Mangrove Soil-Borne Trace Elements in Qi’ao Island: Implications for Understanding Terrestrial Input of Trace Elements into Part of the Pearl River Estuary
Abstract
1. Introduction
2. Materials and Methods
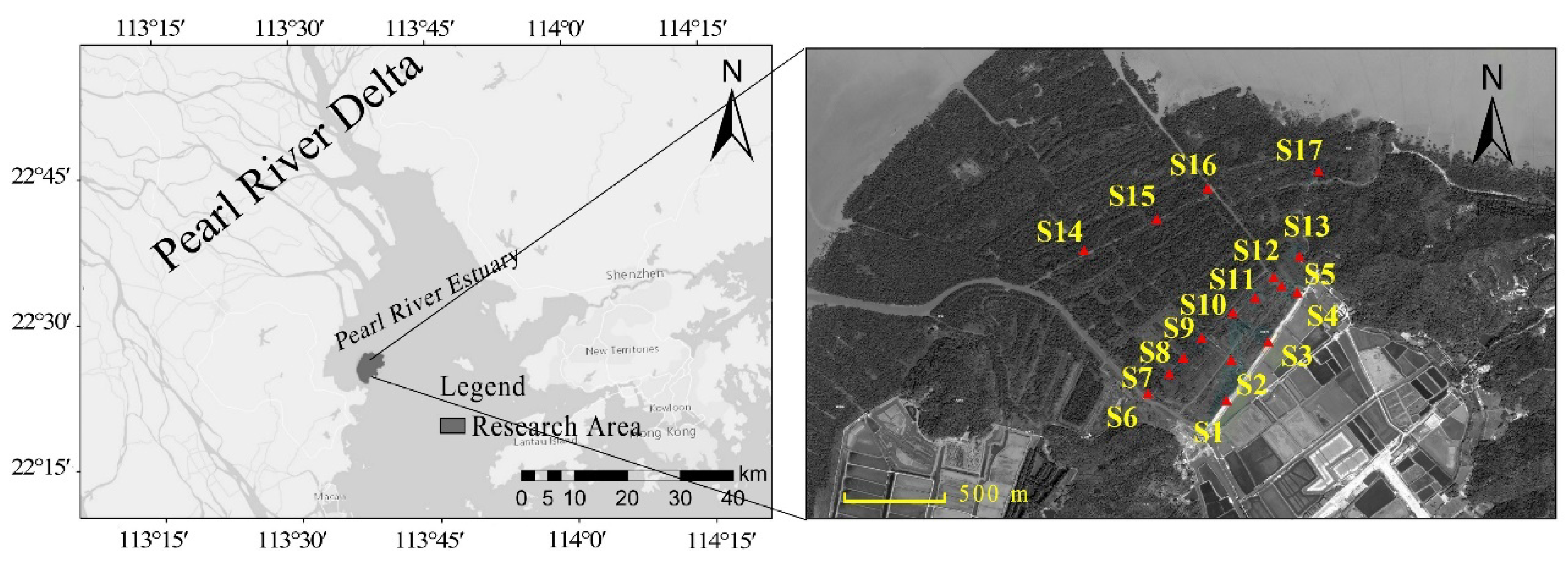
2.1. Study Area
2.2. Soil Sampling Method
2.3. Laboratory Methods
2.4. Evaluation of Soil Environmental Quality
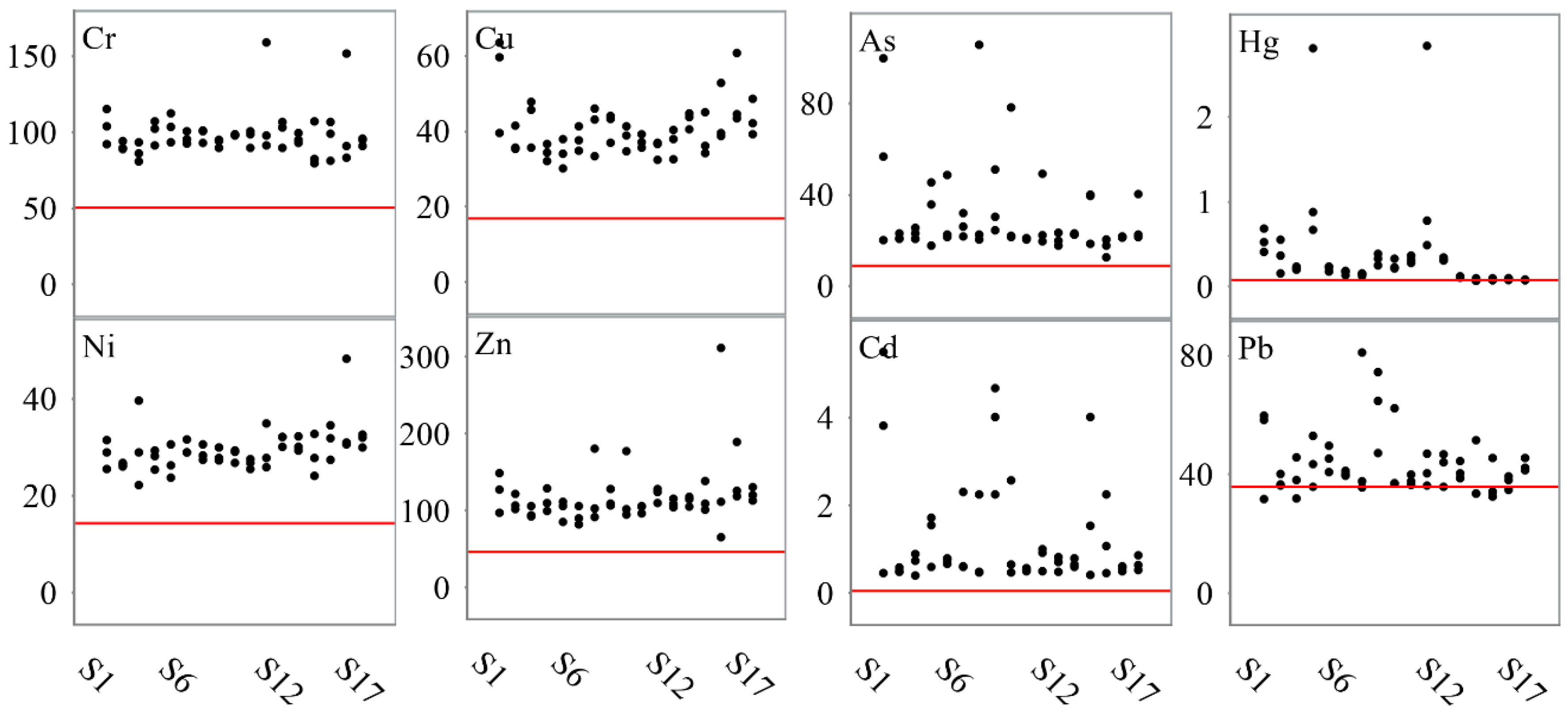
3. Results
3.1. Upper Intertidal Zone
3.2. Mid Intertidal Zone
3.3. Lower Intertidal Zone
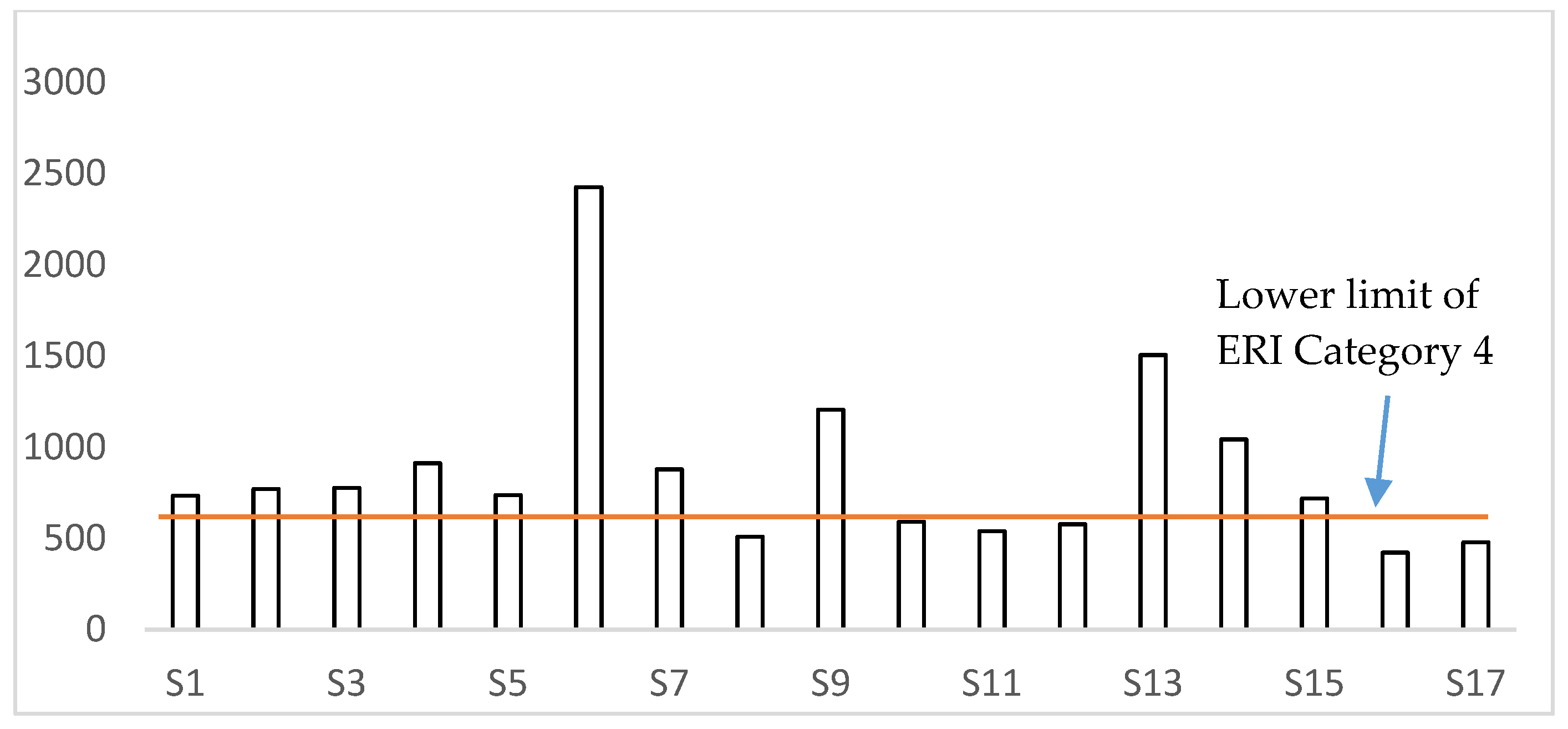
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bayen, S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 2012, 48, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ramanathan, A.L.; Prasad, M.B.K.; Datta, D.; Kumar, M.; Sappal, S.M. Distribution, enrichment, and potential toxicity of trace metals in the surface sediments of Sundarban mangrove ecosystem, Bangladesh: A baseline study before Sundarban oil spill of December, 2014. Environn. Sci. Pollut. R. 2016, 9, 8985–8999. [Google Scholar] [CrossRef] [PubMed]
- Machado, W.; Silva-Filho, E.V.; Oliveira, R.R.; Lacerda, L.D. Trace metal retention in mangrove ecosystems in Guanabara Bay, SE Brazil. Mar. Pollut. Bull. 2002, 11, 1277–1280. [Google Scholar] [CrossRef]
- Soto-Jimenez, M.F.; Paez-Osuna, F. Distribution and normalization of heavy metal concentrations in mangrove and lagoonal sediments from Mazatlan Harbor (SE Gulf of California). Estuar. Coast. Shelf Sci. 2001, 3, 259–274. [Google Scholar] [CrossRef]
- Ahmed, A.; Ohlson, M.; Hoque, S.; Moula, M.G. Chemical composition of leaves of a mangrove tree (Sonneratia apetala buch.-ham.) And their correlation with some soil variables. Bangladesh J. Bot. 2010, 1, 61–69. [Google Scholar] [CrossRef]
- Naidoo, G.; Hiralal, T.; Naidoo, Y. Ecophysiological responses of the mangrove Avicennia marina to trace metal contamination. Flora 2014, 1, 63–72. [Google Scholar] [CrossRef]
- He, B.; Li, R.; Chai, M.; Qiu, G. Threat of heavy metal contamination in eight mangrove plants from the Futian mangrove forest, China. Environ. Geochem. Health 2014, 3, 467–476. [Google Scholar] [CrossRef]
- Liu, Y.; Tam, N.F.Y.; Yang, J.X.; Pi, N.; Wong, M.H.; Ye, Z.H. Mixed heavy metals tolerance and radial oxygen loss in mangrove seedlings. Mar. Pollut. Bull. 2009, 12, 1843–1849. [Google Scholar] [CrossRef]
- Hu, Z. Studies on the Discharging and Distribution of Heavy Metal Pollution in the Pearl River Delta. Ph.D. Thesis, Graduate School of the Chinese Academy of Sciences Guangzhou Institute of Geochemistry, Guangzhou, China, 2004. [Google Scholar]
- Ministry of Ecology and Environment of People’s Republic of China. Bulletin of the State of the Environment of China; Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2011.
- Tam, N.F.Y.; Wong, Y. Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ. Pollut. 2000, 2, 195–205. [Google Scholar] [CrossRef]
- Liang, Y.; Wong, M. Spatial and temporal organic and heavy metal pollution at Mai Po Marshes Nature Reserve, Hong Kong. Chemosphere 2003, 52, 1647–1658. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources-WBR, 2nd ed.; Food and Agriculture Organization of the United Nations: Roma, Italy, 2006. [Google Scholar]
- Wei, G.; Baowen, L.; Fengying, Q.; Xinghua, G.; Jing, H.; Guanghong, T.; Xiongbang, Y. A Preliminary study on controlling the invasive species spartina alterniflora using Sonneratia apetala. Forest Res. 2009, 4, 603–607. [Google Scholar] [CrossRef]
- Guanghong, T.; Mingyan, D.; Xiongbang, Y.; Yanjun, S.; Wenbo, L.; Zhen, L.; Jiajun, G. Characteristics of sarcosperma laurinum community and species diversity from Qi-ao island in Zhuhai city, Guangdong province. Plant Sci. J. 2013, 5, 461–466. [Google Scholar] [CrossRef]
- Yijie, T.; Zhanqiang, F.; Yangting, Z.; Zaiwang, Z.; Kang, C.; Dong, A.; Xiongbang, Y.; Baowen, L. Succession of macrofauna communities in wetlands of Sonneratia apetala artificial mangroves during different ecological restoration stages. Acta Ecol. Sin. 2012, 10, 3160–3169. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, W.; Long, Y.; Bao, X.; Yang, Q. Assessment of heavy metals contamination in urban topsoil from Changchun City, China. J. Geochem. Explor. 2011, 108, 27–38. [Google Scholar] [CrossRef]
- Hou, X.; Huang, J.; Liu, A. Heavy metals pollution and it’s assessment in the wetlands of min river estuary in Fujian province. J. Agro-Environ. Sci. 2009, 11, 2302–2306. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control, a sediment-ecological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Guo, W.; Liu, X.; Liu, Z.; Li, G. Pollution and potential ecological risk evaluation of heavy metals in the sediments around dongjiang harbor, Tianjin. Procedia Environ. Sci. 2010, 2, 729–736. [Google Scholar] [CrossRef]
- Xu, Q.; Ni, S.; Tuo, X.; Zhang, C. Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ. Sci. Technol. 2008, 2, 112–115. [Google Scholar] [CrossRef]
- Ruili, L.; Minwei, C.; Guoyu, Q.; Bei, H. Mercury and copper accumulation during last fifty years and their potential ecological risk assessment in sediment of mangrove wetland of shenzhen, China. Chin. J. Environ Sci. 2012, 12, 4276–4283. [Google Scholar]
- Avudainayagam, S.; Megharaj, M.; Owens, G.; Kookana, R.S.; Chittleborough, D.; Naidu, R. Chemistry of chromium in soils with emphasis on tannery waste sites. Rev. Environ. Contam. Toxicol. 2003, 178, 53–91. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, H.; Sun, Z.; Shi, X. Wu, K.N. Principle component analyses based on soil knowledge as a tool to indicate origin of heavy metals in soils. Sci. Geogr. Sin. 2008, 1, 45–50. [Google Scholar] [CrossRef]
- Sun, H.; Bi, T.; Guo, Y.; Yuan, Y.; Chai, M.; Guo, Z. Source apportionment analysis of trace metal contamination in soils of Guangdong province, China. Acta Sci. Circumstantiae 2018, 2, 704–714. [Google Scholar]
- Zhang, H.; Chen, J.; Zhu, L.; Yang, G.; Li, D. Anthropogenic mercury enrichment factors and contributions in soils of guangdong province, South China. J. Geochem. Explor. 2014, 144, 312–319. [Google Scholar] [CrossRef]
- David, G.; Jiming, H.; Ye, W.; Jingkun, J.; Melissa, C.; Hezhong, T.; Xinbin, F. Anthropogenic mercury emissions in China. Atmos. Environ. 2005, 40, 7789–7806. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhou, J.; Liu, X. Contents and risk assessment of heavy metal sediments in Guangdong coastal areas. China Environ. Sci. 2018, 12, 4653–4660. [Google Scholar] [CrossRef]
- Newton, L. Pollution of the rivers of west wales by lead and zinc mine effluent. Ann. Appl. Biol. 2008, 1, 1–11. [Google Scholar] [CrossRef]
- Xueshu, Z.; Franco, P.; Dexian, Q.; Zhuguo, F.; Guanliang, L.; Hong, N. Baimazhai, Yunnan province, China: A hydrothermally modified magmatic nickel-copper-pge sulfide deposit. Int. Geol. Rev. 2006, 8, 725–741. [Google Scholar] [CrossRef]
- Hutchinson, T.C.; Whitby, L.M. Heavy-metal pollution in the sudbury mining and smelting region of canada, I. soil and vegetation contamination by nickel, copper, and other metals. Environ. Conserv. 1974, 2, 123–132. [Google Scholar] [CrossRef]
- Hashem, M.A.; Nur-A-Tomal, M.S.; Mondal, N.R.; Rahman, M.A. Hair burning and liming in tanneries is a source of pollution by arsenic, lead, zinc, manganese and iron. Environ. Chem. Lett. 2017, 3, 501–506. [Google Scholar] [CrossRef]
- Urszula, A.K.; Dariusz, C. Groundwater hydrochemistry and soil pollution in a catchment affected by an abandoned lead–zinc mine: Functioning of a diffuse pollution source. Environ. Earth Sci. 2012, 4, 1179–1189. [Google Scholar] [CrossRef]
- Jeon, S.-K.; Kwon, M.J.; Yang, J.-S.; Lee, S. Identifying the source of Zn in soils around a Zn smelter using pb isotope ratios and mineralogical analysis. Sci. Total Environ. 2017, 601, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Liuqiang, L.; Zhenhua, D.; Jinling, L.; Huina, L.; Hao, W. Distribution of heavy metals in surficial sediments from main mangrove wetlands of China and their influence factors. Acta Oceanol. Sin. 2008, 5, 159–164. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, Z.; Wu, D. Distribution and bioavailability of seven heavy metals in mangrove wetland sediments in Dongzhai Harbor, Hainan Island, China. Chin. J. Appl. Ecol. 2016, 2, 593–600. [Google Scholar]
- Wei, J.; Wang, Z.; Pan, L. Heavy metal contents and ecological risk assessment of surface sediments in Shankou mangrove wetland of Guangxi. J. Subtrop. Resour. Environ. 2019, 2, 28–33. [Google Scholar] [CrossRef]
- Cai, M.; Wang, Y.; Qiu, C. Heavy metals in surface sediments from mangrove zone in Zhangjiang River estuary, South China. In Proceedings of the 2009 International Conference on Environmental Science and Information Application Technology, Wuhan, China, 4–5 July 2009; Volume 3, pp. 34–38. [Google Scholar]
- Chowdhury, R.; Favas, P.J.C.; Jonathan, M.P.; Venkatachalam, P.; Raja, P.; Sarkar, S.K. Bioremoval of trace metals from rhizosediment by mangrove plants in Indian Sundarban Wetland. Mar. Pollut. Bull. 2017, 124, 1078–1088. [Google Scholar] [CrossRef]
- Kehrig, H.A.; Pinto, F.N.; Moreira, I.; Malm, O. Heavy metals and methylmercury in a tropical coastal estuary and a mangrove in Brazil. Org. Geochem. 2003, 34, 661–669. [Google Scholar] [CrossRef]
- Harikumar, P.S.; Jisha, T.S. Distribution pattern of trace metal pollutants in the sediments of an urban wetland in the southwest coast of India. Int. J. Eng. Sci. Technol. 2010, 2, 840–850. [Google Scholar]
- Baskar, B.; Biraja, K.S.; Jayappriyan, K.R.; Arockia, V.L.; Marimuthu, N.; Nandakumar, P.; Prabakaran, P. Assessment of heavy metal concentrations and associated resistant bacterial communities in bulk and rhizosphere soil of Avicennia marina of Pichavaram mangrove, India. Environ. Earth Sci. 2017, 1, 58. [Google Scholar] [CrossRef]
- Mackey, A.P.; Hodgkinson, M.C. Concentrations and spatial distribution of trace metals in mangrove sediments from the Brisbane River. Aust. Environ. Pollut. 1995, 2, 181–186. [Google Scholar] [CrossRef]
| Pollution Category | INPI | Degree of Pollution |
|---|---|---|
| 1 | <0.7 | No pollution |
| 2 | 0.7–1 | Alert |
| 3 | 1–2 | Slight pollution |
| 4 | 2–3 | Medium pollution |
| 5 | >3 | Heavy pollution |
| Category | ERI | Ecological Risk Level |
|---|---|---|
| 1 | <150 | low |
| 2 | 150–300 | medium |
| 3 | 300–600 | high |
| 4 | >600 | very high |
| Location | Depth | Cr | Ni | Cu | Zn | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 0–10 cm | 115.59 ± 17.82 | 31.57 ± 4.23 | 63.62 ± 5.37 | 94.63 ± 7.63 | 56.78 ± 33.11 | 5.51 ± 2.90 | 0.53 ± 0.03 | 59.90 ± 19.54 |
| 10–20 cm | 104.03 ± 0.07 | 28.97 ± 1.66 | 59.67 ± 3.62 | 107.18 ± 33.22 | 100.00 ± 49.75 | 3.83 ± 1.89 | 0.69 ± 0.27 | 58.51 ± 15.08 | |
| 20–30 cm | 92.52 ± 4.81 | 25.53 ± 1.87 | 39.67 ± 3.05 | 97.84 ± 6.05 | 20.37 ± 2.38 | 0.46 ± 0.04 | 0.41 ± 0.12 | 31.90 ± 4.50 | |
| S2 | 0–10 cm | 89.19 ± 2.88 | 26.50 ± 0.31 | 35.82 ± 1.99 | 122.37 ± 13.55 | 20.92 ± 0.67 | 0.49 ± 0.02 | 0.16 ± 0.01 | 36.32 ± 0.40 |
| 10–20 cm | 90.08 ± 2.48 | 26.09 ± 0.77 | 35.44 ± 1.99 | 102.21 ± 4.76 | 21.12 ± 0.38 | 0.51 ± 0.01 | 0.56 ± 0.45 | 36.69 ± 1.40 | |
| 20–30 cm | 94.39 ± 2.02 | 26.88 ± 0.42 | 41.62 ± 3.00 | 107.18 ± 0.80 | 23.25 ± 1.22 | 0.59 ± 0.05 | 0.37 ± 0.22 | 40.21 ± 1.17 | |
| S3 | 0–10 cm | 80.93 ± 1.69 | 22.24 ± 0.00 | 35.78 ± 1.77 | 93.23 ± 11.35 | 20.90 ± 0.76 | 0.41 ± 0.01 | 0.22 ± 0.00 | 31.97 ± 0.55 |
| 10–20 cm | 86.15 ± 3.41 | 29.00 ± 1.72 | 45.88 ± 7.41 | 122.37 ± 2.62 | 25.73 ± 6.18 | 0.74 ± 0.14 | 0.24 ± 0.03 | 38.20 ± 5.06 | |
| 20–30 cm | 93.79 ± 2.39 | 39.70 ± 11.42 | 47.96 ± 4.63 | 128.05 ± 1.86 | 23.28 ± 0.20 | 0.90 ± 0.30 | 0.20 ± 0.04 | 45.85 ± 8.72 | |
| S4 | 0–10 cm | 91.40 ± 1.10 | 28.22 ± 3.60 | 32.18 ± 0.69 | 100.41 ± 10.08 | 17.78 ± 0.93 | 0.61 ± 0.04 | 2.81 ± 2.19 | 35.89 ± 2.14 |
| 10–20 cm | 102.47 ± 5.42 | 25.42 ± 1.37 | 34.43 ± 2.75 | 110.72 ± 20.42 | 35.76 ± 16.32 | 1.55 ± 0.99 | 0.88 ± 0.35 | 43.69 ± 8.82 | |
| 20–30 cm | 107.20 ± 8.44 | 29.43 ± 3.60 | 36.70 ± 2.57 | 129.14 ± 24.53 | 45.67 ± 24.33 | 1.72 ± 1.02 | 0.67 ± 0.16 | 53.03 ± 11.56 | |
| S5 | 0–10 cm | 93.60 ± 4.61 | 23.82 ± 1.07 | 30.21 ± 0.70 | 85.84 ± 2.54 | 48.82 ± 26.90 | 0.80 ± 0.18 | 0.18 ± 0.03 | 49.83 ± 3.71 |
| 10–20 cm | 112.71 ± 0.93 | 30.63 ± 1.41 | 38.09 ± 1.82 | 106.42 ± 4.06 | 22.79 ± 1.62 | 0.77 ± 0.05 | 0.23 ± 0.03 | 45.53 ± 4.23 | |
| 20–30 cm | 103.66 ± 3.13 | 26.31 ± 0.38 | 34.09 ± 1.27 | 111.96 ± 6.55 | 21.49 ± 0.09 | 0.68 ± 0.05 | 0.24 ± 0.03 | 40.82 ± 1.59 | |
| Average | 97.37 ± 1.90 | 28.02 ± 0.95 | 40.81 ± 1.58 | 108.60 ± 3.56 | 32.90 ± 5.01 | 1.30 ± 0.30 | 0.57 ± 0.17 | 43.34 ± 2.15 |
| Sample | Depth | Cr | Ni | Cu | Zn | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|
| S6 | 0–10 cm | 95.45 ± 2.52 | 31.66 ± 0.32 | 41.39 ± 7.39 | 90.20 ± 1.60 | 26.06 ± 0.52 | 0.62 ± 0.16 | 0.19 ± 0.04 | 39.62 ± 5.41 |
| 10–20 cm | 100.84 ± 1.57 | 29.00 ± 0.88 | 37.78 ± 1.93 | 106.08 ± 4.56 | 32.15 ± 9.64 | 0.60 ± 0.04 | 0.18 ± 0.02 | 40.38 ± 1.83 | |
| 20–30 cm | 92.82 ± 2.31 | 29.02 ± 1.49 | 34.91 ± 0.91 | 82.33 ± 3.44 | 21.85 ± 0.58 | 2.32 ± 1.89 | 0.14 ± 0.01 | 41.27 ± 10.87 | |
| S7 | 0–10 cm | 93.31 ± 1.79 | 27.53 ± 0.05 | 33.48 ± 0.99 | 103.08 ± 2.81 | 20.40 ± 0.96 | 0.49 ± 0.01 | 0.15 ± 0.01 | 37.84 ± 0.38 |
| 10–20 cm | 101.17 ± 4.47 | 30.61 ± 0.43 | 46.08 ± 6.83 | 181.25 ± 72.36 | 106.00 ± 83.20 | 2.26 ± 1.63 | 0.16 ± 0.04 | 81.20 ± 41.36 | |
| 20–30 cm | 100.95 ± 1.15 | 28.31 ± 0.53 | 43.14 ± 6.83 | 92.49 ± 4.69 | 22.68 ± 0.85 | 0.48 ± 0.02 | 0.13 ± 0.03 | 35.67 ± 0.62 | |
| S8 | 0–10 cm | 90.07 ± 2.68 | 29.98 ± 1.50 | 44.21 ± 7.37 | 109.70 ± 9.83 | 30.36 ± 8.56 | 4.03 ± 3.46 | 0.39 ± 0.06 | 64.78 ± 24.19 |
| 10–20 cm | 94.22 ± 4.34 | 27.89 ± 1.68 | 43.36 ± 5.46 | 128.33 ± 7.07 | 51.37 ± 27.64 | 4.68 ± 3.38 | 0.33 ± 0.03 | 74.49 ± 29.23 | |
| 20–30 cm | 95.21 ± 1.74 | 27.33 ± 0.35 | 37.14 ± 1.69 | 107.32 ± 8.56 | 24.66 ± 3.04 | 2.26 ± 1.61 | 0.25 ± 0.06 | 47.33 ± 12.18 | |
| S9 | 0–10 cm | 97.92 ± 1.89 | 26.90 ± 0.33 | 34.77 ± 0.47 | 95.15 ± 0.60 | 21.47 ± 0.16 | 0.48 ± 0.02 | 0.33 ± 0.08 | 37.19 ± 1.16 |
| 10–20 cm | 98.50 ± 4.37 | 29.08 ± 0.78 | 41.45 ± 2.14 | 177.89 ± 32.77 | 78.47 ± 31.50 | 2.58 ± 1.06 | 0.23 ± 0.04 | 62.33 ± 12.11 | |
| 20–30 cm | 98.95 ± 5.53 | 29.41 ± 1.66 | 38.98 ± 2.98 | 102.79 ± 5.64 | 22.19 ± 0.85 | 0.66 ± 0.03 | 0.22 ± 0.04 | 37.00 ± 0.49 | |
| S10 | 0–10 cm | 90.08 ± 0.76 | 25.59 ± 0.88 | 37.30 ± 2.07 | 105.57 ± 9.01 | 21.03 ± 1.42 | 0.50 ± 0.00 | 0.37 ± 0.09 | 40.07 ± 4.72 |
| 10–20 cm | 100.86 ± 5.97 | 26.89 ± 1.13 | 35.79 ± 2.08 | 106.34 ± 10.38 | 20.52 ± 0.90 | 0.53 ± 0.10 | 0.32 ± 0.04 | 36.49 ± 2.20 | |
| 20–30 cm | 98.85 ± 3.83 | 27.64 ± 0.56 | 39.26 ± 2.08 | 97.24 ± 2.00 | 21.03 ± 1.75 | 0.58 ± 0.04 | 0.28 ± 0.02 | 37.86 ± 1.33 | |
| S11 | 0–10 cm | 91.48 ± 6.88 | 25.98 ± 0.29 | 32.51 ± 0.89 | 128.32 ± 32.57 | 19.82 ± 1.06 | 0.50 ± 0.05 | 2.84 ± 2.42 | 36.42 ± 2.21 |
| 10–20 cm | 98.17 ± 4.36 | 27.82 ± 1.24 | 36.72 ± 2.38 | 124.35 ± 27.78 | 49.26 ± 28.19 | 1.01 ± 0.37 | 0.78 ± 0.42 | 47.11 ± 10.30 | |
| 20–30 cm | 159.26 ± 56.12 | 34.98 ± 8.21 | 37.11 ± 2.29 | 110.31 ± 12.89 | 22.35 ± 0.87 | 0.92 ± 0.16 | 0.49 ± 0.19 | 40.52 ± 0.47 | |
| S12 | 0–10 cm | 90.15 ± 9.27 | 30.10 ± 5.03 | 32.75 ± 1.79 | 104.86 ± 2.56 | 17.86 ± 1.80 | 0.49 ± 0.03 | 0.31 ± 0.04 | 36.01 ± 3.13 |
| 10–20 cm | 107.01 ± 2.19 | 32.14 ± 2.67 | 37.97 ± 1.16 | 108.61 ± 5.50 | 19.92 ± 0.44 | 0.71 ± 0.06 | 0.35 ± 0.04 | 44.19 ± 0.12 | |
| 20–30 cm | 103.50 ± 3.03 | 32.20 ± 0.53 | 40.53 ± 0.90 | 115.91 ± 3.32 | 23.56 ± 0.64 | 0.83 ± 0.01 | 0.32 ± 0.03 | 46.89 ± 0.93 | |
| S13 | 0–10 cm | 95.03 ± 6.05 | 30.16 ± 2.46 | 44.76 ± 3.44 | 118.77 ± 6.86 | 23.11 ± 1.50 | 0.60 ± 0.03 | 0.12 ± 0.02 | 40.40 ± 1.88 |
| 10–20 cm | 99.64 ± 6.54 | 32.35 ± 0.37 | 43.79 ± 1.12 | 115.31 ± 14.37 | 22.60 ± 0.39 | 0.80 ± 0.08 | 0.12 ± 0.02 | 44.69 ± 0.87 | |
| 20–30 cm | 93.22 ± 5.56 | 29.44 ± 2.14 | 40.58 ± 0.71 | 105.90 ± 1.81 | 22.60 ± 0.77 | 0.66 ± 0.02 | 0.11 ± 0.01 | 38.80 ± 1.92 | |
| Average | 99.44 ± 2.59 | 29.25 ± 0.47 | 38.99 ± 0.75 | 113.96 ± 4.55 | 31.02 ± 4.44 | 1.23 ± 0.24 | 0.38 ± 0.11 | 45.36 ± 2.55 |
| Sample | Depth | Cr | Ni | Cu | Zn | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|
| S14 | 0–10 cm | 79.60 ± 0.74 | 24.20 ± 0.56 | 34.20 ± 4.12 | 109.30 ± 9.33 | 39.79 ± 22.99 | 4.02 ± 3.70 | 0.07 ± 0.00 | 33.68 ± 3.22 |
| 10–20 cm | 82.70 ± 7.25 | 27.90 ± 0.37 | 36.20 ± 3.35 | 101.99 ± 7.16 | 18.51 ± 0.97 | 0.42 ± 0.03 | 0.07 ± 0.01 | 33.69 ± 2.40 | |
| 20–30 cm | 107.00 ± 18.10 | 32.80 ± 3.68 | 45.20 ± 6.78 | 138.67 ± 21.62 | 40.15 ± 15.17 | 1.54 ± 0.88 | 0.10 ± 0.02 | 51.64 ± 7.93 | |
| S15 | 0–10 cm | 81.60 ± 6.28 | 27.40 ± 0.23 | 38.90 ± 0.93 | 65.74 ± 47.90 | 12.66 ± 7.04 | 2.26 ± 1.90 | 0.08 ± 0.00 | 34.33 ± 3.96 |
| 10–20 cm | 106.00 ± 24.5 | 34.60 ± 6.24 | 39.60 ± 2.98 | 112.05 ± 4.54 | 20.55 ± 1.71 | 0.47 ± 0.05 | 0.08 ± 0.01 | 32.75 ± 2.77 | |
| 20–30 cm | 99.30 ± 9.77 | 31.90 ± 1.81 | 53.00 ± 9.39 | 312.17 ± 173.62 | 17.74 ± 5.84 | 1.08 ± 0.43 | 0.10 ± 0.02 | 45.59 ± 4.72 | |
| S16 | 0–10 cm | 83.50 ± 13.20 | 30.60 ± 0.09 | 43.50 ± 2.27 | 119.37 ± 7.94 | 21.71 ± 0.90 | 0.51 ± 0.10 | 0.10 ± 0.00 | 34.98 ± 4.71 |
| 10–20 cm | 151.00 ± 68.10 | 48.30 ± 17.20 | 44.70 ± 5.16 | 126.34 ± 8.33 | 21.39 ± 0.72 | 0.59 ± 0.04 | 0.08 ± 0.01 | 38.28 ± 2.74 | |
| 20–30 cm | 91.30 ± 1.68 | 30.90 ± 0.74 | 60.80 ± 12.00 | 190.22 ± 44.09 | 21.79 ± 1.81 | 0.62 ± 0.04 | 0.10 ± 0.02 | 39.54 ± 1.65 | |
| S17 | 0–10 cm | 91.30 ± 2.97 | 32.00 ± 1.31 | 39.20 ± 2.07 | 120.63 ± 15.90 | 40.38 ± 19.91 | 0.87 ± 0.36 | 0.09 ± 0.03 | 45.68 ± 9.21 |
| 10–20 cm | 95.30 ± 3.03 | 30.00 ± 0.42 | 42.30 ± 1.64 | 113.64 ± 5.59 | 21.69 ± 0.85 | 0.54 ± 0.04 | 0.08 ± 0.02 | 41.54 ± 1.49 | |
| 20–30 cm | 95.80 ± 5.41 | 32.70 ± 3.11 | 48.70 ± 1.76 | 131.08 ± 2.42 | 22.61 ± 1.31 | 0.65 ± 0.06 | 0.08 ± 0.02 | 42.34 ± 3.19 | |
| Average | 101.55 ± 2.74 | 30.37 ± 0.75 | 40.76 ± 1.08 | 124.84 ± 8.93 | 26.26 ± 2.16 | 0.94 ± 0.13 | 0.28 ± 0.08 | 43.35 ± 1.40 |
| Cr | Ni | Cu | Zn | As | Cd | Hg | Pb | |
|---|---|---|---|---|---|---|---|---|
| Cr | 1 | |||||||
| Ni | 0.129 | 1 | ||||||
| Cu | −0.163 | 0.549 ** | 1 | |||||
| Zn | −0.317 | 0.007 | 0.459 * | 1 | ||||
| As | −0.382 | −0.049 | 0.389 | 0.965 ** | 1 | |||
| Cd | −0.082 | −0.163 | 0.396 | 0.550 ** | 0.495 * | 1 | ||
| Hg | 0.316 | −0.394 | −0.079 | 0.017 | 0.010 | 0.270 | 1 | |
| Pb | 0.171 | −0.267 | −0.089 | 0.450 * | 0.384 | 0.360 | −0.054 | 1 |
| Sampling Location | INPI | Degree of Pollution |
|---|---|---|
| S1 | 12.74 | Heavy pollution |
| S2 | 11.41 | Heavy pollution |
| S3 | 14.52 | Heavy pollution |
| S4 | 16.38 | Heavy pollution |
| S5 | 14.29 | Heavy pollution |
| S6 | 48.42 | Heavy pollution |
| S7 | 16.45 | Heavy pollution |
| S8 | 7.53 | Heavy pollution |
| S9 | 11.72 | Heavy pollution |
| S10 | 9.31 | Heavy pollution |
| S11 | 9.56 | Heavy pollution |
| S12 | 9.90 | Heavy pollution |
| S13 | 17.69 | Heavy pollution |
| S17 | 22.70 | Heavy pollution |
| S18 | 15.18 | Heavy pollution |
| S19 | 8.02 | Heavy pollution |
| S20 | 9.35 | Heavy pollution |
| Study Area | Cr | Ni | Cu | Zn | As | Cd | Hg | Pb |
|---|---|---|---|---|---|---|---|---|
| Sanya, China [35] | 61.5 | - | 21.0 | 126 | 14.3 | 0.09 | - | 42.3 |
| Luoyang Bridge, China [35] | 18.6 | - | 34.0 | 106 | 5.31 | 0.06 | - | 167 |
| Dongzhai Harbor, China [36] | 75.8 | 31.0 | 19.6 | 44.8 | 8.67 | 0.62 | - | 20.7 |
| Yingluo Harbor, China [37] | 56.7 | - | 7.76 | 31.9 | - | 0.11 | 0.04 | 14.2 |
| Shenzhen Bay, China [7] | 96.2 | 44.6 | 88.8 | 358 | 172 | 0.94 | 0.14 | 72 |
| Zhangjiang River Estuary, China [38] | - | - | 21.04 | - | - | 0.33 | 0.03 | 63.2 |
| Sunderban mangrove, India [39] | 41.8 | 47.4 | 60.06 | 88.3 | - | 0.48 | 0.24 | 52.9 |
| Guanabara Bay, Brazil [40] | 42.4 | - | 98.6 | 483 | 1.32 | 1.32 | - | 160 |
| Kottuli, India [41] | 0.26 | 0.38 | 69.3 | 384 | - | 0.03 | - | 6.91 |
| Hong Kong, China [11] | 40.0 | 3.00 | 240 | 40.0 | - | 3.00 | - | 80 |
| Pichavaram, India [42] | 530 | 253 | 150 | 108 | - | 34.5 | - | 133 |
| Brisbane River, Australia [43] | 7.6–116 | 2.4–57.6 | 3.1–34.1 | 40–144 | 0–13.0 | 0–2.0 | - | 7.7–84.7 |
| This Study | 99.5 | 29.2 | 40.8 | 116 | 30.0 | 1.16 | 0.41 | 44.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, A.; Ma, J.; Gao, Y.; Xu, S.; Lin, C. Mangrove Soil-Borne Trace Elements in Qi’ao Island: Implications for Understanding Terrestrial Input of Trace Elements into Part of the Pearl River Estuary. Appl. Sci. 2020, 10, 2439. https://doi.org/10.3390/app10072439
Niu A, Ma J, Gao Y, Xu S, Lin C. Mangrove Soil-Borne Trace Elements in Qi’ao Island: Implications for Understanding Terrestrial Input of Trace Elements into Part of the Pearl River Estuary. Applied Sciences. 2020; 10(7):2439. https://doi.org/10.3390/app10072439
Chicago/Turabian StyleNiu, Anyi, Jiaojiao Ma, Yifei Gao, Songjun Xu, and Chuxia Lin. 2020. "Mangrove Soil-Borne Trace Elements in Qi’ao Island: Implications for Understanding Terrestrial Input of Trace Elements into Part of the Pearl River Estuary" Applied Sciences 10, no. 7: 2439. https://doi.org/10.3390/app10072439
APA StyleNiu, A., Ma, J., Gao, Y., Xu, S., & Lin, C. (2020). Mangrove Soil-Borne Trace Elements in Qi’ao Island: Implications for Understanding Terrestrial Input of Trace Elements into Part of the Pearl River Estuary. Applied Sciences, 10(7), 2439. https://doi.org/10.3390/app10072439






