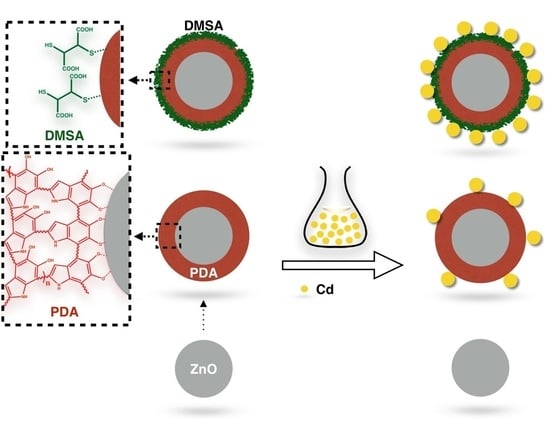
The Application of Self-Assembled of Meso-2,3-Dimercaptosuccinic Acid-Polydopamine-Zinc Oxide for Trace Cadmium Analysis
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DMSA/PDA/ZnO
2.3. Characterization of DMSA/PDA/ZnO
2.4. Adsorption of Cd(II) on DMSA/PDA/ZnO
2.5. Protocol for Preconcentration of Cd(II) with of DMSA/PDA/ZnO
2.6. Data Analysis
3. Results
3.1. Structural Characterization of ZnO, DPOA/ZnO, and DMSA/DPOA/ZnO
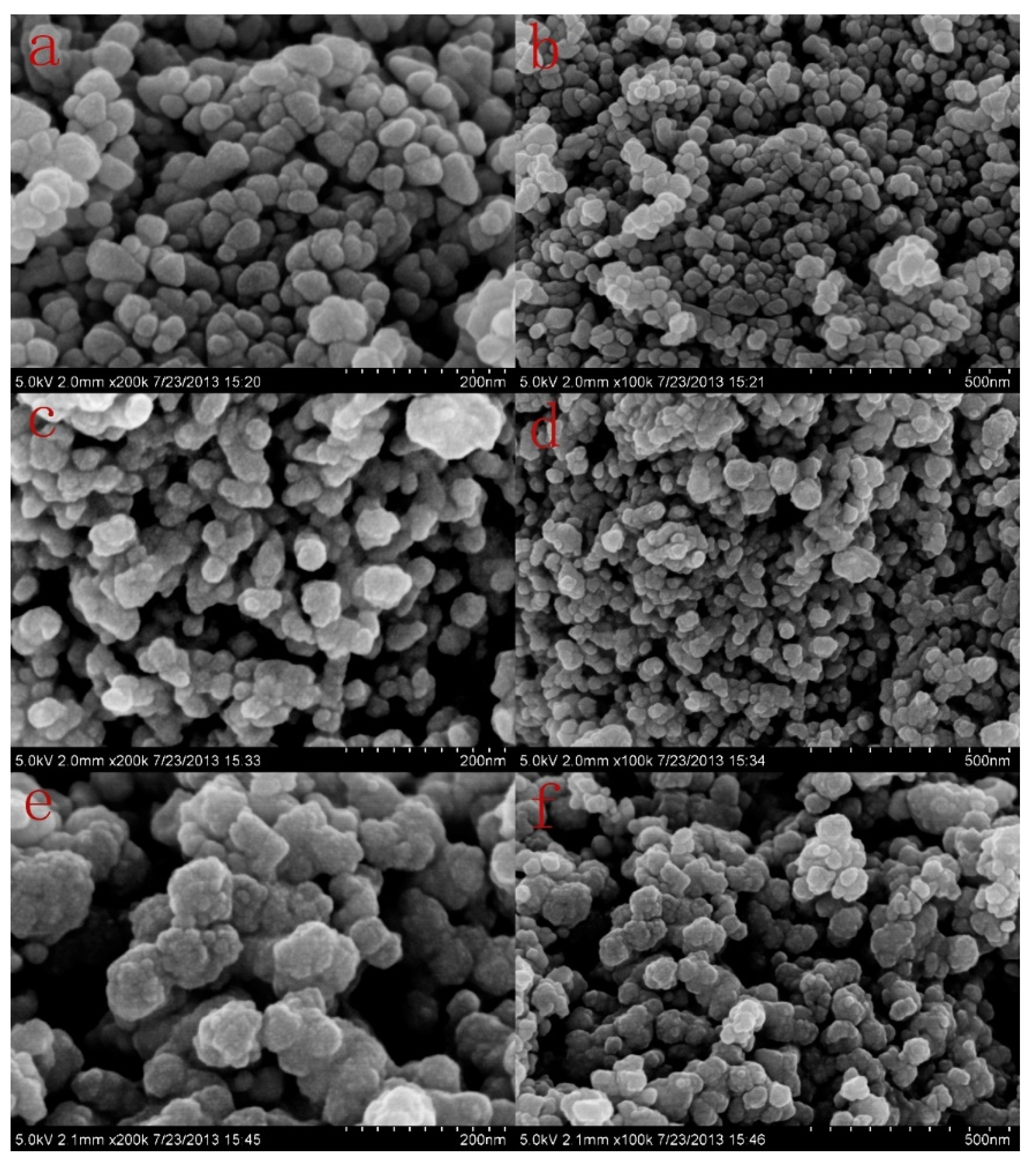
3.2. Morphology of ZnO, DPOA/ZnO, and DMSA/DPOA/ZnO
3.3. FTIR Spectra of ZnO and DMSA/PDA/ZnO
3.4. Quantification of Attached DMSA and PDA in DMSA/PDA/ZnO
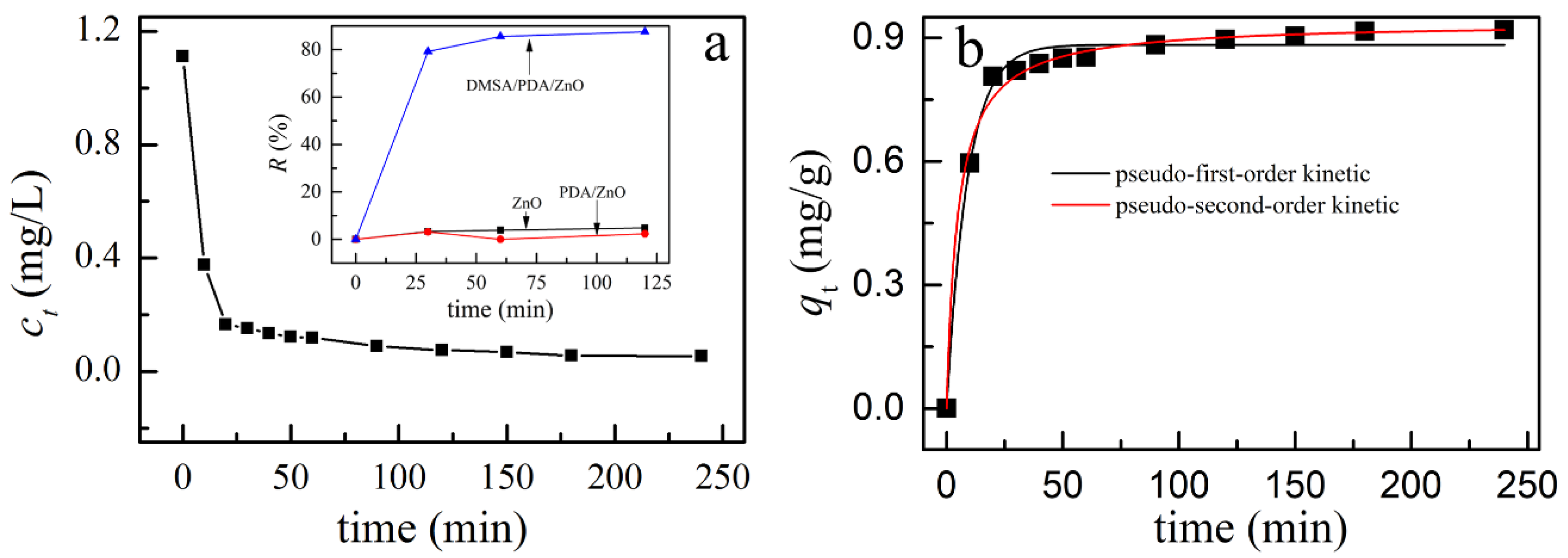
3.5. Adsorption of Cd(II) on ZnO, PDA/ZnO, and DMSA/PDA/ZnO
3.6. Effects of Solution pH and Adsorbent Dosage on Adsorption of Cd on DMSA/PDA/ZnO
3.7. Performance of DMSA/PDA/ZnO in the Analysis of Ultra-Trace Cd(II)
3.7.1. Linear Region
3.7.2. Accuracy of the Method
3.7.3. Limit of Detection
3.8. Analysis of Cd(II) in Natural Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Canton, J.H.; Slooff, W. Toxicity and accumulation studies of cadmium (Cd2+) with freshwater organisms of different trophic levels. Ecotox. Environ. Safe. 1982, 6, 113–128. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, H.; Hao, R.; Chu, H.; Zhu, Y. Physiological changes in chlamydomonas reinhardtii after 1000 generations of selection of cadmium exposure at environmentally relevant concentrations. Environ. Sci. Proc. Imp. 2018, 20, 923–933. [Google Scholar] [CrossRef] [PubMed]
- W.H.O. Cadmium in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Sun, J.; Yang, Z.; Lee, H.; Wang, L. Simultaneous speciation and determination of arsenic, chromium and cadmium in water samples by high performance liquid chromatography with inductively coupled plasma mass spectrometry. Anal. Methods 2015, 7, 2653–2658. [Google Scholar] [CrossRef]
- Behbahani, M.; Veisi, A.; Omidi, F.; Noghrehabadi, A.; Esrafili, A.; Ebrahimi, M.H. Application of a dispersive micro-solid-phase extraction method for pre-concentration and ultra-trace determination of cadmium ions in water and biological samples. Appl. Organomet. Chem. 2018, 32, e4134. [Google Scholar] [CrossRef]
- Ali, M.M.; Ali, M.L.; Islam, M.S.; Rahman, M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2016, 5, 27–35. [Google Scholar] [CrossRef]
- Barreto, I.S.; Andrade, S.I.E.; Cunha, F.A.S.; Lima, M.B.; Araujo, M.C.U.; Almeida, L.F. A robotic magnetic nanoparticle solid phase extraction system coupled to flow-batch analyzer and GFAAS for determination of trace cadmium in edible oils without external pretreatment. Talanta 2018, 178, 384–391. [Google Scholar] [CrossRef]
- Akkaya, E.; Aylin Kasa, N.; Çetin, G.; Bakirdere, S. A new method for the determination of cadmium at ultratrace levels using slotted quartz tube-flame atomic absorption spectrometry after preconcentration with stearic acid coated magnetite nanoparticles. J. Anal. At. Spectrom. 2017, 32, 2433–2438. [Google Scholar] [CrossRef]
- Hideo, A.; Hiroshi, K.; Eiji, Y. Spectrophotometric determination of cadmium(II) with dithizone and 1,10-phenanthroline. Bull. Chem. Soc. Jpn. 1979, 52, 3718–3720. [Google Scholar]
- Wen, X.; Yang, Q.; Yan, Z.; Deng, Q. Determination of cadmium and copper in water and food samples by dispersive liquid–liquid microextraction combined with UV–vis spectrophotometry. Microchem. J. 2011, 97, 249–254. [Google Scholar] [CrossRef]
- Yilmaz, V.; Yilmaz, H.; Arslan, Z.; Leszczynski, J. Novel Imprinted polymer for the preconcentration of cadmium with determination by inductively coupled plasma mass spectrometry. Anal. Lett. 2017, 50, 482–499. [Google Scholar] [CrossRef]
- Safari, M.; Yamini, Y.; Masoomi, M.Y.; Morsali, A.; Mani-Varnosfaderani, A. Magnetic metal-organic frameworks for the extraction of trace amounts of heavy metal ions prior to their determination by ICP-AES. Microchim. Acta. 2017, 184, 1555–1564. [Google Scholar] [CrossRef]
- Dos Santos, W.N.L.; Costa, J.L.O.; Araujo, R.G.O.; de Jesus, D.S.; Costa, A.C.S. An on-line pre-concentration system for determination of cadmium in drinking water using FAAS. J. Hazard. Mater. 2006, 137, 1357–1361. [Google Scholar] [CrossRef]
- Lemos, V.A.; do Nascimento, G.S.; Nunes, L.S. A new functionalized resin for preconcentration and determination of cadmium, cobalt, and nickel in sediment samples. Water Air Soil Poll. 2015, 226, 2. [Google Scholar] [CrossRef]
- Minaberry, Y.S.; Tudino, M. An ion imprinted amino-functionalized mesoporous sorbent for the selective minicolumn preconcentration of cadmium ions and determination by GFAAS. Anal. Methods 2018, 10, 5305–5312. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Miró, M.; da Silva, E.G.P.; Matos, G.D.; dos Reis, P.S.; Brandao, G.C.; dos Santos, W.N.L.; Duarte, A.T.; Vale, M.G.R.; Araujo, R.G.O. Slurry Sampling—An Analytical Strategy for the Determination of Metals and Metalloids by Spectroanalytical Techniques. Appl. Spectrosc. Rev. 2010, 45, 44–62. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine modified superparamagnetic iron oxide nanoparticles as multifunctional nanocarrier for targeted prostate cancer treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef]
- Park, J.Y.; Back, S.H.; Chang, S.-J.; Lee, S.J.; Lee, K.G.; Park, T.J. Dopamine-assisted synthesis of carbon-coated silica for PCR Enhancement. ACS Appl. Mater. Interfaces 2015, 7, 15633–15640. [Google Scholar] [CrossRef]
- Soyekwo, F.; Zhang, Q.; Zhen, L.; Ning, L.; Zhu, A.; Liu, Q. Borate crosslinking of polydopamine grafted carbon nanotubes membranes for protein separation. Biochem. Eng. J. 2018, 337, 110–121. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized Dopamine coating deposited on hydrophobic Polymer Films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Wen, X.; Wu, W. Efficient removal of cadmium using facile functionalized of mesoporous silica via a biomimetic coating. RSC Adv. 2016, 6, 18340–18347. [Google Scholar] [CrossRef]
- Song, Q.; Li, M.; Huang, L.; Wu, Q.; Zhou, Y.; Wang, Y. Bifunctional polydopamine@Fe3O4 core–shell nanoparticles for electrochemical determination of lead(II) and cadmium(II). Anal. Chim. Acta 2013, 787, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Gluhcheva, Y.; Kamenova, K.; Dorkov, P.; Lobanova, Y.; Skalnaya, M.; Ivanova, J. Comparative effects of meso-2,3-dimercaptosuccinic acid, monensin and salinomycin on the concentrations of cadmium and some essential elements in skeletal muscles of Cd-exposed mice. J. Trace. Elem. Med. Biol. 2018, 50, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, S.; Shahab, S.; Sheikhi, M.; Ahmadianarog, M. DFT study on the selective complexation of meso-2,3-dimercaptosuccinic acid with toxic metal ions (Cd2+, Hg2+ and Pb2+) for pharmaceutical and biological applications. J. Mol. Struct. 2019, 1176, 901–907. [Google Scholar] [CrossRef]
- Andersen, O.; Nielsen, J.B. Oral Cadmium Chloride Intoxication in Mice: Effects of Penicillamine, Dimercaptosuccinic Acid and Related Compounds. Pharmacol. Toxicol. 1988, 63, 386–389. [Google Scholar] [CrossRef]
- Fang, X.; Hua, F.; Fernando, Q. Comparison of rac- and meso-2,3-Dimercaptosuccinic Acids for Chelation of Mercury and Cadmium Using Chemical Speciation Models. Chem. Res. Toxicol. 1996, 9, 284–290. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Abd. Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhang, L.; Yuan, J.; Yan, S.; Wang, C. Low-temperature synthesis of ZnO nanoparticles by solid-state pyrolytic reaction. Nanotechnology 2002, 14, 11–15. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Wang, K.; Fu, J.; Wang, S.; Gao, M.; Zhu, J.; Wang, Z.; Xu, Q. Polydopamine-coated magnetic nanochains as efficient dye adsorbent with good recyclability and magnetic separability. J. Colloid. Interface Sci. 2018, 516, 263–273. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Biochem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, D.; Qi, X.; Guo, Y.; Yue, F.; Wang, X.; Han, M. Polydopamine-based surface modification of paclitaxel nanoparticles for osteosarcoma targeted therapy. Nanotechnology 2019, 30, 255101. [Google Scholar] [CrossRef] [PubMed]



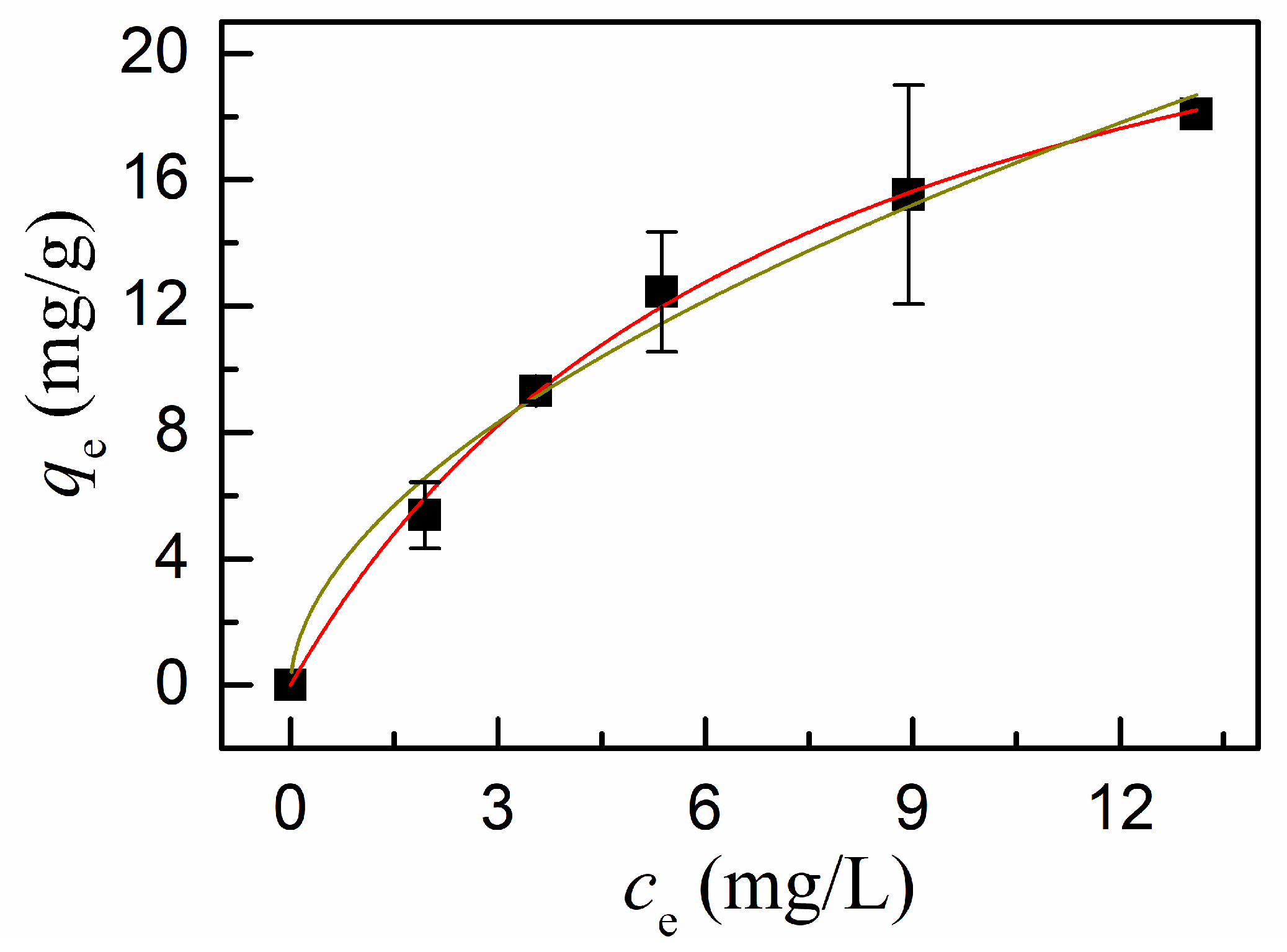


| Element | C | N | H | S |
|---|---|---|---|---|
| ZnO | 0.03 | 0 | 0.041 | 0.010 |
| PDA/ZnO | 8.63 | 1.24 | 0.941 | 0.012 |
| DMSA/PDA/ZnO | 8.08 | 1.18 | 1.076 | 0.834 |
| Model | Kinetics | Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|
| Pseudo-First-Order | Pseudo-Second-Order | Langmuir | Freundlich | |||||
| parameters | k1 | ce | k2 | ce | KL | qmax | KF | 1/n |
| value | 0.11 | 0.88 | 0.22 | 0.94 | 0.14 | 28.5 | 4.55 | 0.55 |
| correlation coefficient | 0.989 | 0.993 | 0.997 | 0.961 | ||||
| Sample | Added (μg/L) | Found (μg/L) | Recovery % |
|---|---|---|---|
| Xinghu Lake | 0 | 0.6 ± 0.02 | — |
| 5.0 | 4.9 ± 0.04 | 86 | |
| Yangtze River | 0 | N.D. | — |
| 2.0 | 1.8 ± 0.05 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Zhang, X.; Chen, N. The Application of Self-Assembled of Meso-2,3-Dimercaptosuccinic Acid-Polydopamine-Zinc Oxide for Trace Cadmium Analysis. Appl. Sci. 2020, 10, 2462. https://doi.org/10.3390/app10072462
Lu M, Zhang X, Chen N. The Application of Self-Assembled of Meso-2,3-Dimercaptosuccinic Acid-Polydopamine-Zinc Oxide for Trace Cadmium Analysis. Applied Sciences. 2020; 10(7):2462. https://doi.org/10.3390/app10072462
Chicago/Turabian StyleLu, Min, Xu Zhang, and Nuo Chen. 2020. "The Application of Self-Assembled of Meso-2,3-Dimercaptosuccinic Acid-Polydopamine-Zinc Oxide for Trace Cadmium Analysis" Applied Sciences 10, no. 7: 2462. https://doi.org/10.3390/app10072462
APA StyleLu, M., Zhang, X., & Chen, N. (2020). The Application of Self-Assembled of Meso-2,3-Dimercaptosuccinic Acid-Polydopamine-Zinc Oxide for Trace Cadmium Analysis. Applied Sciences, 10(7), 2462. https://doi.org/10.3390/app10072462