagasse cellulose nanofibrils reinforced thermoplastic cassava starch. Carbohydr. Polym. 2009, 78, 422–431. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of edible films based on nanoreinforced gelatinized starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Cerruti, P.; Santagata, G.; d’Ayala, G.G.; Ambrogi, V.; Carfagna, C.; Malinconico, M.; Persico, P. Effect of a natural polyphenolic extract on the properties of a biodegradable starch-based polymer. Polym. Degrad. Stab. 2011, 96, 839–846. [Google Scholar] [CrossRef]
- Mendes, J.; Paschoalin, R.; Carmona, V.; Neto, A.R.S.; Marques, A.; Marconcini, J.; Mattoso, L.; Medeiros, E.; Oliveira, J. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Musa, M.; Yoo, M.; Kang, T.; Kolawole, E.; Ishiaku, U.; Yakubu, M.; Whang, D. Characterization and thermomechanical properties of thermoplastic potato starch. J. Eng. Technol 2013, 2, 9–16. [Google Scholar]
- Dang, K.M.; Yoksan, R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; El-Saeed, A.M.; Al-Shafey, H.I.; Abdul-Raheim, A.-R.M.; El-Sockary, M.A. Rosin based epoxy coating: Synthesis, identification and characterization. Eur. Polym. J. 2015, 69, 403–415. [Google Scholar] [CrossRef]
- Campos, A.; Teodoro, K.; Teixeira, E.; Corrêa, A.; Marconcini, J.; Wood, D.; Williams, T.; Mattoso, L. Properties of thermoplastic starch and TPS/polycaprolactone blend reinforced with sisal whiskers using extrusion processing. Polym. Eng. Sci. 2013, 53, 800–808. [Google Scholar] [CrossRef]
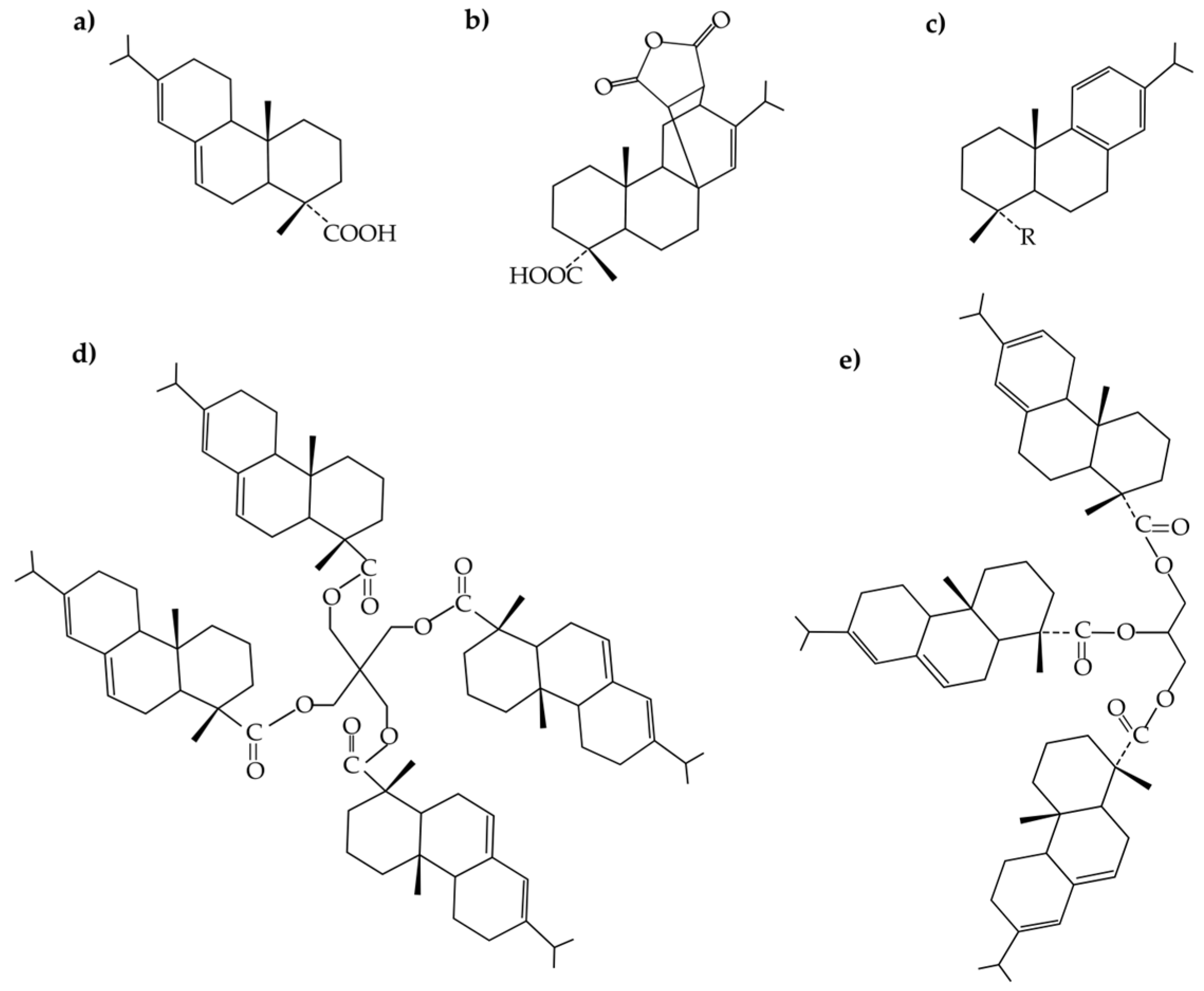
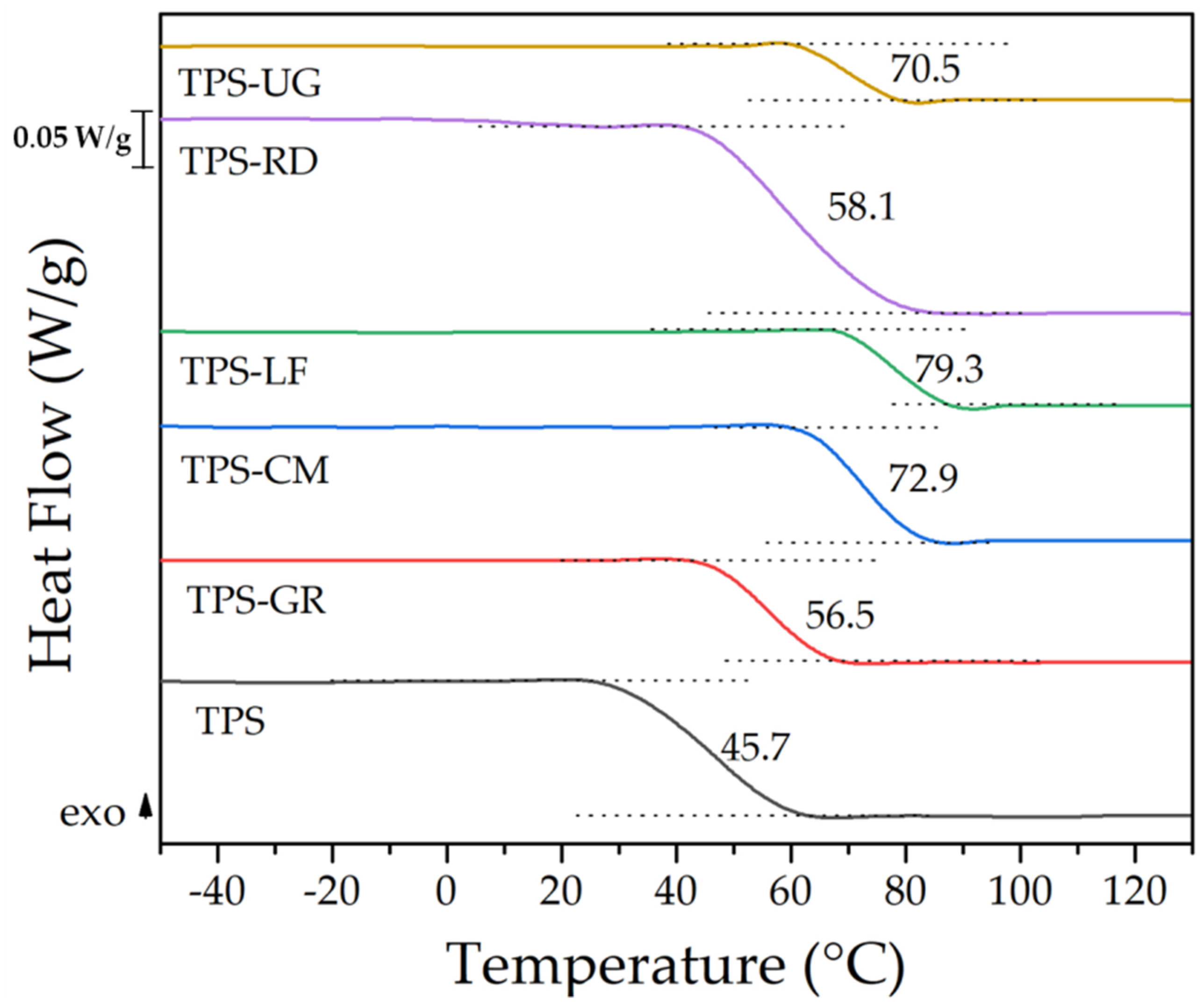
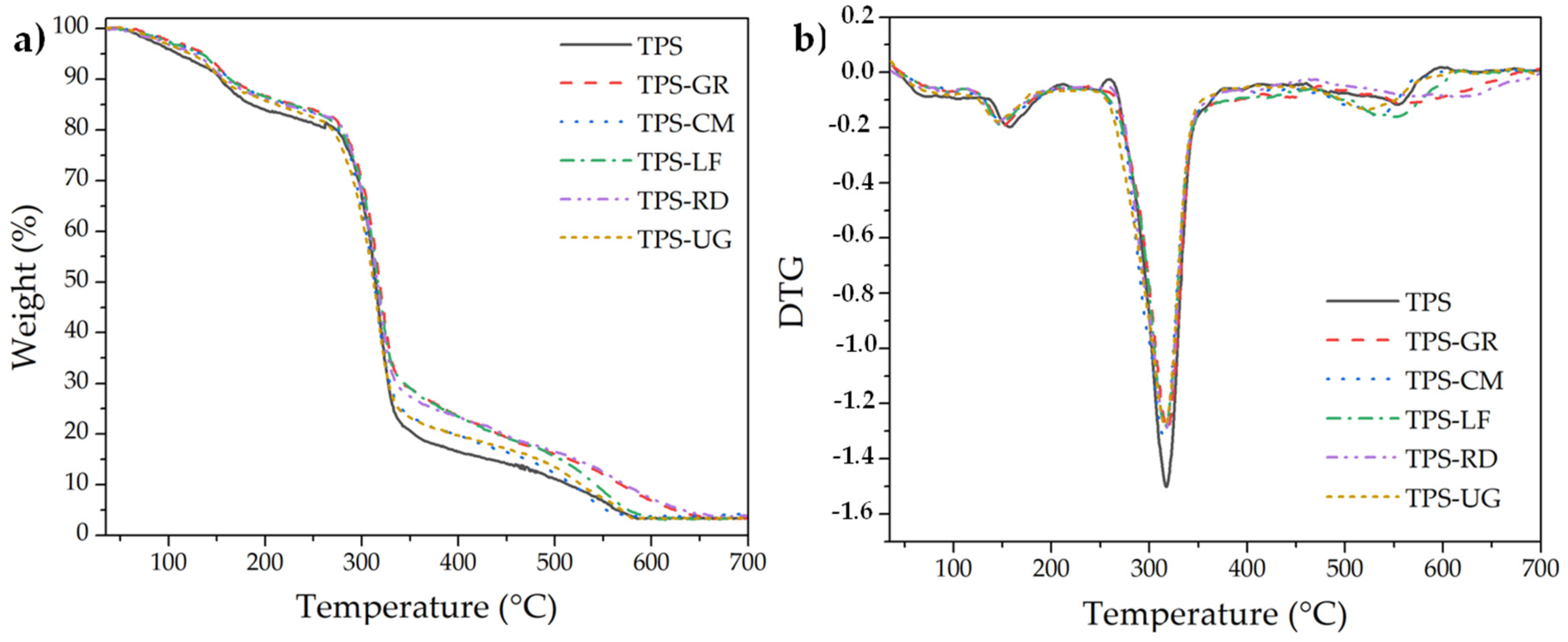
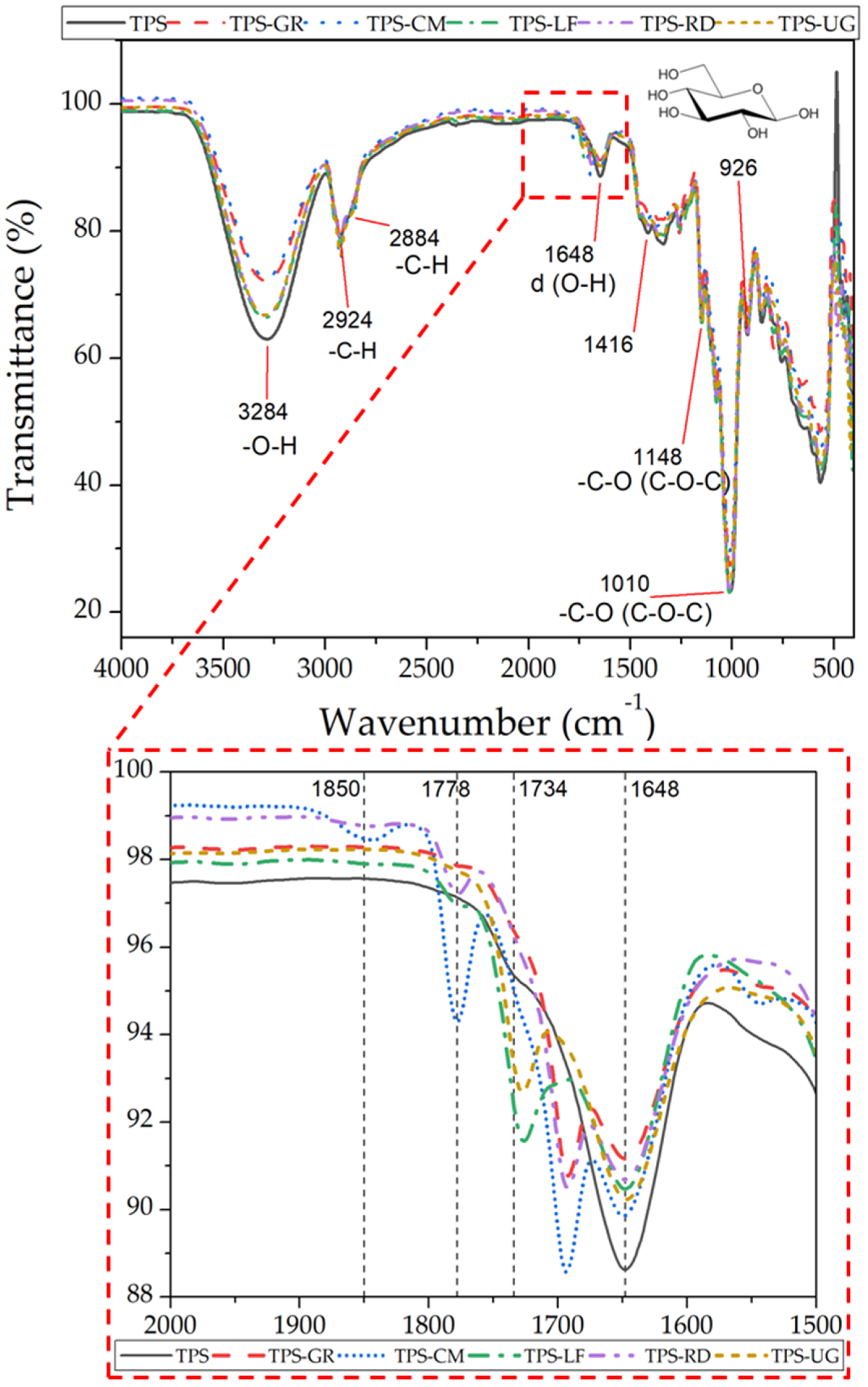
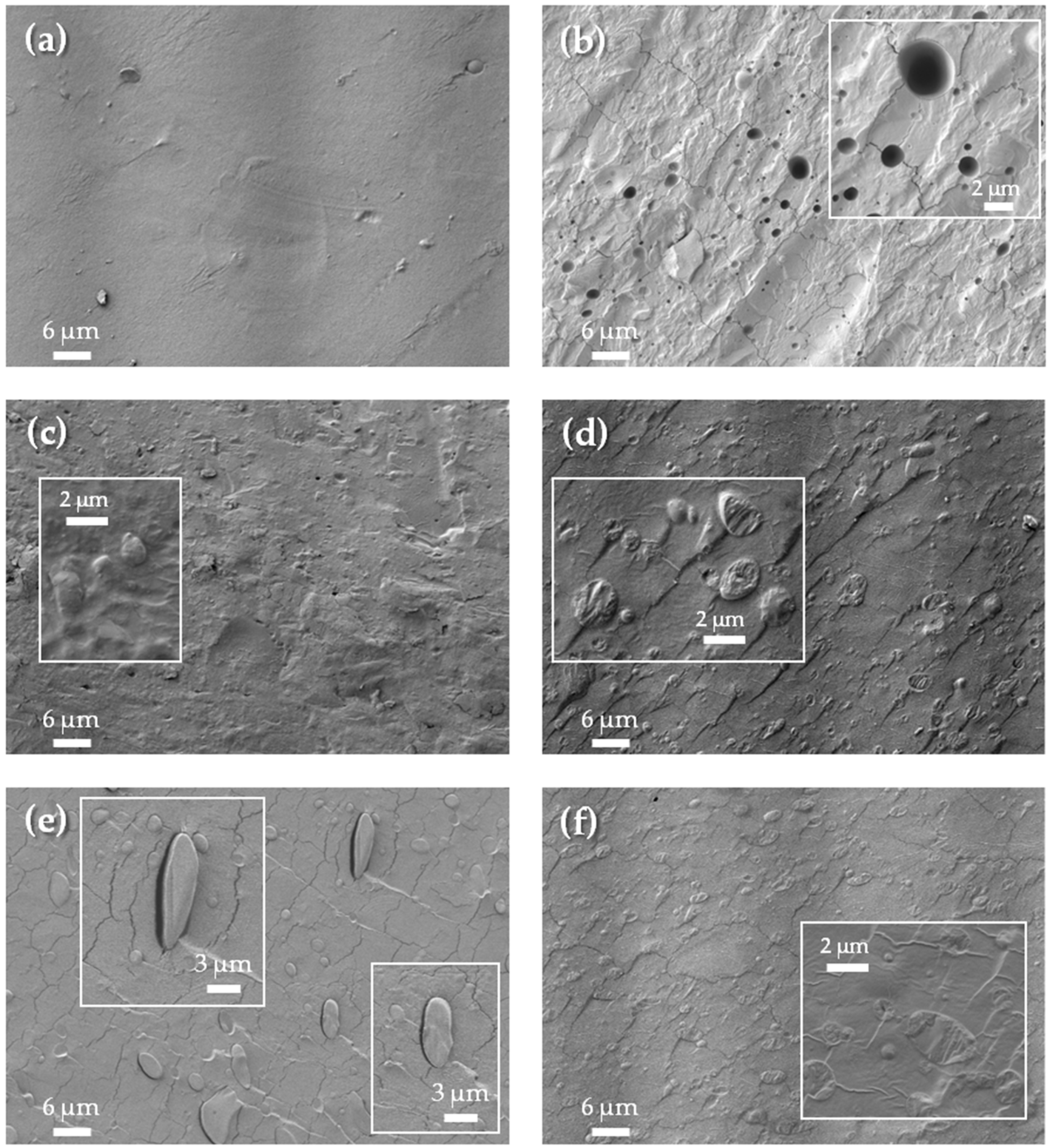
| Formulations | Type of Resin in the Blend | Resin Commercial Name | TPS (wt. %) | Resin (wt. %) |
|---|---|---|---|---|
| TPS | - | - | 100 | 0 |
| TPS-GR | Gum rosin or unmodified colophony | Gum rosin | 90 | 10 |
| TPS-CM | Maleic anhydride modified gum rosin | Colmodif R-330 | 90 | 10 |
| TPS-LF | Pentaerythritol ester of gum rosin | Lurefor 125 | 90 | 10 |
| TPS-RD | Disproportionated gum rosin | Residis 455 | 90 | 10 |
| TPS-UG | Glycerol ester of gum rosin | Unik Gum G88 | 90 | 10 |
| Material | T5% (°C) | Tmax (°C) | T95% (°C) |
|---|---|---|---|
| TPS | 109.3 ± 2.1 | 317.3 ± 1.9 | 564.8 ± 2.3 |
| TPS-GR | 138.3 ± 1.9 | 318.3 ± 1.7 | 624.3 ± 2.1 |
| TPS-CM | 132.8 ± 2.0 | 315.8 ± 1.9 | 553.8 ± 1.9 |
| TPS-LF | 133.8 ± 1.9 | 316.3 ± 1.7 | 576.3 ± 2.3 |
| TPS-RD | 126.3 ± 1.8 | 316.8 ± 2.0 | 632.2 ± 1.8 |
| TPS-UG | 121.8 ± 2.0 | 315.8 ± 1.8 | 570.2 ± 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldas, M.; Pavon, C.; López-Martínez, J.; Arrieta, M.P. Pine Resin Derivatives as Sustainable Additives to Improve the Mechanical and Thermal Properties of Injected Moulded Thermoplastic Starch. Appl. Sci. 2020, 10, 2561. https://doi.org/10.3390/app10072561
Aldas M, Pavon C, López-Martínez J, Arrieta MP. Pine Resin Derivatives as Sustainable Additives to Improve the Mechanical and Thermal Properties of Injected Moulded Thermoplastic Starch. Applied Sciences. 2020; 10(7):2561. https://doi.org/10.3390/app10072561
Chicago/Turabian StyleAldas, Miguel, Cristina Pavon, Juan López-Martínez, and Marina Patricia Arrieta. 2020. "Pine Resin Derivatives as Sustainable Additives to Improve the Mechanical and Thermal Properties of Injected Moulded Thermoplastic Starch" Applied Sciences 10, no. 7: 2561. https://doi.org/10.3390/app10072561
APA StyleAldas, M., Pavon, C., López-Martínez, J., & Arrieta, M. P. (2020). Pine Resin Derivatives as Sustainable Additives to Improve the Mechanical and Thermal Properties of Injected Moulded Thermoplastic Starch. Applied Sciences, 10(7), 2561. https://doi.org/10.3390/app10072561








