Cell-Laden Thermosensitive Chitosan Hydrogel Bioinks for 3D Bioprinting Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Chitosan Hydrogel
2.2. Characterization of the Chitosan Hydrogel
2.2.1. Gelation Time Determination
2.2.2. Rheological Analysis
2.2.3. Field Emission Scanning Electron Microscopy
2.3. Biocompatibility of Chitosan Bioinks (CBIs)
2.3.1. Cell Culture
2.3.2. Water Soluble Tetrazolium Salt (WST) Assay
2.3.3. Live and Dead Assay
2.4. 3D Bioprinting Cell-Laden Constructs
2.4.1. 3D Bioprinter
2.4.2. 3D Bioprinting of Polycaprolactone and CBI Constructs
2.4.3. Cell Viability of Cell-Laden Constructs
2.5. Statistical Data Analysis
3. Results and Discussion
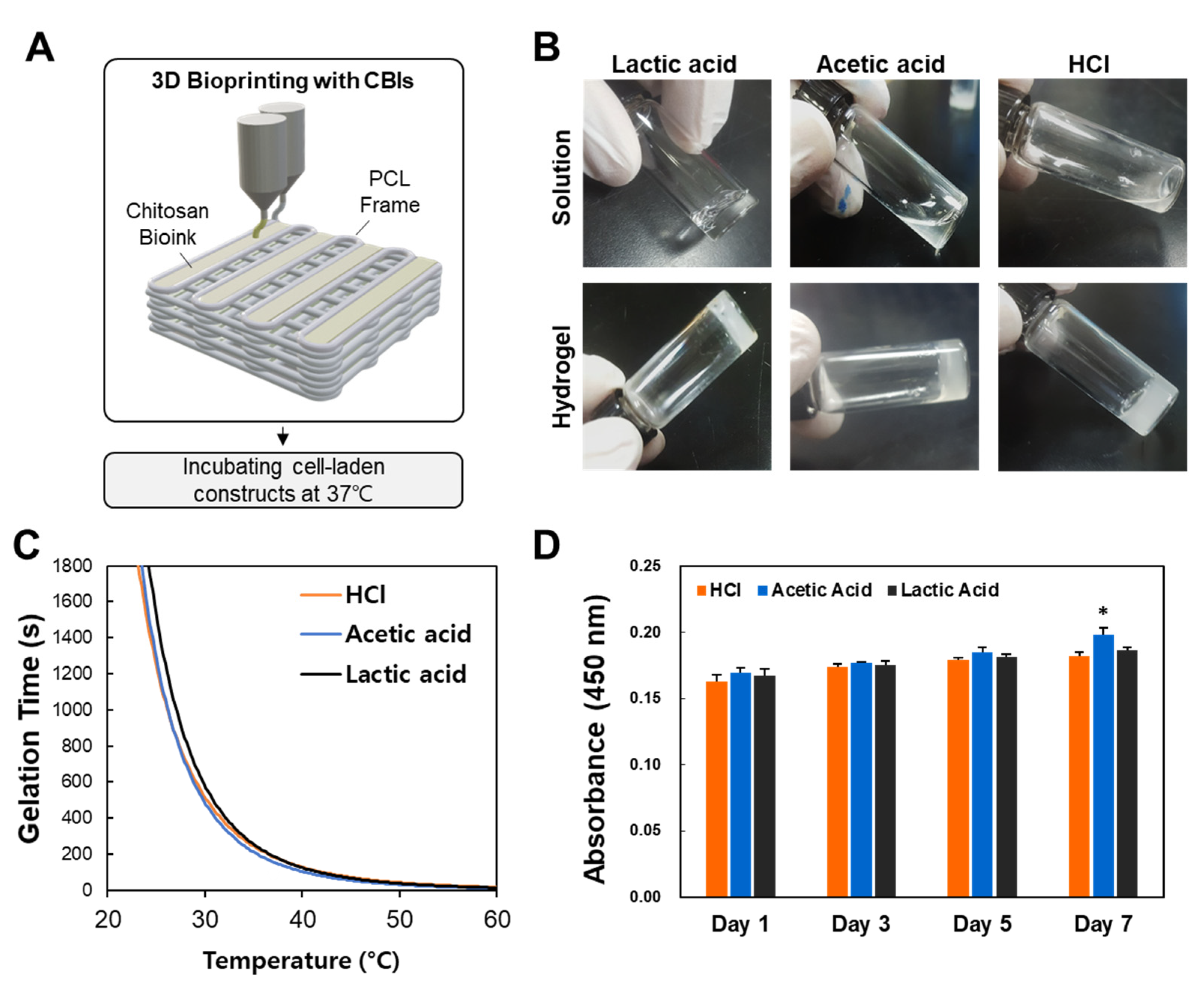
3.1. Effect of Solvents on Chitosan Hydrogels
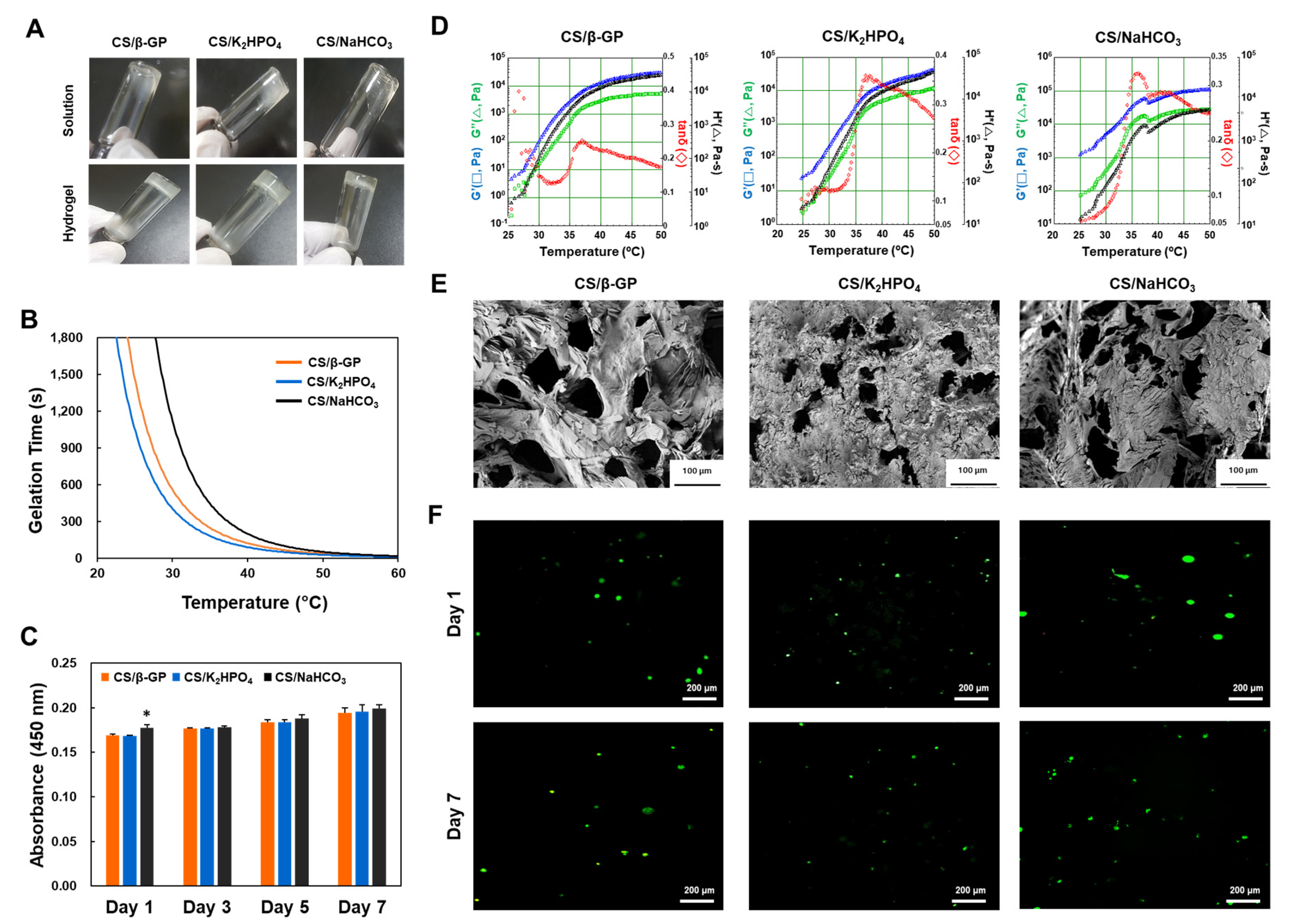
3.2. Effects of Gelling Agents on Chitosan Hydrogels
3.3. 3D Printing Cell-Laden Constructs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Saunders, R.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Tissue Engineering Applications of Three-Dimensional Bioprinting. Cell Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef]
- Hribar, K.C.; Soman, P.; Warner, J.; Chung, P.; Chen, S. Light-assisted direct-write of 3D functional biomaterials. Lab Chip 2014, 14, 268–275. [Google Scholar] [CrossRef]
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.G.; Kaplan, D.L. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef]
- Wang, S.; Lee, J.M.; Yeong, W.Y. Smart hydrogels for 3D bioprinting. Int. J. Bioprint. 2015, 1, 3–14. [Google Scholar] [CrossRef]
- Van Miller, J.P.; Hermansky, S.J.; Losco, P.; Ballantyne, B. Chronic toxicity and oncogenicity study with glutaraldehyde dosed in the drinking water of Fischer 344 rats. Toxicology 2002, 175, 177–189. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Dang, Q.F.; Zou, S.H.; Chen, X.; Liu, C.; Li, J.J.; Zhou, X.; Liu, Y.; Cheng, X.J. Characterizations of chitosan-based highly porous hydrogel—The effects of the solvent. J. Appl. Polym. Sci. 2012, 125, E88–E98. [Google Scholar] [CrossRef]
- Liu, L.; Tang, X.; Wang, Y.; Guo, S. Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system. Int. J. Pharm. 2011, 414, 6–15. [Google Scholar] [CrossRef]
- Ta, H.T.; Han, H.; Larson, I.; Dass, C.R.; Dunstan, D.E. Chitosan-dibasic orthophosphate hydrogel: A potential drug delivery system. Int. J. Pharm. 2009, 371, 134–141. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.; Hoemann, C.D.; Leroux, J.; Atkinson, B.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Chung, Y.-M.; Simmons, K.L.; Gutowska, A.; Jeong, B. Sol−Gel Transition Temperature of PLGA-g-PEG Aqueous Solutions. Biomacromolecules 2002, 3, 511–516. [Google Scholar] [CrossRef]
- Seonwoo, H.; Jang, K.-J.; Lee, D.; Park, S.; Lee, M.; Park, S.; Lim, K.-T.; Kim, J.; Chung, J.H. Neurogenic Differentiation of Human Dental Pulp Stem Cells on Graphene-Polycaprolactone Hybrid Nanofibers. Nanomaterials 2018, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanian, S.; Qasaimeh, M.A.; Akbari, M.; Tamayol, A.; Juncker, D. Microfluidic direct writer with integrated declogging mechanism for fabricating cell-laden hydrogel constructs. Biomed. Microdevices 2014, 16, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Seonwoo, H.; Kim, S.W.; Shin, B.; Jang, K.-J.; Lee, M.; Choo, O.-S.; Choi, M.-J.; Kim, J.; Lim, K.-T.; Jang, J.H.; et al. Latent stem cell-stimulating therapy for regeneration of chronic tympanic membrane perforations using IGFBP2-releasing chitosan patch scaffolds. J. Biomater. Appl. 2019, 34, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Du, Y.; Fan, L. Dialdehyde starch-crosslinked chitosan films and their antimicrobial effects. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 993–997. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.W.; Choi, S.J.; Lim, K.-T.; Bin Lee, J.; Seonwoo, H.; Choung, P.-H.; Park, K.; Cho, C.-S.; Choung, Y.-H.; et al. A Healing Method of Tympanic Membrane Perforations Using Three-Dimensional Porous Chitosan Scaffolds. Tissue Eng. Part A 2011, 17, 2763–2772. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.-T.; Seonwoo, H.; Park, S.; Cho, S.-P.; Hong, B.H.; Choung, Y.-H.; Chung, J.H.; et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N. Chitosan in Nanostructured Thin Films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Schauer, C. Cross-Linking Chitosan Nanofibers. Biomacromolecules 2007, 8, 594–601. [Google Scholar] [CrossRef]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. 2004, 69, 216–222. [Google Scholar] [CrossRef]
- Denkbaş, E.B.; Oztürk, E.; Ozdemir, N.; Keçeci, K.; Agalar, C. Norfloxacin-loaded chitosan sponges as wound dressing material. J. Biomater. Appl. 2004, 18, 291–303. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Seonwoo, H.; Shin, B.; Jang, K.; Lee, M.; Choo, O.; Park, S.; Kim, Y.C.; Choi, M.; Kim, J.; Garg, P.; et al. Epidermal Growth Factor–Releasing Radially Aligned Electrospun Nanofibrous Patches for the Regeneration of Chronic Tympanic Membrane Perforations. Adv. Healthc. Mater. 2018, 8, 1801160. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, J.; Seonwoo, H.; Park, S.; Jang, K.-J.; Lee, J.; Lee, M.; Lim, J.W.; Kim, J.; Chung, J.H. Cell-Laden Thermosensitive Chitosan Hydrogel Bioinks for 3D Bioprinting Applications. Appl. Sci. 2020, 10, 2455. https://doi.org/10.3390/app10072455
Ku J, Seonwoo H, Park S, Jang K-J, Lee J, Lee M, Lim JW, Kim J, Chung JH. Cell-Laden Thermosensitive Chitosan Hydrogel Bioinks for 3D Bioprinting Applications. Applied Sciences. 2020; 10(7):2455. https://doi.org/10.3390/app10072455
Chicago/Turabian StyleKu, Jongbeom, Hoon Seonwoo, Sangbae Park, Kyoung-Je Jang, Juo Lee, Myungchul Lee, Jae Woon Lim, Jangho Kim, and Jong Hoon Chung. 2020. "Cell-Laden Thermosensitive Chitosan Hydrogel Bioinks for 3D Bioprinting Applications" Applied Sciences 10, no. 7: 2455. https://doi.org/10.3390/app10072455
APA StyleKu, J., Seonwoo, H., Park, S., Jang, K.-J., Lee, J., Lee, M., Lim, J. W., Kim, J., & Chung, J. H. (2020). Cell-Laden Thermosensitive Chitosan Hydrogel Bioinks for 3D Bioprinting Applications. Applied Sciences, 10(7), 2455. https://doi.org/10.3390/app10072455






