Mesoporous Carbon as Galvanic-Corrosion Activator Accelerates Fe Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fabrication Process
2.3. Microstructure Characterization
2.4. Electrochemical Experiments
2.5. Immersion Tests
2.6. In Vitro Cell Experiments
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microstructure and Phase Composition
3.2. Mechanical Properties
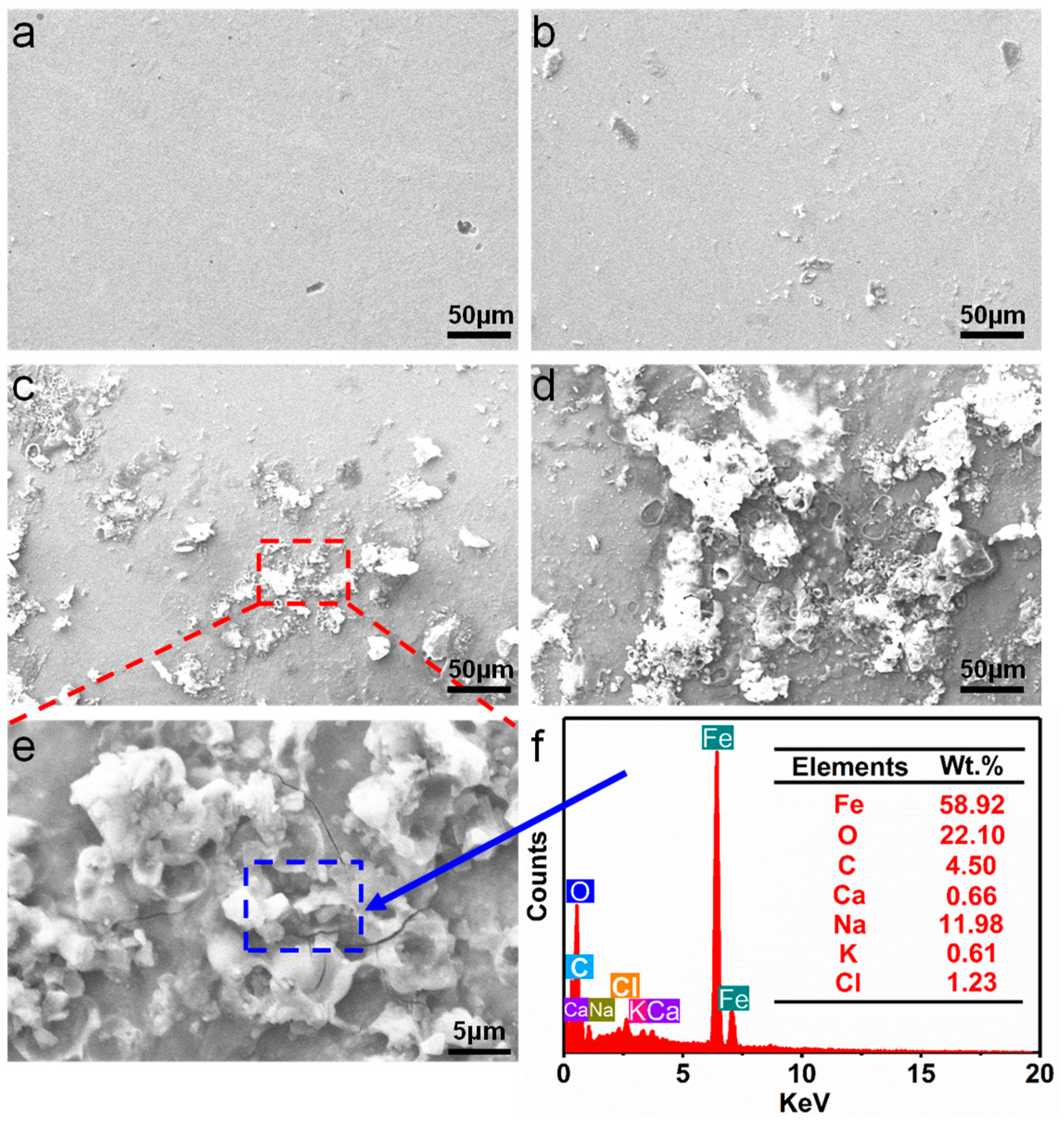
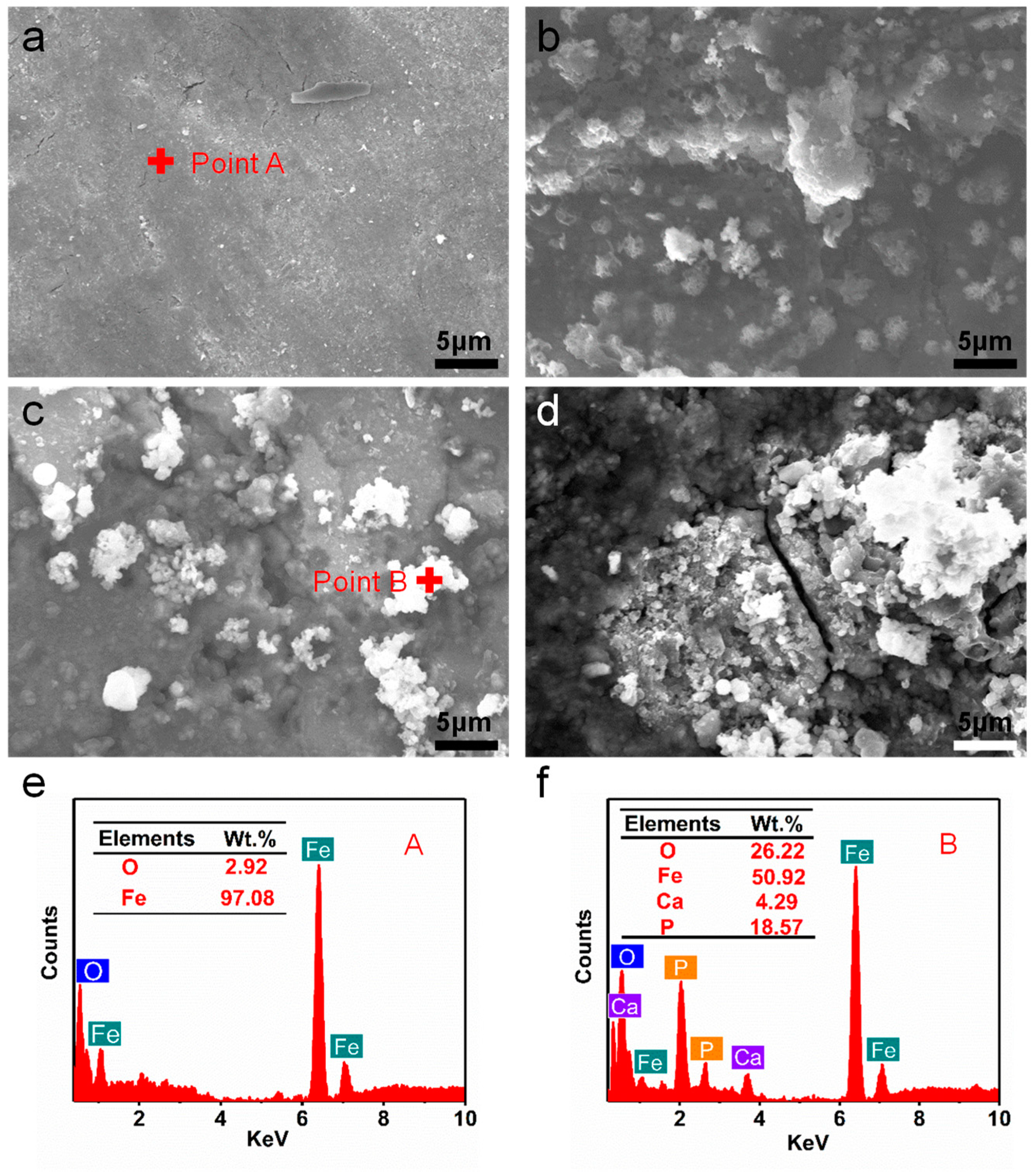
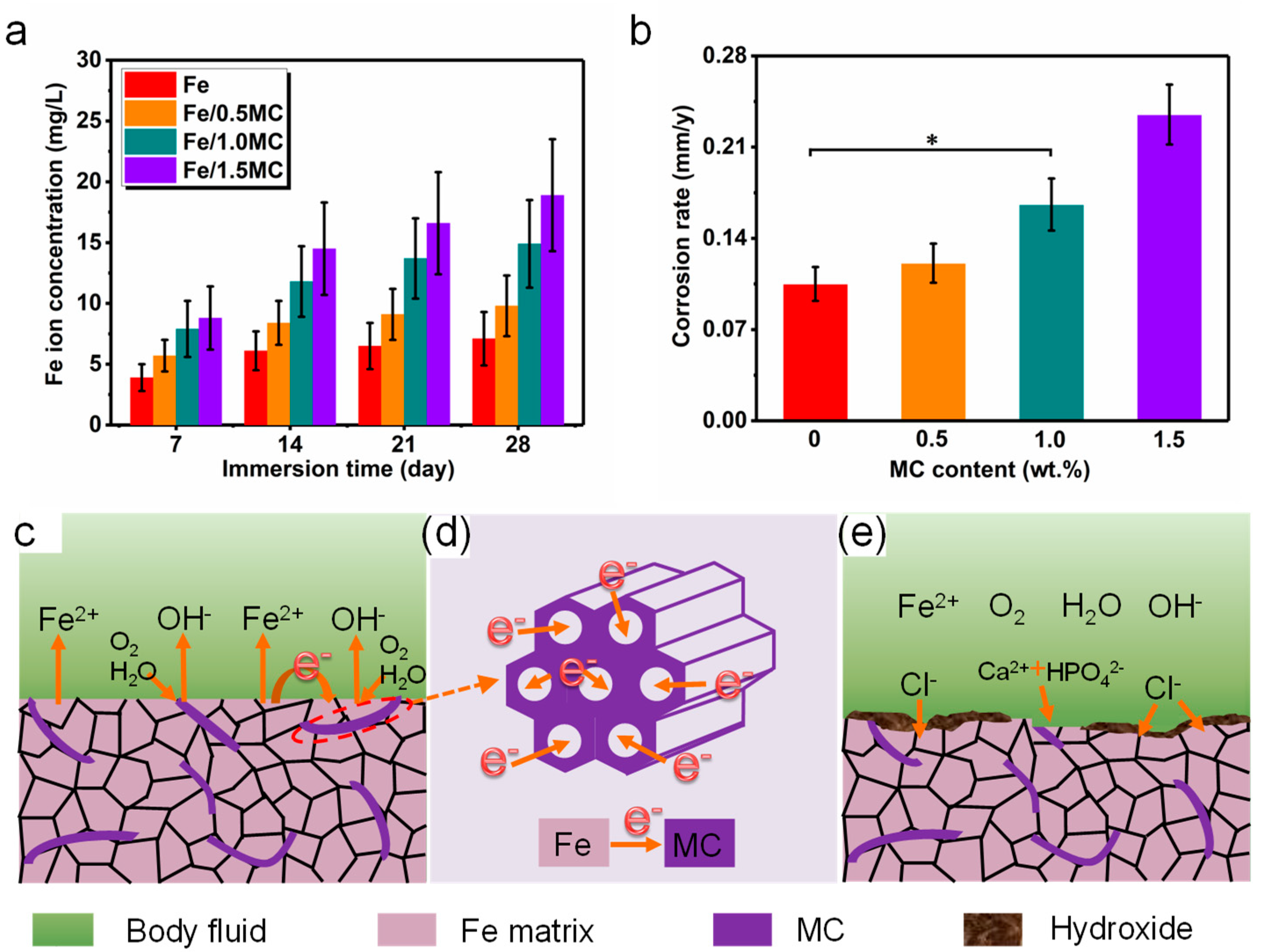
3.3. Degradation Behavior
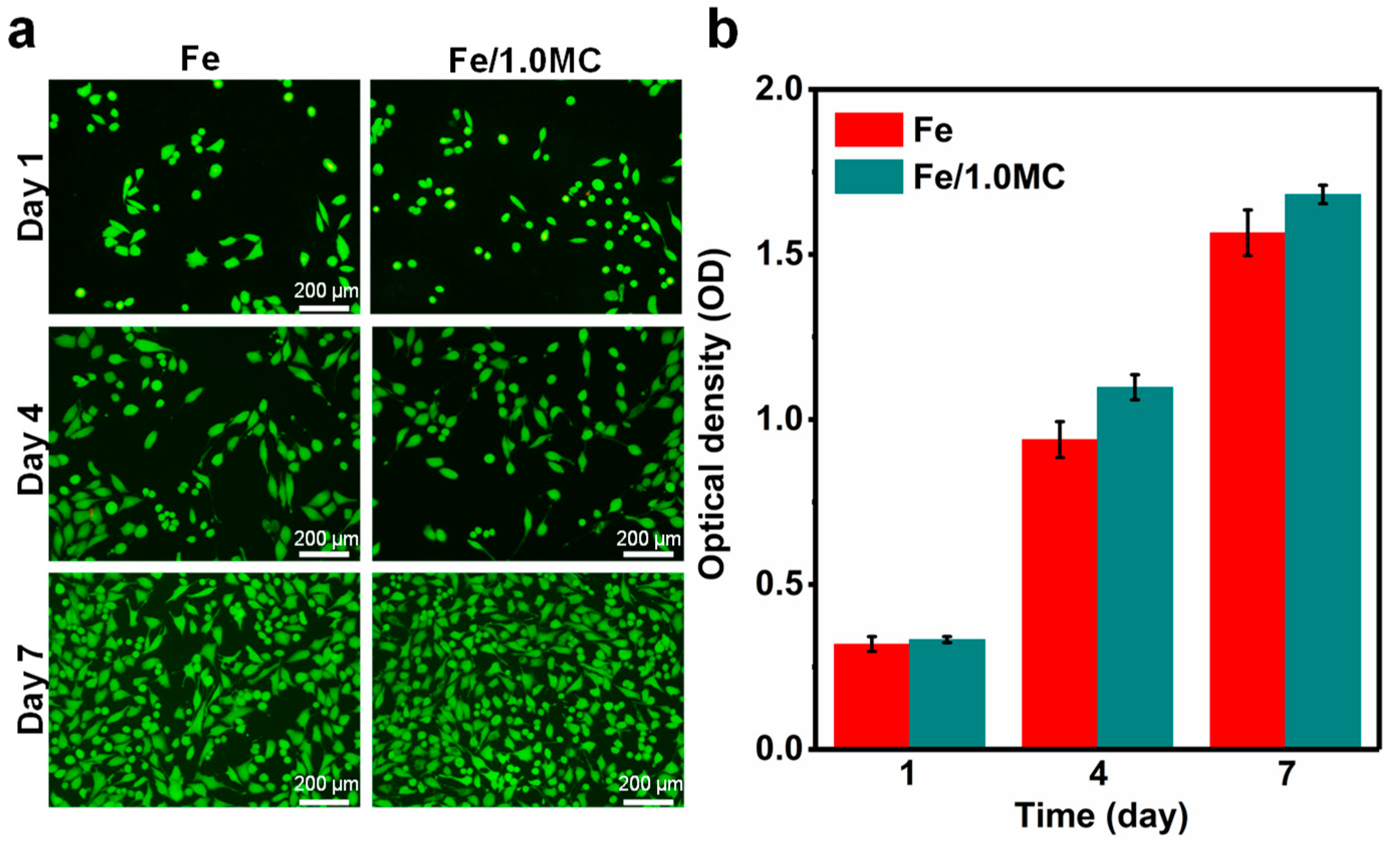
3.4. Cytocompatibility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. 3D printed Fe scaffolds with HA nanocoating for bone regeneration. Acs Biomater. Sci. Eng. 2018, 4, 608–616. [Google Scholar] [CrossRef]
- Tong, X.; Shi, Z.; Xu, L.; Lin, J.; Zhang, D.; Wang, K.; Li, Y.; Wen, C. Degradation behavior, cytotoxicity, hemolysis, and antibacterial properties of electro-deposited Zn–Cu metal foams as potential biodegradable bone implants. Acta Biomater. 2019, 102, 481–492. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, B.; Bin, S.; Peng, S.; Gao, C. Interfacial strengthening by reduced graphene oxide coated with MgO in biodegradable Mg composites. Mater. Des. 2020, 191, 108612. [Google Scholar] [CrossRef]
- Gao, C.; Yao, M.; Shuai, C.; Peng, S.; Deng, Y. Technology, Nano-SiC reinforced Zn biocomposites prepared via laser melting: Microstructure, mechanical properties and biodegradability. J. Mater. Sci. Technol. 2019, 201935, 2608–2617. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; E, D.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Pratesa, Y.; Suharno, B.; Wardhana, A.C.; Harjanto, S. Application of carbamide as foaming agent of FE-MN-C alloy for degradable biomaterial candidate with powder metallurgy process. J. Teknol. 2019, 81. [Google Scholar] [CrossRef]
- He, F.; Qian, G.; Ren, W.; Ke, J.; Fan, P.; Shi, X.; Cheng, Y.; Wu, S.; Deng, X.; Ye, J. Preparation and characterization of iron/β-tricalcium phosphate bio-cermets for load-bearing bone substitutes. Ceram. Int. 2017, 43, 8348–8355. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Y.; Fu, Z.; Li, Y.; Shi, J.; Ma, D.; Liu, S.; Luo, D. Fe/Zn-modified tricalcium phosphate (TCP) biomaterials: Preparation and biological properties. Rsc Adv. 2019, 9, 781–789. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Jiang, C.; Li, Y.; Fu, Y. In vitro corrosion behavior and cytocompatibility of pure Fe implanted with Ta. Surface and Coatings Technology 2017, 320, 201–205. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Liu, J.; Jiang, C.; Li, Y. In vitro corrosion properties and cytocompatibility of Fe-Ga alloys as potential biodegradable metallic materials. Mater. Sci. Eng. C 2017, 71, 60–66. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Chevallier, P.; Turgeon, S.; Paternoster, C.; Mostaed, E.; Vedani, M.; Mantovani, D. Long-term in vitro degradation behaviour of Fe and Fe/Mg 2 Si composites for biodegradable implant applications. Rsc Adv. 2018, 8, 9627–9639. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Peng, Q. High degradation rate of Fe-20Mn-based bio-alloys by accumulative cryo-rolling and annealing. Mater. Sci. Eng. C. 2017, 79, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cheng, J.; Zheng, Y. In vitro degradation and biocompatibility of Fe–Pd and Fe–Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. C 2014, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, A.; Swain, S.; Gotman, I.; Starosvetsky, D.; Psakhie, S.; Unger, R.; Gutmanas, E. Mechanical, degradation and drug-release behavior of nano-grained Fe-Ag composites for biomedical applications. J. Mech. Behav. Biomed. Mater. 2018, 86, 240–249. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Yang, Y.; Pan, H.; He, C.; Qi, F.; Xie, D.; Liang, H. Selective laser melted Fe-Mn bone scaffold: Microstructure, corrosion behavior and cell response. Mater. Res. Express 2020, 7, 015404. [Google Scholar] [CrossRef]
- Čapek, J.; Msallamová, Š.; Jablonská, E.; Lipov, J.; Vojtěch, D. A novel high-strength and highly corrosive biodegradable Fe-Pd alloy: Structural, mechanical and in vitro corrosion and cytotoxicity study. Mater. Sci. Eng. C 2017, 79, 550–562. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Tang, Y.; Zhao, M.-C.; Liu, C.; Liu, L.; Gao, C.-D.; Shuai, C.; Atrens, A. Study on Fe-xGO Composites Prepared by Selective Laser Melting: Microstructure, Hardness, Biodegradation and Cytocompatibility. JOM 2019, 72, 1163–1174. [Google Scholar] [CrossRef]
- Atkins, P.; Overton, T. Shriver and Atkins’ Inorganic Chemistry; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Ma, T.-Y.; Liu, L.; Yuan, Z.-Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Wang, G.; Yang, Y.; Peng, S.; Gao, C. Strong corrosion induced by carbon nanotubes to accelerate Fe biodegradation. Mater. Sci. Eng. C 2019, 104, 109935. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, C. Area Ratio of Cathode/Anode Effect on the Galvanic Corrosion of High Potential Difference Coupling in Seawater; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 022046. [Google Scholar]
- Hu, S.; Liu, R.; Liu, L.; Cui, Y.; Oguzie, E.E.; Wang, F. Effect of hydrostatic pressure on the galvanic corrosion of 90/10 Cu-Ni alloy coupled to Ti6Al4V alloy. Corros. Sci. 2019, 163, 108242. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Guan, B.Y.; Lou, X.W. Mesoporous Carbon@ Titanium Nitride Hollow Spheres as an Efficient SeS2 Host for Advanced Li–SeS2 Batteries. Angew. Chem. Int. Ed. 2017, 56, 16003–16007. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Ding, B.; Chang, Z.; Zhang, X.; Yamauchi, Y.; Wu, K.C.W. Confined Self-Assembly in Two-Dimensional Interlayer Space: Monolayered Mesoporous Carbon Nanosheets with In-Plane Orderly Arranged Mesopores and a Highly Graphitized Framework. Angewandte Chemie International Edition 2018, 57, 2894–2898. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Ordered mesoporous materials in the context of drug delivery systems and bone tissue engineering. Chem.–A Eur. J. 2006, 12, 5934–5943. [Google Scholar]
- Shuai, C.; Yuan, X.; Yang, W.; Peng, S.; He, C.; Feng, P.; Qi, F.; Wang, G. Cellulose nanocrystals as biobased nucleation agents in poly-l-lactide scaffold: Crystallization behavior and mechanical properties. Polym. Test. 2020, 85, 106458. [Google Scholar] [CrossRef]
- Shuai, C.; Cheng, Y.; Yang, W.; Feng, P.; Yang, Y.; He, C.; Qi, F.; Peng, S. Magnetically actuated bone scaffold: Microstructure, cell response and osteogenesis. Compos. Part B Eng. 2020, 192, 107986. [Google Scholar] [CrossRef]
- Shuai, C.; Zeng, Z.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Graphene oxide assists polyvinylidene fluoride scaffold to reconstruct electrical microenvironment of bone tissue. Mater. Des. 2020, 190, 108564. [Google Scholar] [CrossRef]
- Yang, Y.; Zan, J.; Yang, W.; Qi, F.; he, C.; Huang, S.; Peng, S.; Shuai, C. Metal organic framework as compatible reinforcement in biopolymer bone scaffold. Mater. Chem. Front. 2020, 4, 973–984. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, Y. In vitro study on newly designed biodegradable Fe-X composites (X= W, CNT) prepared by spark plasma sintering. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2013, 101, 485–497. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Shen, Q.; Zhang, L. Investigation on reactive sintering process of boron carbide ceramics by XRD. Mater. Sci. Technol. 2009, 25, 809–812. [Google Scholar] [CrossRef]
- Yuan, Q.; Xu, G.; Liu, S.; Liu, M.; Hu, H.; Li, G. Effect of rolling reduction on microstructure and property of ultrafine grained low-carbon steel processed by cryorolling martensite. Metals 2018, 8, 518. [Google Scholar] [CrossRef]
- Abdizadeh, H.; Baghchesara, M. Investigation into the mechanical properties and fracture behavior of A356 aluminum alloy-based ZrO 2-particle-reinforced metal-matrix composites. Mech. Compos. Mater. 2013, 49, 571–576. [Google Scholar] [CrossRef]
- Lai, D.; Kong, G.; Che, C. Synthesis and corrosion behavior of ZnO/SiO2 nanorod-sub microtube superhydrophobic coating on zinc substrate. Surf. Coat. Technol. 2017, 315, 509–518. [Google Scholar] [CrossRef]
- Solmaz, R. Gold-supported activated NiZn coatings: Hydrogen evolution and corrosion studies. Int. J. Energy Res. 2017, 41, 1452–1459. [Google Scholar] [CrossRef]
- Meng, J.; Sun, W.; Tian, Z.; Qiu, X.; Zhang, D. Corrosion performance of magnesium (Mg) alloys containing rare-earth (RE) elements. In Corrosion Prevention of Magnesium Alloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 38–60. [Google Scholar]
- Zhou, Y.; Du, C.; Han, G.; Gao, Y.; Yin, G. Ultra-low Pt decorated PdFe alloy nanoparticles for formic acid electro-oxidation. Electrochimica Acta 2016, 217, 203–209. [Google Scholar] [CrossRef]
- Shuai, C.; Zan, J.; Yang, Y.; Peng, S.; Yang, W.; Qi, F.; Shen, L.; Tian, Z. Surface modification enhances interfacial bonding in PLLA/MgO bone scaffold. Mater. Sci. Eng. C 2020, 108, 110486. [Google Scholar] [CrossRef]
- Prabhu, T.R. An overview of high-performance aircraft structural Al alloy-AA7085. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 909–921. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Dong, C.; Liu, G. Tuning the functionalization degree of graphene: Determining critical conditions for inhibiting the corrosion promotion activity of graphene/epoxy nanocomposite coatings. Mater. Lett. 2019, 240, 262–266. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, T.; Zheng, Y. Microstructure, mechanical property, biodegradation behavior, and biocompatibility of biodegradable Fe–Fe2O3 composites. J. Biomed. Mater. Res. Part A 2014, 102, 2277–2287. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef]
- Feng, P.; Kong, Y.; Yu, L.; Li, Y.; Gao, C.; Peng, S.; Pan, H.; Zhao, Z.; Shuai, C. Molybdenum disulfide nanosheets embedded with nanodiamond particles: Co-dispersion nanostructures as reinforcements for polymer scaffolds. Appl. Mater. Today 2019, 17, 216–226. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Peng, S.; Yang, W.; Bin, S.; Shuai, C. Graphene oxide-driven interfacial coupling in laser 3D printed PEEK/PVA scaffolds for bone regeneration. Virtual Phys. Prototyp. 2020. [Google Scholar] [CrossRef]
- Qian, G.; Lu, T.; Zhang, J.; Liu, R.; Wang, Z.; Yu, B.; Li, H.; Shi, H.; Ye, J. Promoting bone regeneration of calcium phosphate cement by addition of PLGAmicrospheres and zinc silicate via synergistic effect of in-situ pore generation, bioactive ion stimulation and macrophage immunomodulation. Appl. Mater. Today 2020, 19, 100615. [Google Scholar] [CrossRef]
- Wang, G.; He, C.; Yang, W.; Qi, F.; Qian, G.; Peng, S.; Shuai, C. Surface-Modified Graphene Oxide with Compatible Interface Enhances Poly-L-Lactic Acid Bone Scaffold. J. Nanomater. 2020, 2020, 5634096. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Yang, W.; He, C.; Wang, G.; Liu, Z.; Qi, F.; Peng, S. Functionalized BaTiO3 enhances piezoelectric effect towards cell response of bone scaffold. Colloids Surf. B Biointerfaces 2020, 185, 110587. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yang, S.; Zhang, Y.; Li, X.; Gao, D.; Zhong, Y.; Cao, L.; Ma, H.; Liu, Y.; Li, G.; et al. LncRNA ODIR1 Inhibits Osteogenic Differentiation of hUC-MSCs through the FBXO25/H2BK120ub/H3K4me3/OSX Axis. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
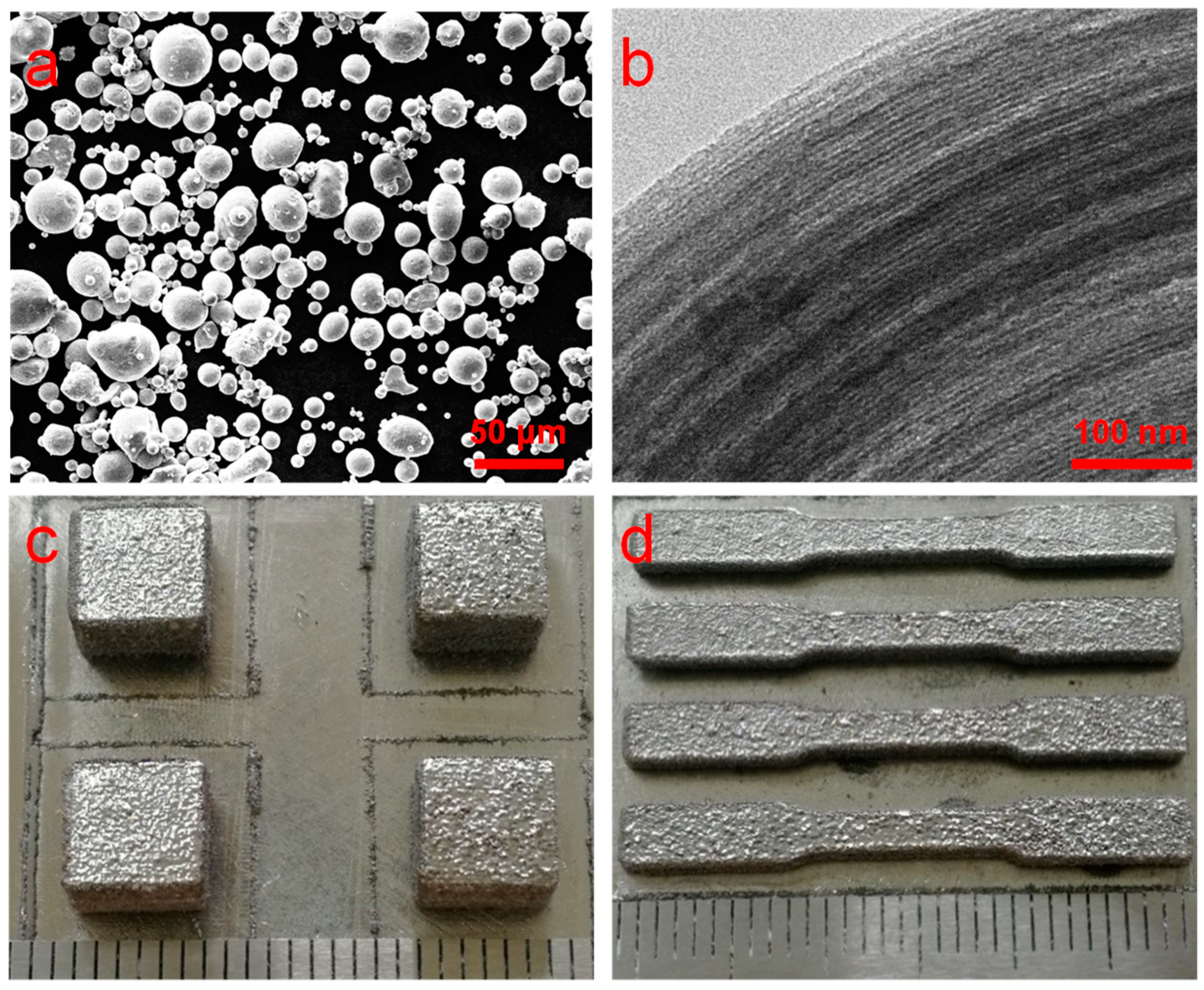
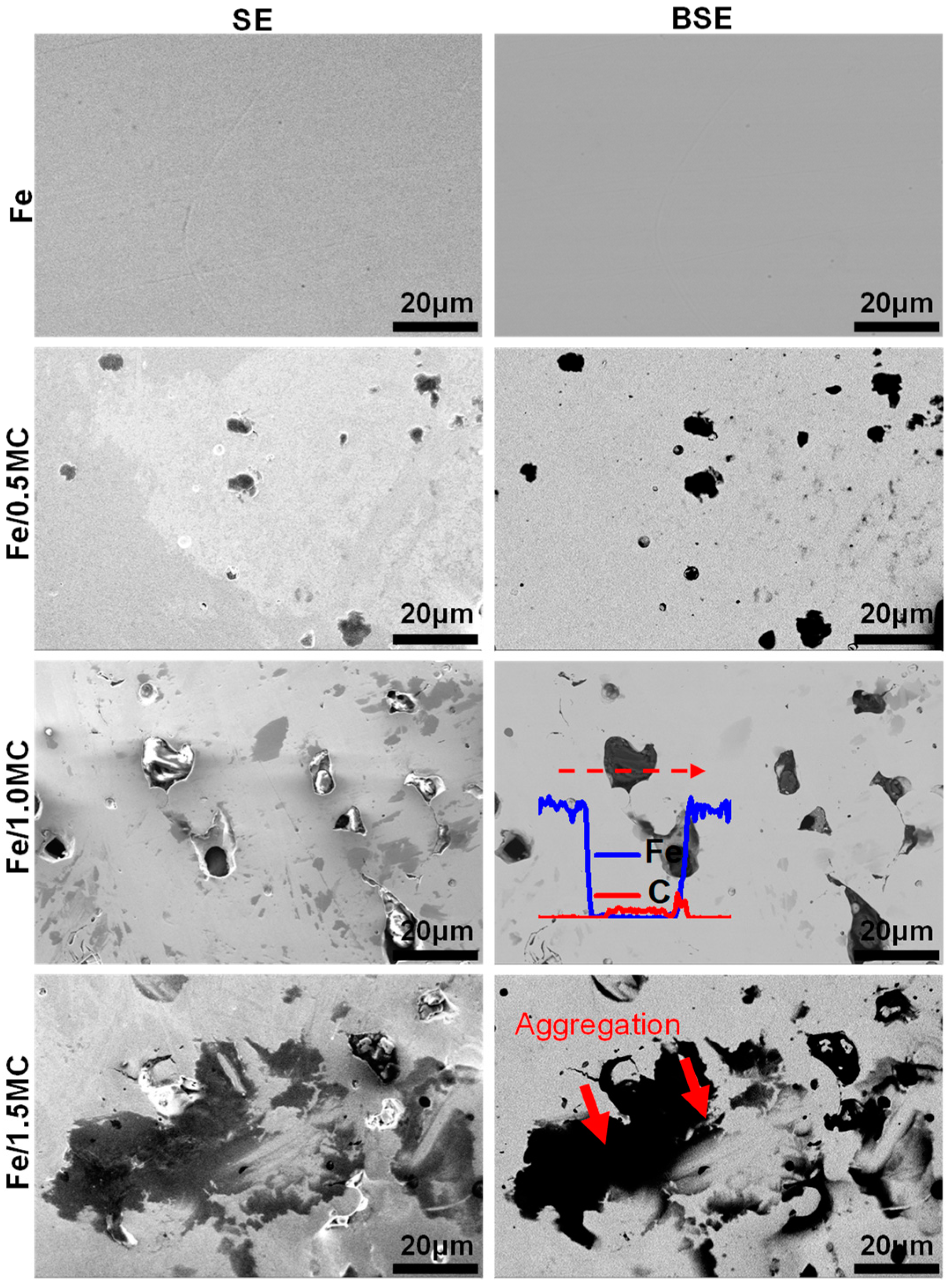
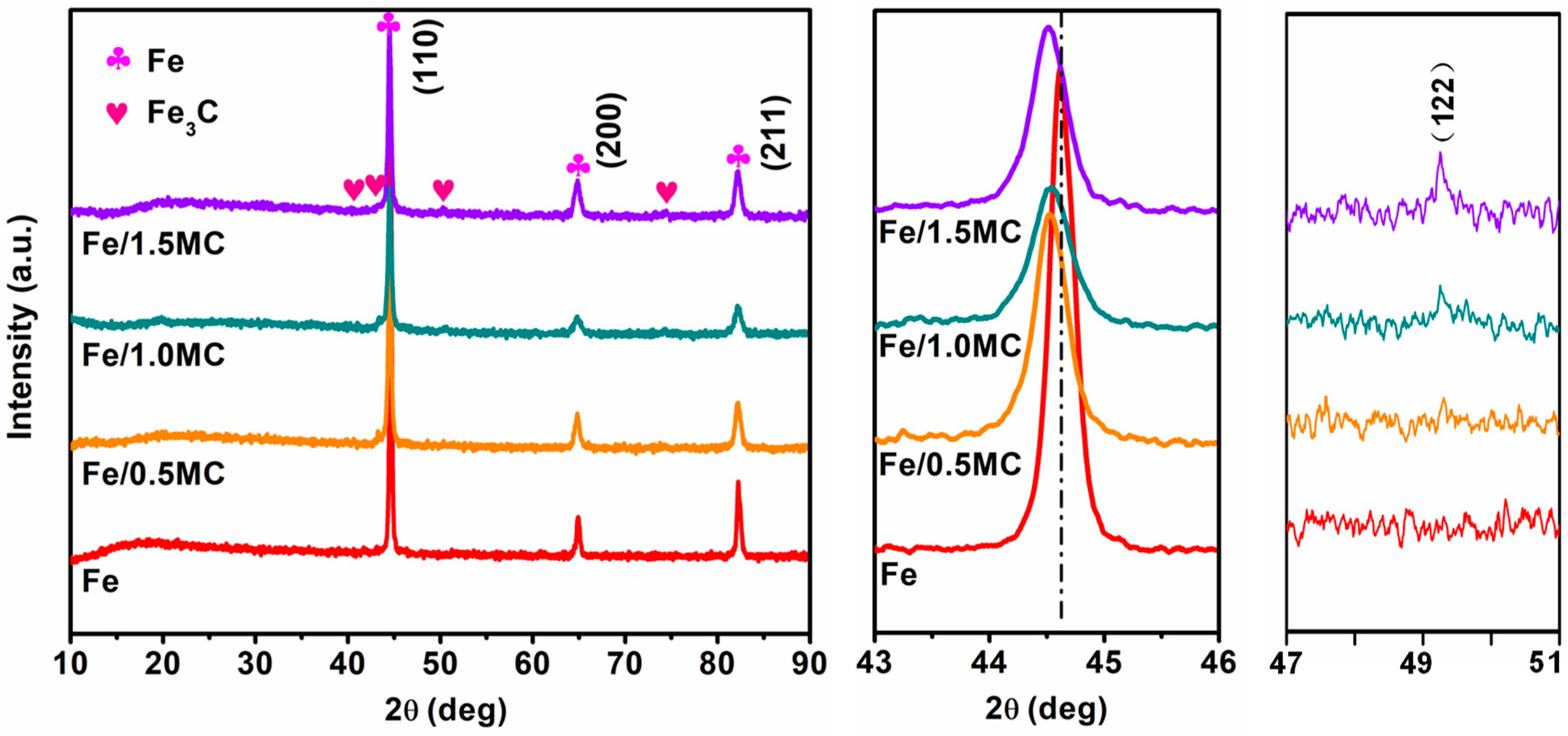
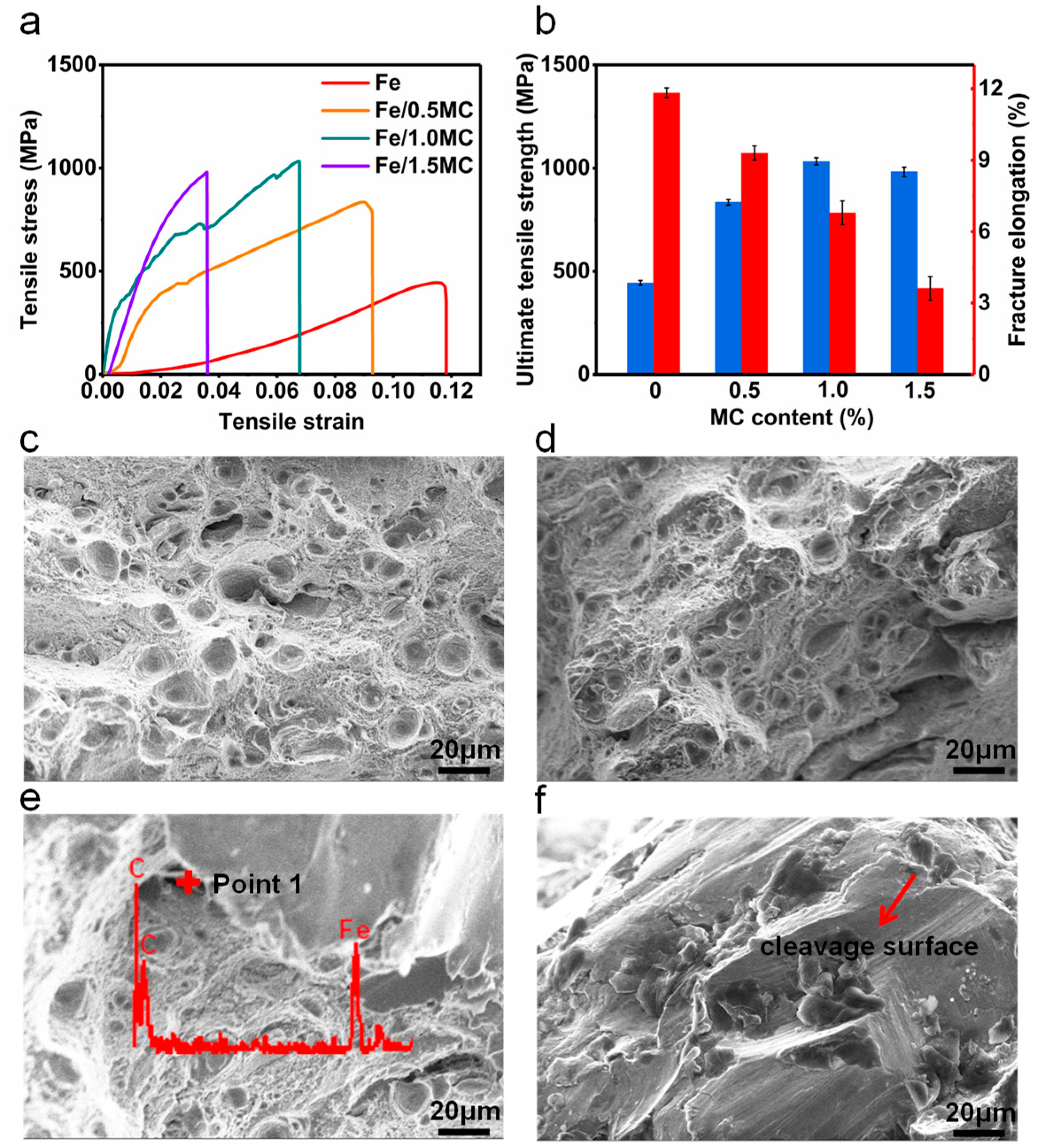

| Micro Porosity (%) | Pore Diameter (nm) | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|
| 3.7 | 3.9 | 1201 | 1.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Li, Y.; Deng, F.; Yang, Y.; Peng, S.; Qi, F.; Shen, L. Mesoporous Carbon as Galvanic-Corrosion Activator Accelerates Fe Degradation. Appl. Sci. 2020, 10, 2487. https://doi.org/10.3390/app10072487
Shuai C, Li Y, Deng F, Yang Y, Peng S, Qi F, Shen L. Mesoporous Carbon as Galvanic-Corrosion Activator Accelerates Fe Degradation. Applied Sciences. 2020; 10(7):2487. https://doi.org/10.3390/app10072487
Chicago/Turabian StyleShuai, Cijun, Yulong Li, Fang Deng, Youwen Yang, Shuping Peng, Fangwei Qi, and Lida Shen. 2020. "Mesoporous Carbon as Galvanic-Corrosion Activator Accelerates Fe Degradation" Applied Sciences 10, no. 7: 2487. https://doi.org/10.3390/app10072487
APA StyleShuai, C., Li, Y., Deng, F., Yang, Y., Peng, S., Qi, F., & Shen, L. (2020). Mesoporous Carbon as Galvanic-Corrosion Activator Accelerates Fe Degradation. Applied Sciences, 10(7), 2487. https://doi.org/10.3390/app10072487






