Silver Nanoparticles Ecofriendly Synthesized by Achyranthes aspera and Scoparia dulcis Leaf Broth as an Effective Fungicide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
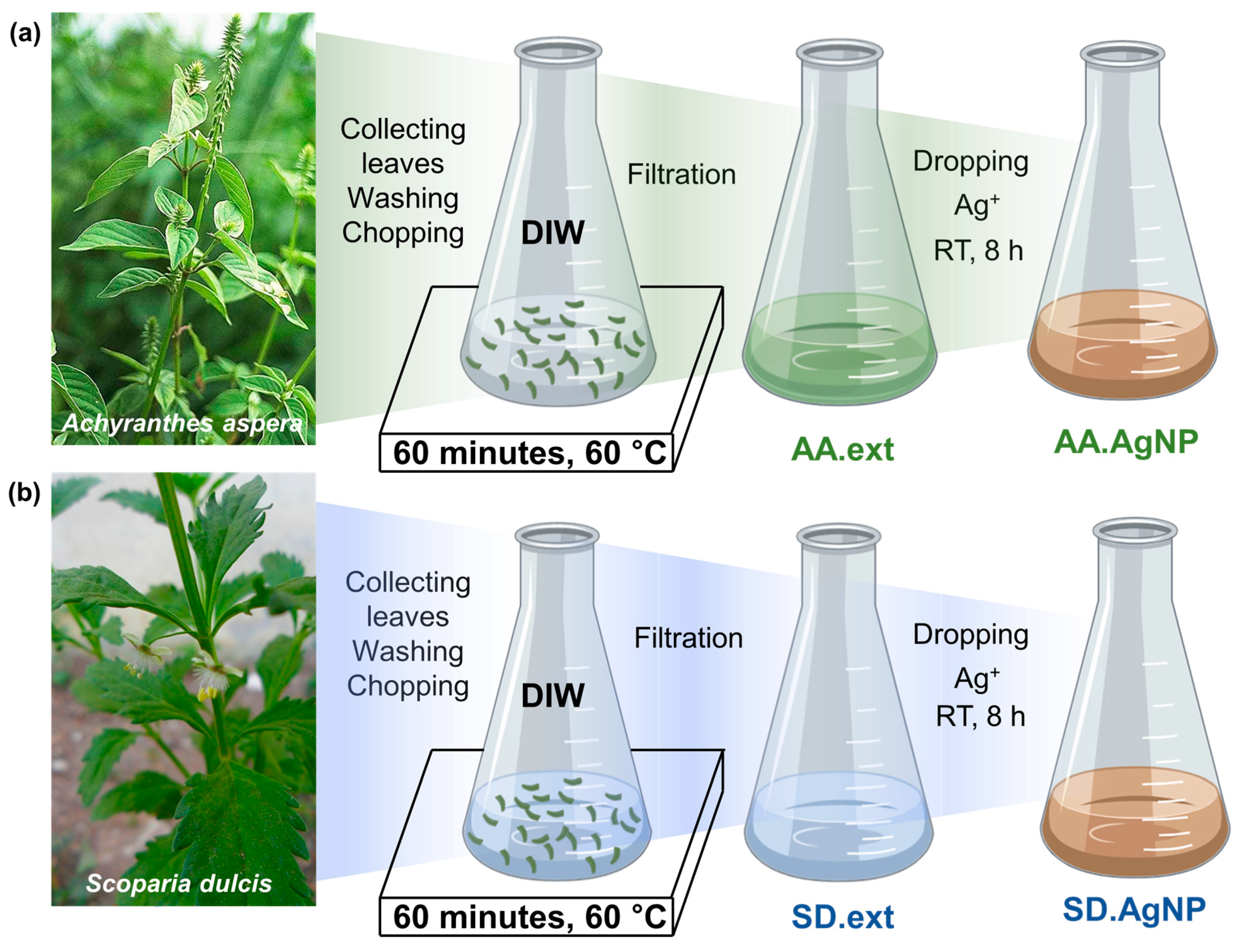
2.2. Biosynthesis of Silver Nanoparticles Using the A. aspera and S. dulcis Leaf Extracts
2.3. Characterization of AA.AgNP and SD.AgNP Nanoparticles
2.4. Antifungal Activity of AA.AgNP and SD.AgNP Nanoparticles
3. Results and Discussion
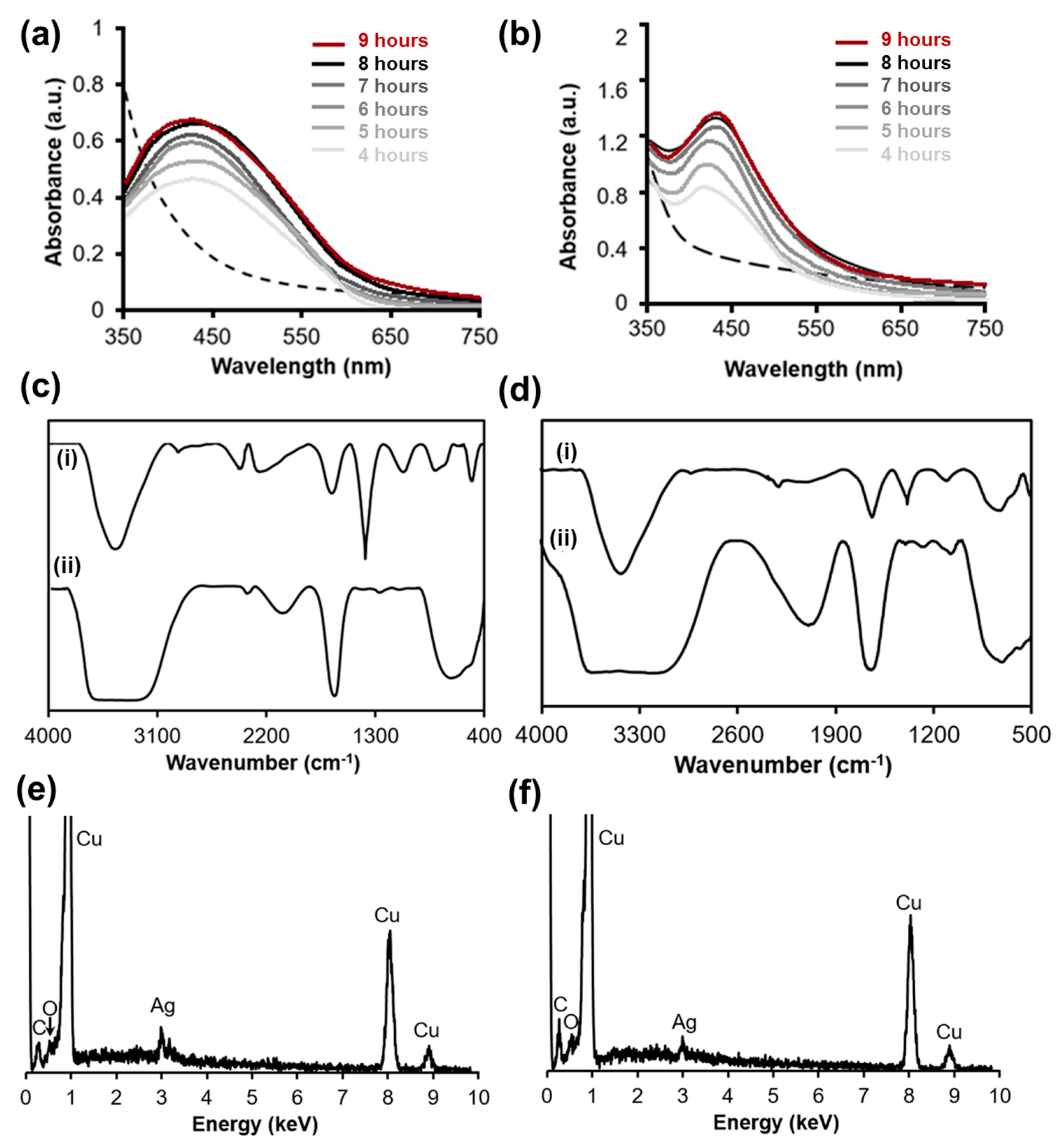
3.1. The Formation of Biosynthesized AgNPs Including AA.AgNPs and SD.AgNPs
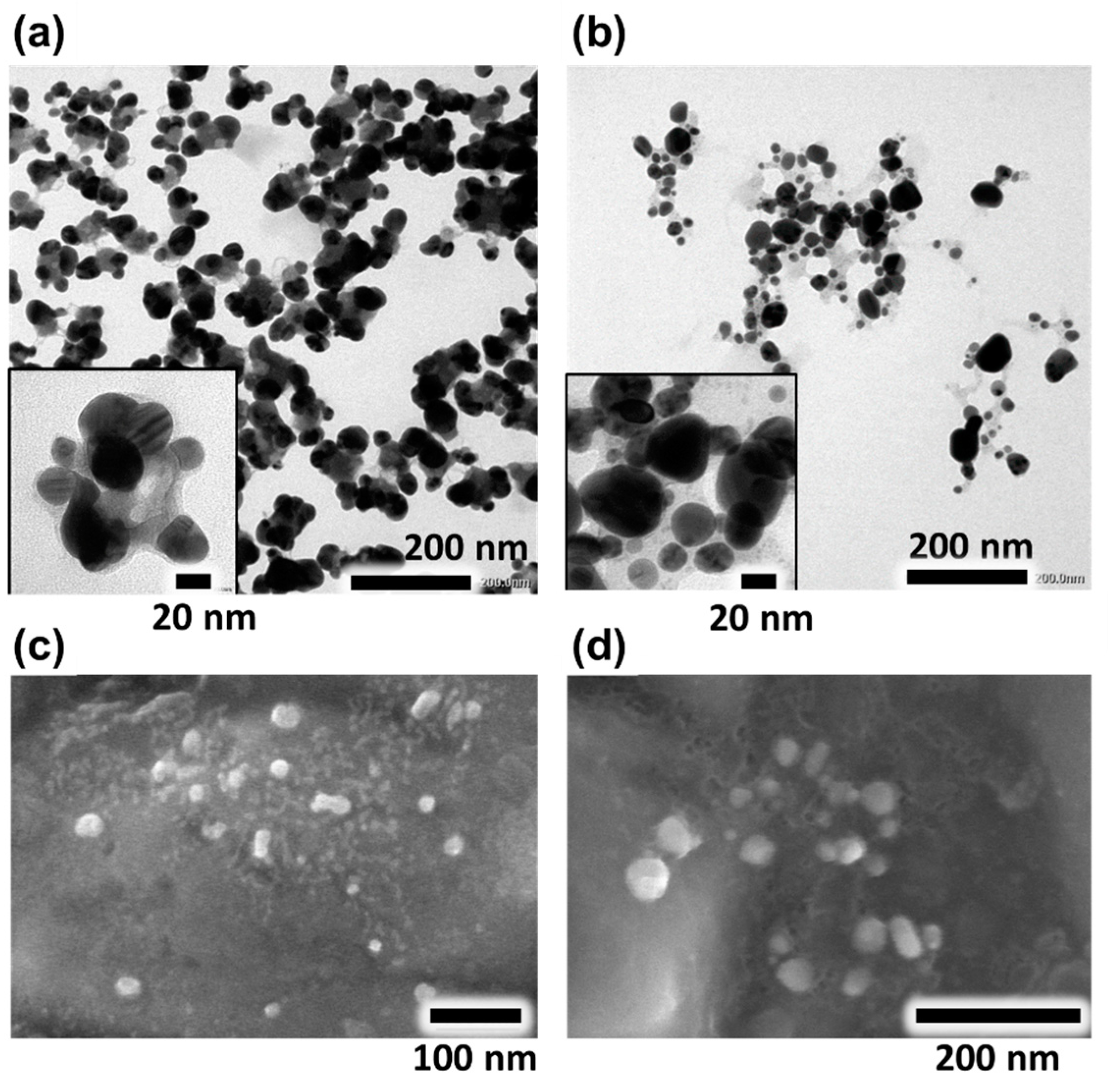
3.2. The Morphology and Dimension of AA.AgNPs and SD.AgNPs
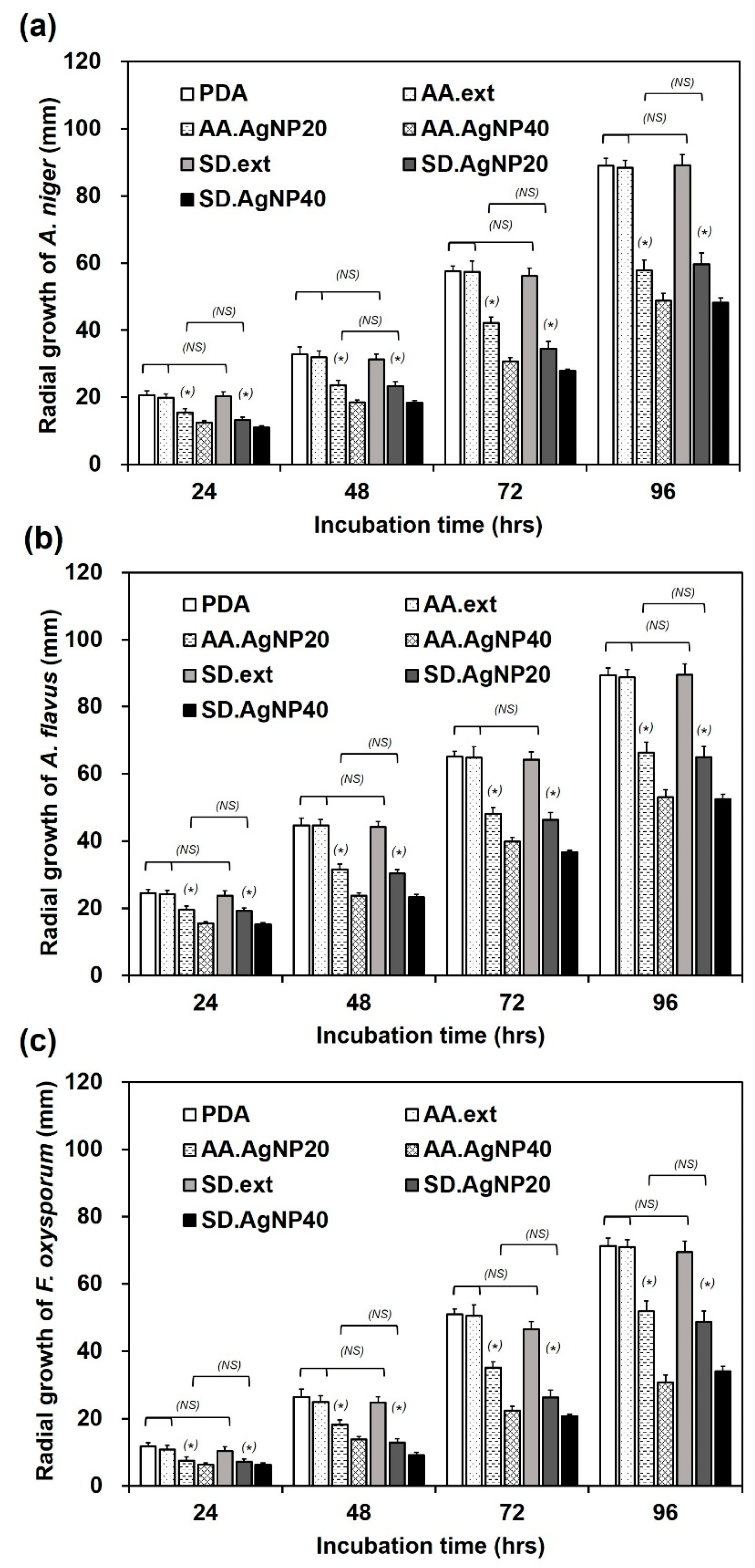
3.3. Antifungal Tests
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hendrickson, J.A.; Hu, C.; Aitken, S.L.; Beyda, N. Antifungal Resistance: A Concerning Trend for the Present and Future. Curr. Infect Dis. Rep. 2019, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, A.; Sharma, M.; Bhalla, N.; Estrela, P.; Jain, A.; Thakur, P.; Thakur, A. Nanomaterial Fungicides: In Vitro and In Vivo Antimycotic Activity of Cobalt and Nickel Nanoferrites on Phytopathogenic Fungi. Glob. Chall. 2017, 1, 1700041. [Google Scholar] [CrossRef] [PubMed]
- Thanh, V.M.; Bui, L.M.; Bach, L.G.; Nguyen, N.T.; Thi, H.L.; Hoang Thi, T.T. Origanum majorana L. Essential Oil-Associated Polymeric Nano Dendrimer for Antifungal Activity against Phytophthora infestans. Materials 2019, 12, 1446. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Cao, X.; Ma, C.; Zhang, Z.; Zhao, N.; Ali, A.; Hou, T.; Xiang, Z.; Zhuang, J.; Wu, S.; et al. Potential Applications and Antifungal Activities of Engineered Nanomaterials against Gray Mold Disease Agent Botrytis cinerea on Rose Petals. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Grace, J.L.; Huang, J.X.; Cheah, S.-E.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Quinn, J.F.; Velkov, T.; Whittaker, M.R. Antibacterial low molecular weight cationic polymers: Dissecting the contribution of hydrophobicity, chain length and charge to activity. RSC Adv. 2016, 6, 15469–15477. [Google Scholar] [CrossRef]
- Grace, J.L.; Elliott, A.G.; Huang, J.X.; Schneider, E.K.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Quinn, J.F.; Velkov, T.; et al. Cationic acrylate oligomers comprising amino acid mimic moieties demonstrate improved antibacterial killing efficiency. J. Mater. Chem. B 2017, 5, 531–536. [Google Scholar] [CrossRef]
- Barbon, S.M.; Truong, N.P.; Elliott, A.G.; Cooper, M.A.; Davis, T.P.; Whittaker, M.R.; Hawker, C.J.; Anastasaki, A. Elucidating the effect of sequence and degree of polymerization on antimicrobial properties for block copolymers. Polym. Chem. 2020, 11, 84–90. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Lee, Y.; Le Thi, P.; Park, K.D. Nitric oxide-releasing injectable hydrogels with high antibacterial activity through in situ formation of peroxynitrite. Acta Biomater. 2018, 67, 66–78. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef]
- Baker, S.; Nagendra Prasad, M.N.; Chouhan, R.S.; Mohan Kumar, K.; Satish, S. Development of bioconjugated nano-molecules against targeted microbial pathogens for enhanced bactericidal activity. Mater. Chem. Phys. 2020, 242, 122292. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener Techniques for the Synthesis of Silver Nanoparticles Using Plant Extracts, Enzymes, Bacteria, Biodegradable Polymers, and Microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and In Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef]
- Hassan, S.A.; Hanif, E.; Khan, U.H.; Tanoli, A.K. Antifungal activity of silver nanoparticles from Aspergillus niger. Pak. J. Pharm Sci. 2019, 32, 1163–1166. [Google Scholar] [PubMed]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tripathi, P.; Kumar, S. One-pot synthesis of silver nanocomposites from Achyranthes aspera: An eco-friendly larvicide against Aedes aegypti L. Asian Pac. J. Trop. Biomed. 2020, 10, 54–64. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Park, K.D.; Ching, Y.C.; Nguyen, X.T.; Phan, V.H.G.; Hoang Thi, T.T. Green Silver Nanoparticles Formed by Phyllanthus urinaria, Pouzolzia zeylanica, and Scoparia dulcis Leaf Extracts and the Antifungal Activity. Nanomaterials 2020, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Venkataranganna, M.V.; Prasad, N.B.L.; Godavarthi, A. Achyranthes aspera Attenuates epilepsy in experimental animals: Possible involvement of GABAergic mechanism. Metab. Brain Dis. 2017, 32, 867–879. [Google Scholar] [CrossRef]
- Khandagle, A.J.; Tare, V.S.; Raut, K.D.; Morey, R.A. Bioactivity of essential oils of Zingiber officinalis and Achyranthes aspera against mosquitoes. Parasitol. Res. 2011, 109, 339–343. [Google Scholar] [CrossRef]
- Mali, R.G.; Dhake, A.S. A review on herbal antiasthmatics. Orient. Pharm. Exp. Med. 2011, 11, 77–90. [Google Scholar] [CrossRef]
- Edwin, S.; Jarald, E.E.; Deb, L.; Jain, A.; Kinger, H.; Dutt, K.R.; Raj, A.A. Wound Healing and Antioxidant Activity ofAchyranthes aspera. Pharm. Biol. 2009, 46, 824–828. [Google Scholar] [CrossRef]
- Bhoomika, R.G.; Ramesh, K.G.; Anita, A.M. Phyto-pharmacology of Achyranthes aspera: A Review. Pharmacogn. Rev. 2007, 1, 143. [Google Scholar]
- Liu, Q.; Yang, Q.M.; Hu, H.J.; Yang, L.; Yang, Y.B.; Chou, G.X.; Wang, Z.T. Bioactive diterpenoids and flavonoids from the aerial parts of Scoparia dulcis. J. Nat. Prod. 2014, 77, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Meera, P.; Kavitha, V.; Krishnaja, K.R. Scoparia dulcis: A review on its phytochemical and pharmacological profile. Innoriginal Int. J. Sci. 2017, 4, 18–22. [Google Scholar]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- Doan, P.; Cao, V.; Nguyen, D.H.; Tran, N.Q. Metallic nanoparticles: Potential ecofungicide for controlling growth of plant-pathogenic fungi. J. Chem. Soc. Pak. 2018, 40, 664–675. [Google Scholar]
- Ruchi, S. Pathogenecity of Aspergillus niger in plants. Cibtech J. Microbiol. 2012, 1, 47–51. [Google Scholar]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Pineider, F.; Sangregorio, C. Chapter 6—Nanomaterials for Magnetoplasmonics. In Novel Magnetic Nanostructures; Domracheva, N., Caporali, M., Rentschler, E., Eds.; Elsevier: New York, NY, USA, 2018; pp. 191–220. [Google Scholar] [CrossRef]
- Gerami, M.; Ramezani, M.; Majlesi, Z. Investigation on some main glycosides content of Stevia rebaudian B. under different concentrations of commercial and synthesized silver nanoparticles. Pharm. Biomed. Res. 2018. [Google Scholar] [CrossRef]
- Sutradhar, P.; Saha, M. Size-controlled synthesis of silver nanoparticles using Zizyphus mauritiana fruit extract. Main Group Chem. 2015, 15, 47–55. [Google Scholar] [CrossRef]
- Mirgorod, Y.A.; Borodina, V.G.; Borsch, N.A. Investigation of interaction between silver ions and rutin in water by physical methods. Biophysics 2014, 58, 743–747. [Google Scholar] [CrossRef]
- Halder, A.; Das, S.; Bera, T.; Mukherjee, A. Rapid synthesis for monodispersed gold nanoparticles in kaempferol and anti-leishmanial efficacy against wild and drug resistant strains. RSC Adv. 2017, 7, 14159–14167. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Chikkanna, M.M.; Neelagund, S.E. Effect of Sheep and Goat Fecal Mediated Synthesis and Characterization of Silver Nanoparticles (AgNPs) and Their Antibacterial Effects. J. Nanofluids 2018, 7, 309–315. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Elumalai, D.; Kaleena, P.K.; Ashok, K.; Suresh, A.; Hemavathi, M. Green synthesis of silver nanoparticle using Achyranthes aspera and its larvicidal activity against three major mosquito vectors. Eng. Agric. Environ. Food 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Parvataneni, R. Biogenic synthesis and characterization of silver nanoparticles using aqueous leaf extract of Scoparia dulcis L. and assessment of their antimicrobial property. Drug Chem. Toxicol. 2019, 43, 307–321. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Nayfeh, E.M. Fundamentals and Applications of Nano Silicon in Plasmonics and Fullerines: Current and Future Trends, Chapter 8: Nanometal. Micro Nano Technol. 2018, 169–203. [Google Scholar] [CrossRef]
- Colloidal Silver Study Results: Bacteriology. Available online: https://www.silver-colloids.com/biostudies/ (accessed on 30 March 2020).
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopartic. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Muna, A.; Halima, Z.H. Antifungal effect of silver nanoparticles (AgNPs) against Aspergillus flavus. EC Microbiol. 2017, 6, 63–66. [Google Scholar]
- Villamizar-Gallardo, R.; Cruz, J.F.O.; Ortíz-Rodriguez, O.O. Fungicidal effect of silver nanoparticles on toxigenic fungi in cocoa. Pesquisa Agropecuária Brasileira 2016, 51, 1929–1936. [Google Scholar] [CrossRef]
- Marta, A.-T.S.; Adam, S.; Jacek, O.; Stanisław, D. Effects of copper and silver nanoparticles on growth of selected species of pathogenic and wood-decay fungi in vitro. For. Chron. 2018, 94, 109–116. [Google Scholar]
- Augustine, R.; Hasan, A. Emerging applications of biocompatible phytosynthesized metal/metal oxide nanoparticles in healthcare. J. Drug Deliv. Sci. Technol. 2020, 56, 101516. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Ramezani, T.; Hosseini, N.; Mohamad, R. Green Synthesis of Silver Nanoparticles using Achillea biebersteinii Flower Extract and its Anti-Angiogenic Properties in the Rat Aortic Ring Model. Molecules 2014, 19, 4624–4634. [Google Scholar] [CrossRef]
- Amin, M.; Hameed, S.; Ali, A.; Anwar, F.; Shahid, S.A.; Shakir, I.; Yaqoob, A.; Hasan, S.; Khan, S.A.; Sajjad Ur, R. Green Synthesis of Silver Nanoparticles: Structural Features and In Vivo and In Vitro Therapeutic Effects against Helicobacter pylori Induced Gastritis. Bioinorg. Chem. Appl. 2014, 2014, 135824. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, N.T.T.; Nguyen, D.H.; Nguyen, N.H.; Ching, Y.C.; Pham Nguyen, D.Y.; Ngo, C.Q.; Nhat, H.N.T.; Hoang Thi, T.T. Silver Nanoparticles Ecofriendly Synthesized by Achyranthes aspera and Scoparia dulcis Leaf Broth as an Effective Fungicide. Appl. Sci. 2020, 10, 2505. https://doi.org/10.3390/app10072505
Le NTT, Nguyen DH, Nguyen NH, Ching YC, Pham Nguyen DY, Ngo CQ, Nhat HNT, Hoang Thi TT. Silver Nanoparticles Ecofriendly Synthesized by Achyranthes aspera and Scoparia dulcis Leaf Broth as an Effective Fungicide. Applied Sciences. 2020; 10(7):2505. https://doi.org/10.3390/app10072505
Chicago/Turabian StyleLe, Ngoc Thuy Trang, Dai Hai Nguyen, Ngoc Hoi Nguyen, Yern Chee Ching, Dong Yen Pham Nguyen, Cuong Quoc Ngo, Hang Nguyen Thi Nhat, and Thai Thanh Hoang Thi. 2020. "Silver Nanoparticles Ecofriendly Synthesized by Achyranthes aspera and Scoparia dulcis Leaf Broth as an Effective Fungicide" Applied Sciences 10, no. 7: 2505. https://doi.org/10.3390/app10072505
APA StyleLe, N. T. T., Nguyen, D. H., Nguyen, N. H., Ching, Y. C., Pham Nguyen, D. Y., Ngo, C. Q., Nhat, H. N. T., & Hoang Thi, T. T. (2020). Silver Nanoparticles Ecofriendly Synthesized by Achyranthes aspera and Scoparia dulcis Leaf Broth as an Effective Fungicide. Applied Sciences, 10(7), 2505. https://doi.org/10.3390/app10072505




