Comparison of Equilibrium-Stage and Rate-Based Models of a Packed Column for Tar Absorption Using Vegetable Oil
Abstract
:1. Introduction
1.1. Tar Treatments
1.2. Modelling Approaches for the Packed Column Used for Absorption Processes
2. Description of the Experiment
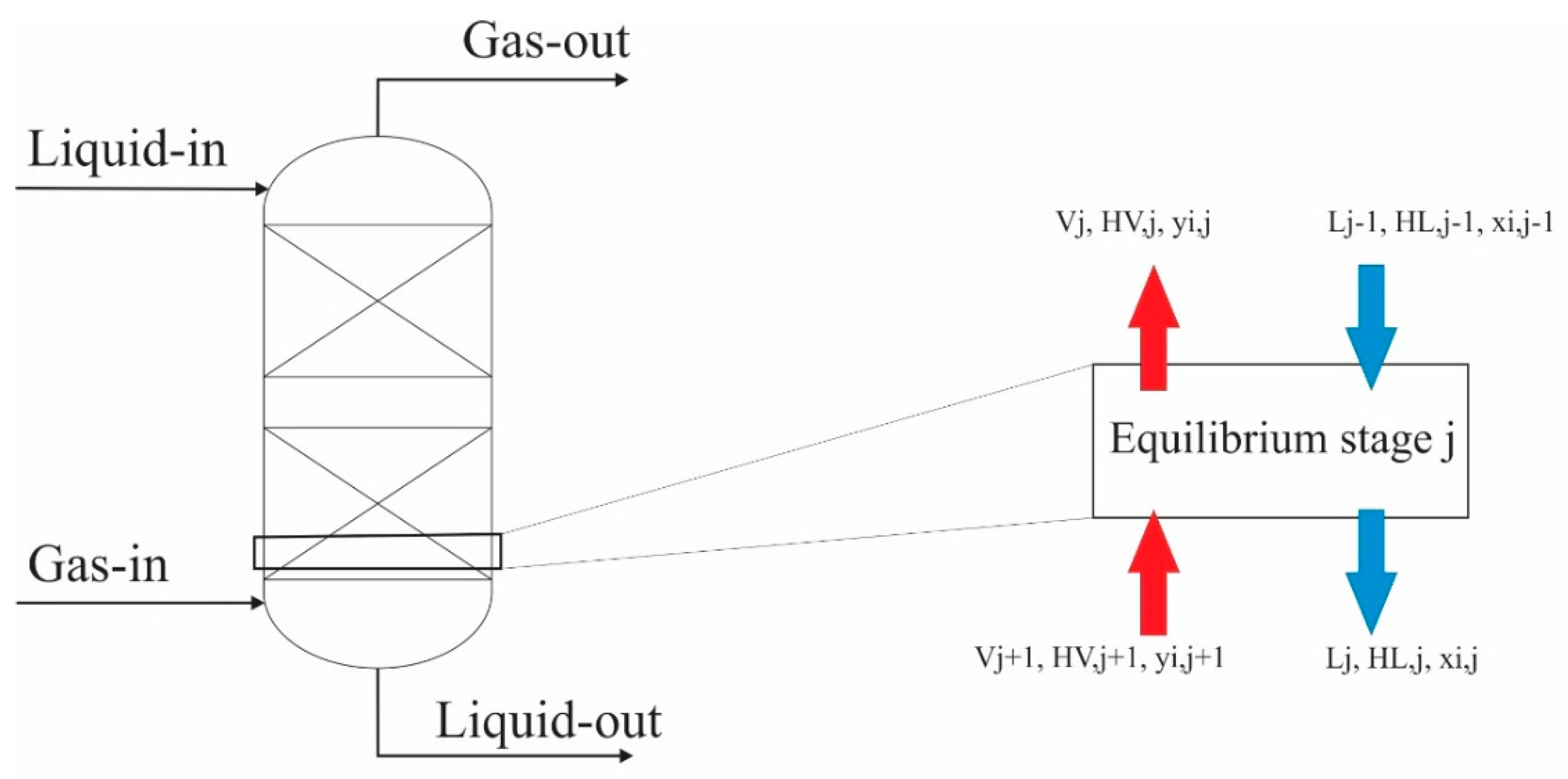
2.1. Mathematical Models of the Packed Column Used for Tar Absorption Processes
2.2. Equilibrium Model
- Phase equilibrium is presented at each stage;
- There are no chemical reactions between the components of vapor and liquid;
- Entrainment of liquid drops in vapor and occlusion of vapor bubbles in the liquid is negligible.
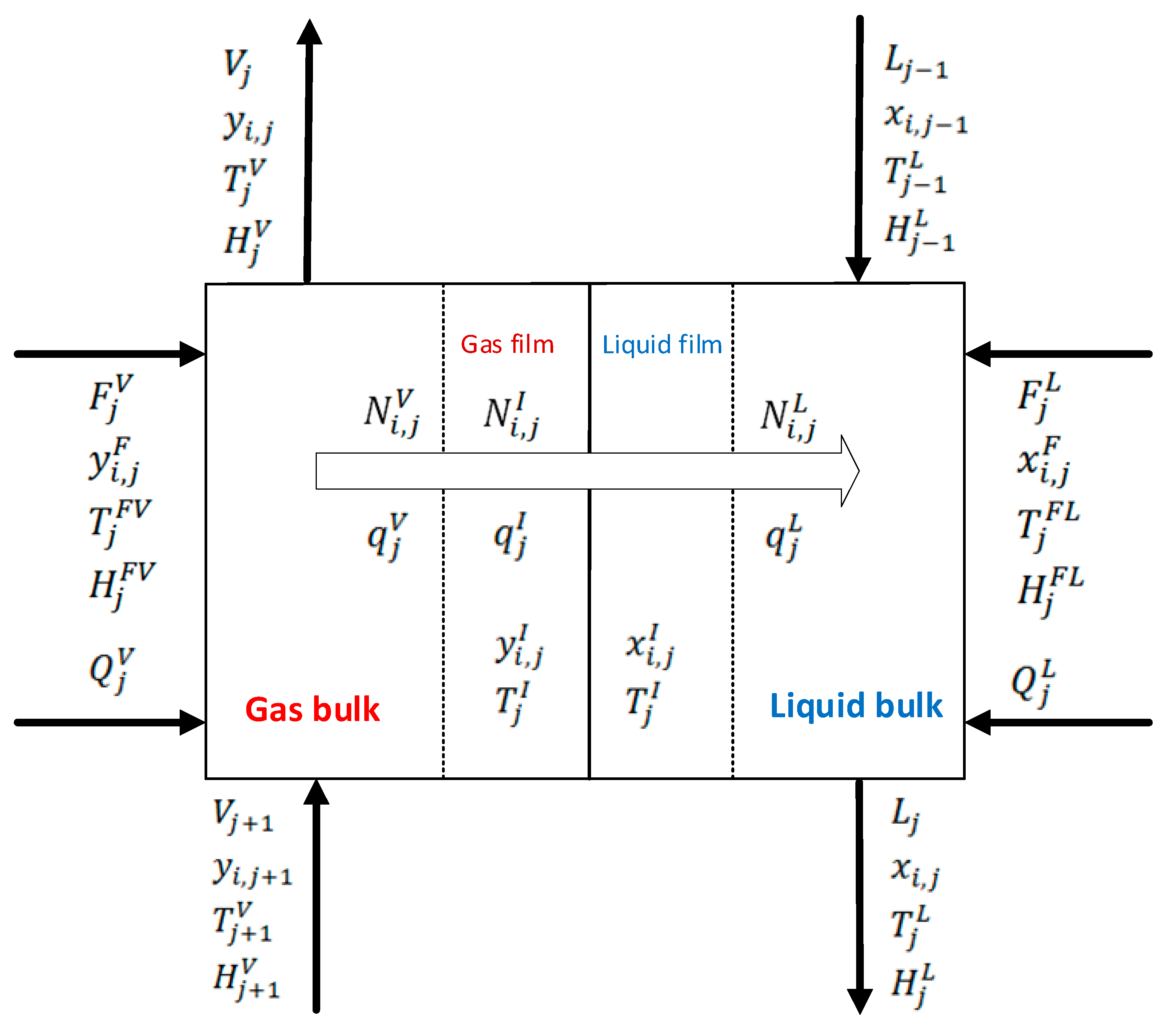
2.3. Rate-Based Model Description
2.4. Assumptions and Mathematical Model for Rate-Based Model
- The reaction is quick and occurs in the liquid film;
- The absorption column is supposed to be adiabatic;
- The interfacial surface area is identical for both heat and mass transfer;
- The liquid-side heat transfer resistance is small compared to the gas phase, and the interface temperature is, therefore, identical as the bulk temperature;
- The type of flow is plug flow, and concentration and temperature change in the radial direction is negligible;
- Both liquid and gas phases are formally discussed as ideal mixtures
2.5. Material and Energy Balances
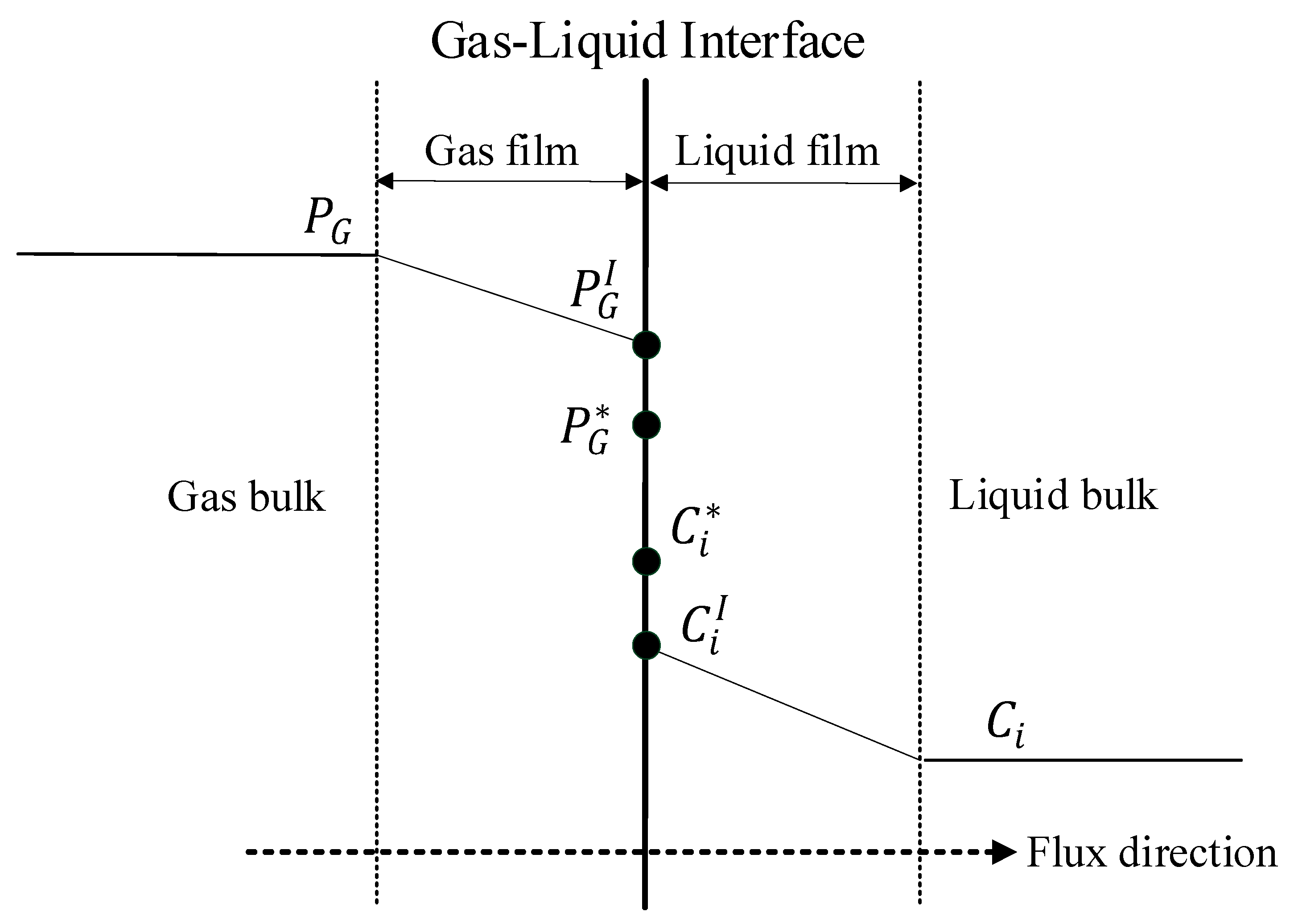
2.6. Mass Transfer through the Interfacial Area
2.7. Heat Transfer through the Interfacial Area
2.8. Thermodynamics Approaches for Prediction of Phase Behavior
2.9. Model Specification
3. Results and Discussion
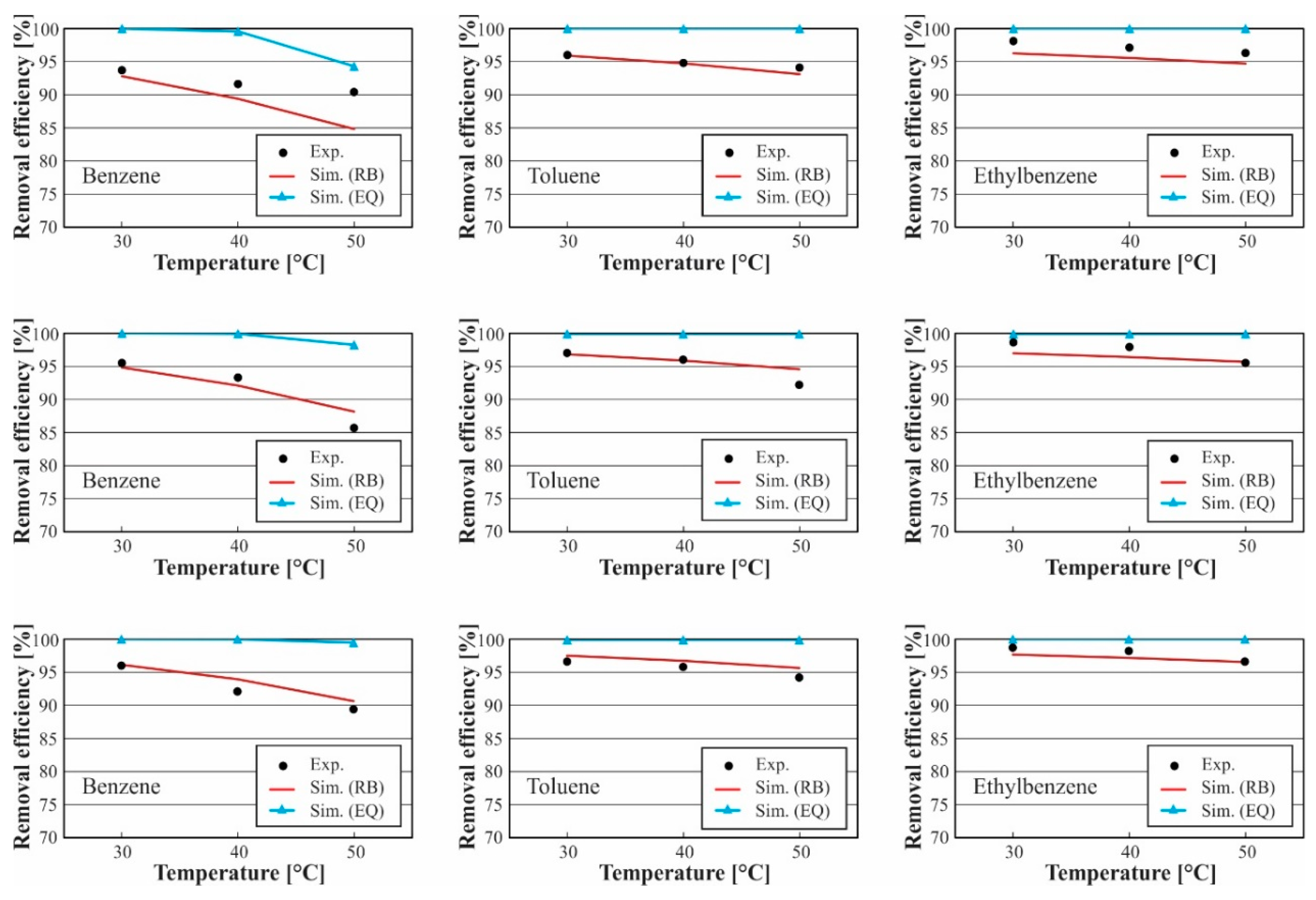
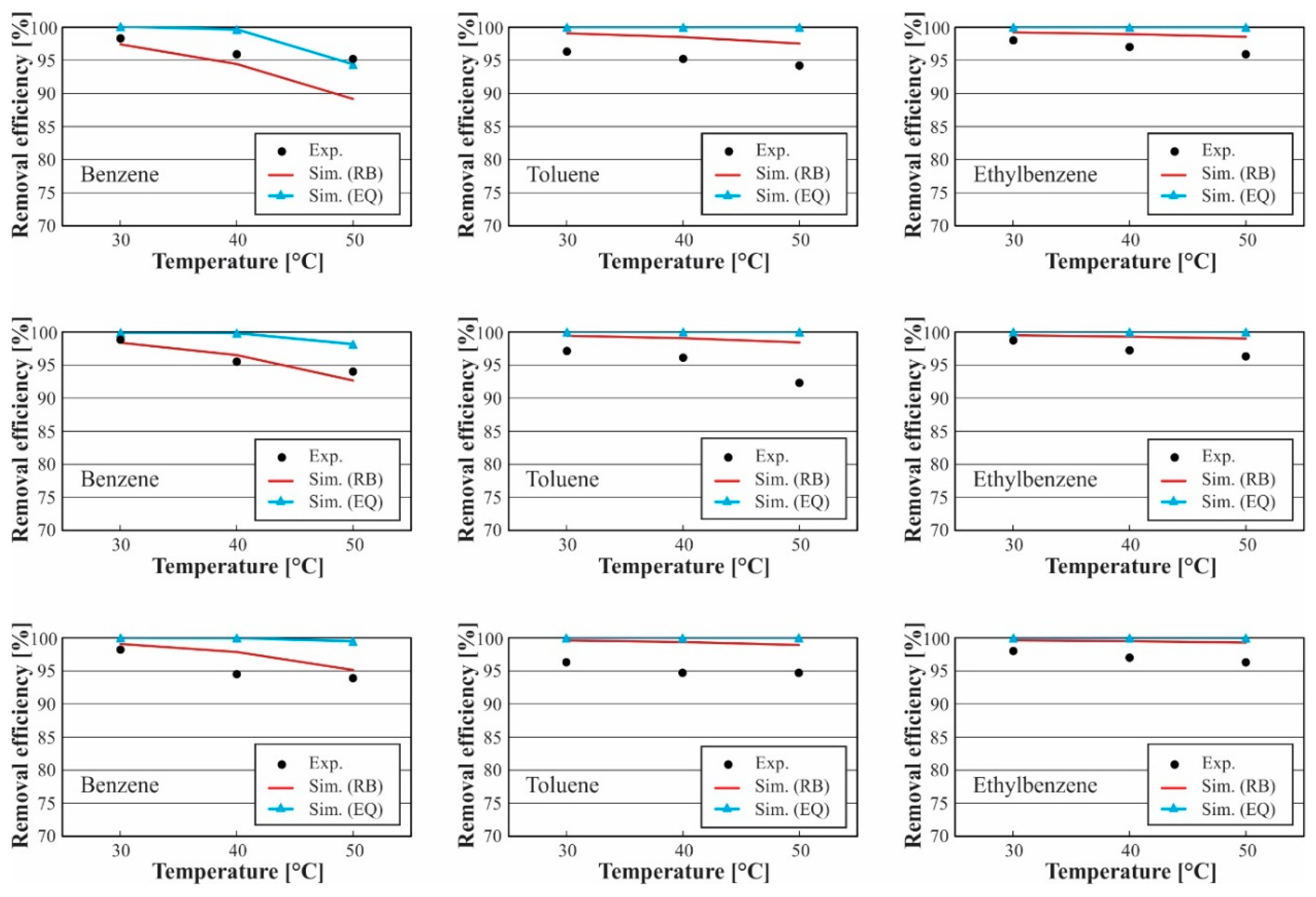
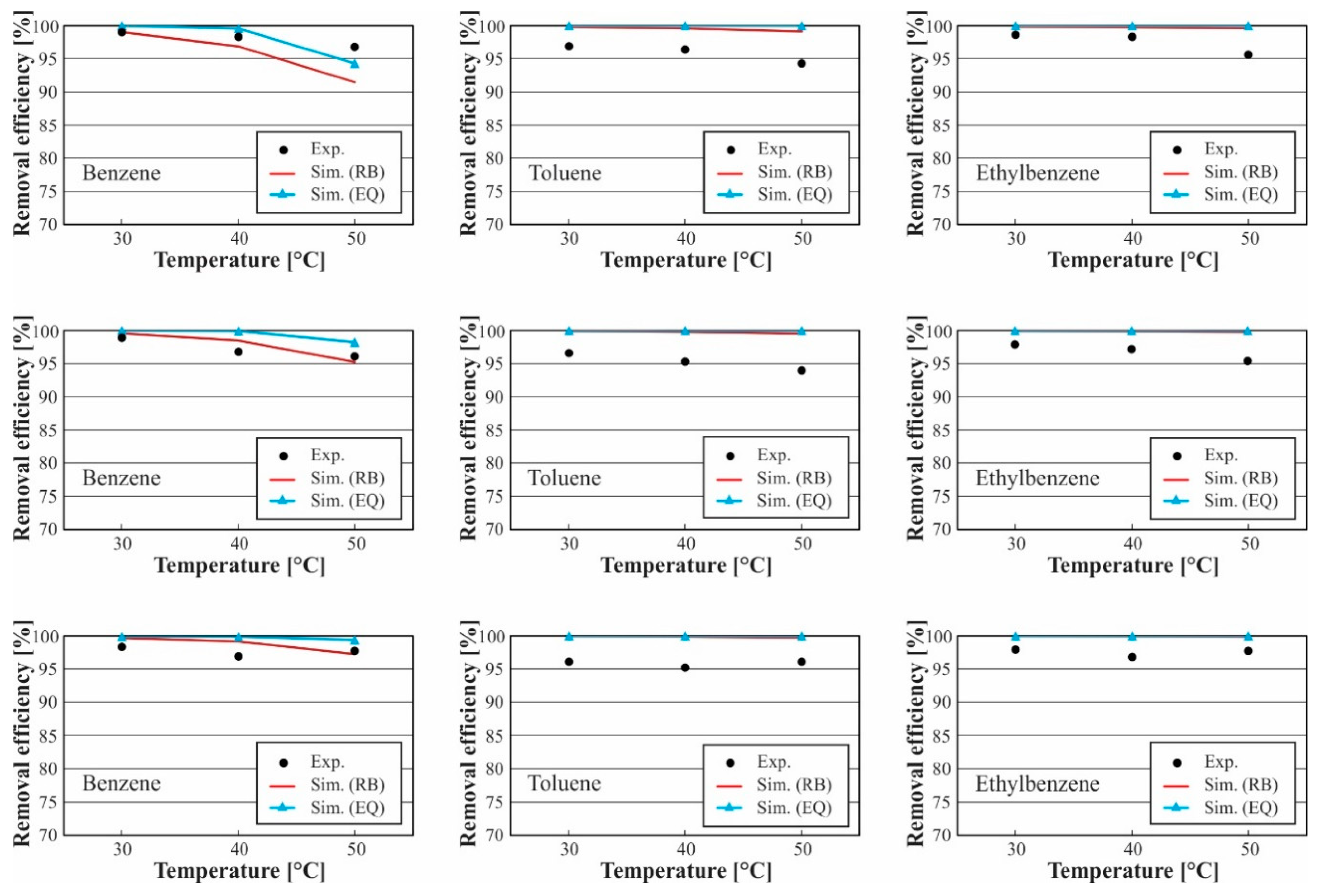
3.1. Model Validation
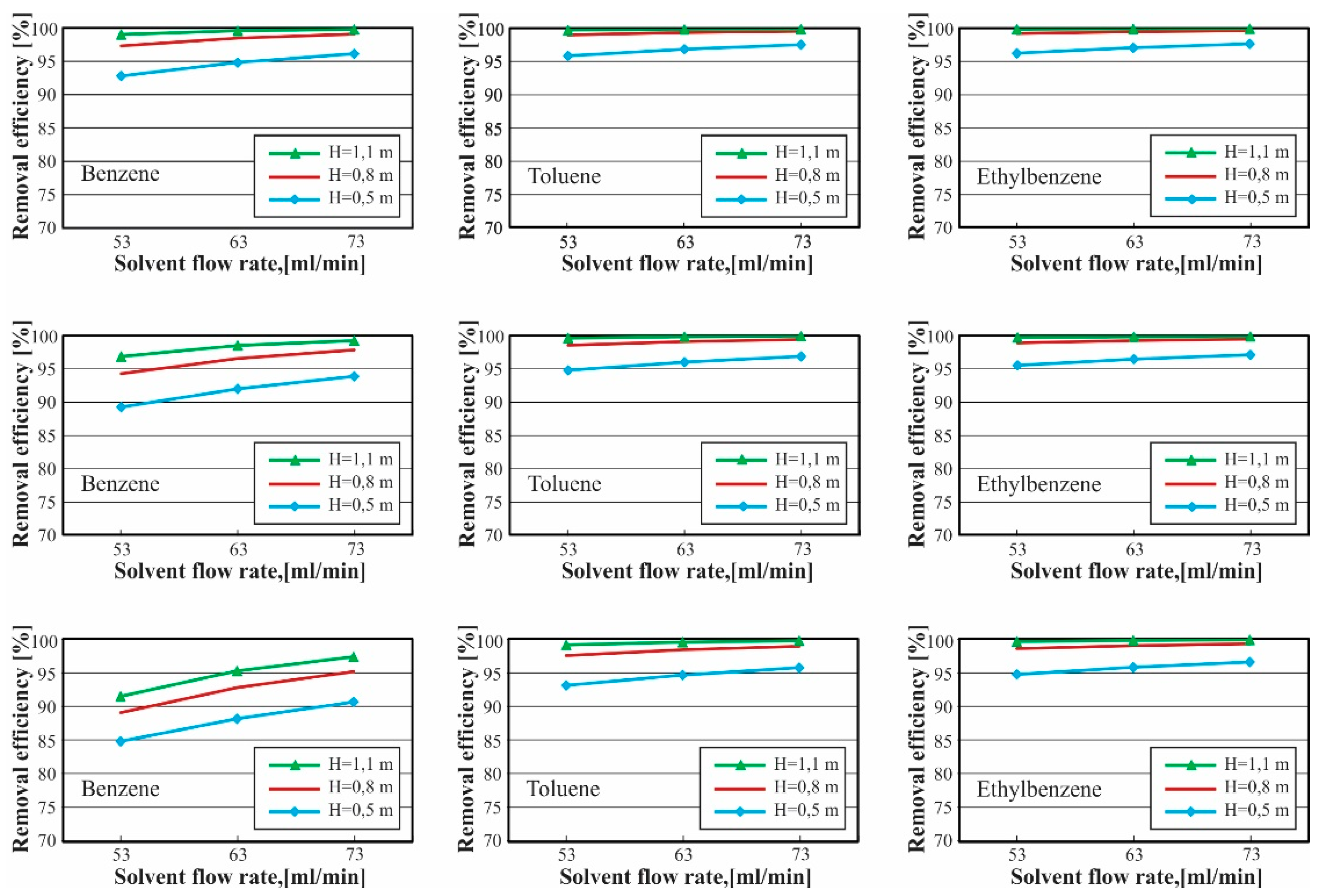
3.2. Analysis of Tar Absorption Process
3.2.1. Effect of Solvent Temperature
3.2.2. Effect of Bed Height and Solvent Volumetric Flow Rate
3.2.3. Optimum Operation Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclatures
| the molar flow rate of liquid | |
| the molar flow rate of vapor | |
| the molar flow rate of feed | |
| molar transfer rate | |
| molar transfer rate per unit of square area | |
| Molar concentration | |
| Equilibrium ratio | |
| Gas pressure | |
| Reaction rate | |
| Enthalpy | |
| Heat input to a stage | |
| Heat transfer rate | |
| T | Temperature |
| Liquid mole fraction | |
| Vapor mole fraction | |
| Binary mass transfer coefficient for the liquid | |
| Binary mass transfer coefficient for the gas | |
| Liquid mass transfer coefficient parameter, characteristic of the shape and structure of the packing | |
| Vapor mass transfer coefficient parameter, characteristic of the shape and structure of the packing | |
| Gravitational constant | |
| The density of the liquid, vapor | |
| The viscosity of liquid, vapor | |
| Diffusivity of the liquid, vapor | |
| Hydraulic diameter | |
| velocity for the liquid, vapor | |
| Void fraction of the packing | |
| Specific surface area | |
| Effective surface area per unit volume of the column | |
| Equivalent diameter | |
| Liquid surface tension | |
| Heat transfer coefficient for liquid | |
| Heat transfer coefficient for vapor | |
| The number of components | |
| Specific molar heat capacity | |
| Thermal conductivity | |
| Volumetric liquid holdup | |
| Fugacity of component i in the vapor phase | |
| Fugacity of component i in the liquid phase | |
| V | Total volume |
| Fugacity coefficient of component i | |
| Pressure | |
| Gas constant =0.08314 | |
| Temperature | |
| Mole number of component i | |
| Compression factor | |
| Subscripts | |
| Feed | |
| Film | |
| Interface | |
| Liquid | |
| Vapor | |
| Liquid or Vapor | |
| G | Gas |
| Component | |
| Number of components | |
| Stage number | |
| Abbreviations | |
| ASPEN PLUS | Simulation software program |
| GC | Gas chromatography |
| NRTL | Non-random two-liquid model |
| ICE | Internal combustion engine |
| MESH | Equations of material, energy balances, summation of composition, and equilibrium relation |
| MERSHQ | Equations of material, energy balances, rate of mass and heat transfer, summation of composition, hydraulic equation of pressure drop, and equilibrium |
| RB | Rate-based model |
| EQ | Equilibrium-stage model |
| MAPE | The mean absolute percentage error |
References
- Van Paasen, S.; Kiel, J. Tar formation in fluidised-bed gasification-impact of gasifier operating conditions. Acknowledgement/Preface 2004, 14, 26. [Google Scholar]
- Anis, S.; Zainal, Z. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Abatzaglou, N. Biomass Gasifier “Tars”: Their Nature, Formation, and Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.; Van Paasen, S.V.; Bergman, P.C.; Kiel, J.H. Catalytic decomposition of biomass tars: Use of dolomite and untreated olivine. Renew. Energy 2005, 30, 565–587. [Google Scholar] [CrossRef]
- Boerrigter, H.; Van Paasen, S.; Bergman, P.; Könemann, J.; Emmen, R.; Wijnands, A. OLGA tar removal technology. Energy Res. Centre Neth. ECN-C--05-009 2005. [Google Scholar]
- Unger, C.; Ising, M. Mechanismen und Bedeutung der Teerbildung und Teerbeseitigung bei der termochemischen Umwandlung fester Kohlenstoffträger. DGMK-Tagungsbericht 2002, 2, 131–142. [Google Scholar]
- Li, C.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Neeft, J.; Knoef, H.; Onaji, P. Behaviour of Tar in Biomass Gasification Systems: Tar Related Problems and their Solutions; Novem: Sittard, The Netherlands, 1999. [Google Scholar]
- Kübel, M. Teerbildung und Teerkonversion bei der Biomassevergasung-Anwendung der Nasschemischen Teerbestimmung nach CEN-Standard; Cuvillier: Göttingen, Germany, 2007. [Google Scholar]
- Wolfesberger, U.; Aigner, I.; Hofbauer, H. Tar content and composition in producer gas of fluidized bed gasification of wood-Influence of temperature and pressure. Environ. Prog. Sustain. Energy 2009, 28, 372–379. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Nakamura, S.; Kitano, S.; Yoshikawa, K. Biomass gasification process with the tar removal technologies utilizing bio-oil scrubber and char bed. Appl. Energy 2016, 170, 186–192. [Google Scholar] [CrossRef]
- Dyment, J.; Watanasiri, S. Acid Gas Cleaning Using DEPG Physical Solvents: Validation with Experimental and Plant Data; Aspen Technology Inc.: Bedford, MA, USA, 2015. [Google Scholar]
- Pilling, M.; Holden, B.S. Choosing Trays and Packings for Distillation; American Institute of Chemical Engineers CEP: New York, NY, USA, 2009; pp. 44–50. [Google Scholar]
- Mudhasakul, S.; Ku, H.-M.; Douglas, P.L. A simulation model of a CO2 absorption process with methyldiethanolamine solvent and piperazine as an activator. Int. J. Greenh. Gas Control. 2013, 15, 134–141. [Google Scholar] [CrossRef]
- Phuphuakrat, T.; Namioka, T.; Yoshikawa, K. Absorptive removal of biomass tar using water and oily materials. Bioresour. Technol. 2011, 102, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.C.; van Paasen, S.V.; Boerrigter, H. The novel “OLGA” technology for complete tar removal from biomass producer gas. In Pyrolysis and Gasification of Biomass and Waste, Expert Meeting; osti.gov: Strasbourg, France, 2002. [Google Scholar]
- Paethanom, A.; Nakahara, S.; Kobayashi, M.; Prawisudha, P.; Yoshikawa, K. Performance of tar removal by absorption and adsorption for biomass gasification. Fuel Process. Technol. 2012, 104, 144–154. [Google Scholar] [CrossRef]
- Bhoi, P.R. Wet Scrubbing of Biomass Producer Gas Tars Using Vegetable Oil; Oklahoma State University: Stillwater, OK, USA, 2014. [Google Scholar]
- Ozturk, B.; Yilmaz, D. Absorptive Removal of Volatile Organic Compounds from Flue Gas Streams. Process. Saf. Environ. Prot. 2006, 84, 391–398. [Google Scholar] [CrossRef]
- Mofarahi, M.; Khojasteh, Y.; Khaledi, H.; Farahnak, A. Design of CO2 absorption plant for recovery of CO2 from flue gases of gas turbine. Energy 2008, 33, 1311–1319. [Google Scholar] [CrossRef]
- Pandya, J. Adiabatic gas absorption and stripping with chemical reaction in packed towers. Chem. Eng. Commun. 1983, 19, 343–361. [Google Scholar] [CrossRef]
- Tontiwachwuthikul, P.; Meisen, A.; Lim, C. CO2 absorption by NaOH, monoethanolamine and 2-amino-2-methyl-1-propanol solutions in a packed column. Chem. Eng. Sci. 1992, 47, 381–390. [Google Scholar] [CrossRef]
- Tobiesen, F.A.; Svendsen, H.F.; Juliussen, O. Experimental validation of a rigorous absorber model for CO2 postcombustion capture. AIChE J. 2007, 53, 846–865. [Google Scholar] [CrossRef]
- Mores, P.L.; Scenna, N.; Mussati, S. A rate based model of a packed column for CO2 absorption using aqueous monoethanolamine solution. Int. J. Greenh. Gas Control. 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Afkhamipour, M.; Mofarahi, M. Comparison of rate-based and equilibrium-stage models of a packed column for post-combustion CO2 capture using 2-amino-2-methyl-1-propanol (AMP) solution. Int. J. Greenh. Gas Control. 2013, 15, 186–199. [Google Scholar] [CrossRef]
- Bhoi, P.; Huhnke, R.L.; Kumar, A.; Payton, M.E.; Patil, K.N.; Whiteley, J.R. Vegetable oil as a solvent for removing producer gas tar compounds. Fuel Process. Technol. 2015, 133, 97–104. [Google Scholar] [CrossRef]
- Seader, J.; Henley, E.J.; Roper, D.K. Separation Process Principles: Chemical and Biochemical Operations; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ramesh, K.; Aziz, N.; Shukor, S.A.; Ramasamy, M. Dynamic rate-based and equilibrium model approaches for continuous tray distillation column. J. Appl. Sci. Res. 2007, 3, 2030–2041. [Google Scholar]
- Billet, R.; Schultes, M. Prediction of Mass Transfer Columns with Dumped and Arranged Packings. Chem. Eng. Res. Des. 1999, 77, 498–504. [Google Scholar] [CrossRef]
- Taylor, R.; Krishna, R. Multicomponent Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 1993. [Google Scholar]
- Whitman, W.G. The two film theory of gas absorption. Int. J. Heat Mass Transf. 1962, 5, 429–433. [Google Scholar] [CrossRef]
- Ngo, T.H. Gas Absorption into Emulsions (Doctoral Dissertation Published by Universitätsbibliothek Braunschweig. Available online: https://nbn-resolving.org/urn:nbn:de:gbv:084-13043009095 (accessed on 5 March 2013).
- Khan, F.; Krishnamoorthi, V.; Mahmud, T. Modelling reactive absorption of CO2 in packed columns for post-combustion carbon capture applications. Chem. Eng. Res. Des. 2011, 89, 1600–1608. [Google Scholar] [CrossRef]
- Simon, L.L.; Elias, Y.; Puxty, G.; Artanto, Y.; Hungerbuhler, K. Rate based modeling and validation of a carbon-dioxide pilot plant absorbtion column operating on monoethanolamine. Chem. Eng. Res. Des. 2011, 89, 1684–1692. [Google Scholar] [CrossRef]
- Bhoi, P.; Huhnke, R.L.; Kumar, A.; Patil, K.N.; Whiteley, J.R. Design and development of a bench scale vegetable oil based wet packed bed scrubbing system for removing producer gas tar compounds. Fuel Process. Technol. 2015, 134, 243–250. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N.; Meredith, R.E. Reviewer Transport Phenomena. J. Electrochem. Soc. 1961, 108, 78C. [Google Scholar] [CrossRef]
- Gebreyohannes, S.; Yerramsetty, K.; Neely, B.J.; Gasem, K.A.M. Improved QSPR generalized interaction parameters for the nonrandom two-liquid activity coefficient model. Fluid Phase Equilibria 2013, 339, 20–30. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Chilton, T.H.; Colburn, A.P. Mass Transfer (Absorption) Coefficients Prediction from Data on Heat Transfer and Fluid Friction. Ind. Eng. Chem. 1934, 26, 1183–1187. [Google Scholar] [CrossRef]
- Plus, A. Aspen Plus Documentation Version V7. 3; Aspen Tech: Cambridge, MA, USA, 2011. [Google Scholar]
- Cateni, B.G. Effects of Feed Composition and Gasification Parameters on Product Gas from a Pilot Scale Fluidized Bed Gasifier; Oklahoma State University: Stillwater, OK, USA, 2007. [Google Scholar]
- Hlina, M.; Hrabovsky, M.; Kavka, T.; Konrad, M. Tar measurement in synthetic gas produced by plasma gasification by solid phase microextraction (SPME). In Proceedings of the ISPC Conference, Philadelphia, PA, USA, 24–29 July 2011; pp. 24–29. [Google Scholar]




| Absorbent | Water | Diesel Fuel | Biodiesel Fuel | Vegetable Oil | Engine Oil |
|---|---|---|---|---|---|
| Benzene | 24.1 | 77.0 | 86.1 | 77.6 | 61.7 |
| Toluene | 22.5 | 63.2 | 94.7 | 91.1 | 82.3 |
| Xylene | 22.1 | −730.1 | 97.8 | 96.4 | 90.7 |
| Styrene | 23.5 | 57.7 | 98.1 | 97.1 | 91.1 |
| Phenol | 92.8 | −111.1 | 99.9 | 99.7 | 97.7 |
| Indene | 28.2 | 97.9 | 97.2 | 97.6 | 88.7 |
| Naphthalene | 38.9 | 97.4 | 90.3 | 93.5 | 76.2 |
| Component | N-HEXADECANOIC-ACID | STEARIC-ACID | OLEIC-ACID | LINOLEIC-ACID | LINOLENIC-ACID | BENZENE | TOLUENE | ETHYLBENZENE | AIR |
|---|---|---|---|---|---|---|---|---|---|
| N-HEXADECANOIC-ACID | 0 | 4.31171 × 107 | 4.68659 × 107 | 4.64699 × 107 | 4.49859 × 107 | 1.06954 × 106 | 9.4904 × 107 | 8.58892 × 107 | 2.02164 × 106 |
| STEARIC-ACID | 4.31171 × 107 | 0 | 4.38352 × 107 | 4.38287 × 107 | 4.40934 × 107 | 1.10896 × 106 | 9.96534 × 107 | 9.036 × 107 | 2.07824 × 106 |
| OLEIC-ACID | 4.68659 × 107 | 4.38352 × 107 | 0 | 4.55134 × 107 | 4.54058 × 107 | 1.12982 × 106 | 9.97283 × 107 | 9.01854 × 107 | 2.14434 × 106 |
| LINOLEIC-ACID | 4.64699 × 107 | 4.38287 × 107 | 4.55134 × 107 | 0 | 4.53858 × 107 | 1.12457 × 106 | 9.93516 × 107 | 8.98562 × 107 | 2.13301 × 106 |
| LINOLENIC-ACID | 4.49859 × 107 | 4.40934 × 107 | 4.54058 × 107 | 4.53858 × 107 | 0 | 1.11272 × 106 | 9.88611 × 107 | 8.94878 × 107 | 2.10185 × 106 |
| BENZENE | 1.06954 × 106 | 1.10896 × 106 | 1.12982 × 106 | 1.12457 × 106 | 1.11272 × 106 | 0 | 5.44604 × 107 | 4.90094 × 107 | 6.55351 × 107 |
| TOLUENE | 9.4904 × 107 | 9.96534 × 107 | 9.97283 × 107 | 9.93516 × 107 | 9.88611 × 107 | 5.44604 × 107 | 0 | 5.42316 × 107 | 4.14606 × 107 |
| ETHYLBENZENE | 8.58892 × 107 | 9.036 × 107 | 9.01854 × 107 | 8.98562 × 107 | 8.94878 × 107 | 4.90094 × 107 | 5.42316 × 107 | 0 | 3.32784 × 107 |
| AIR | 2.02164 × 106 | 2.07824 × 106 | 2.14434 × 106 | 2.13301 × 106 | 2.10185 × 106 | 6.55351 × 107 | 4.14606 × 107 | 3.32784 × 107 | 0 |
| Component | N-HEXADECANOIC-ACID | STEARIC-ACID | OLEIC-ACID | LINOLEIC-ACID | LINOLENIC-ACID | BENZENE | TOLUENE | ETHYLBENZENE | AIR |
|---|---|---|---|---|---|---|---|---|---|
| N-HEXADECANOIC-ACID | 0 | 4.9358 × 107 | 5.1459 × 107 | 5.12412 × 107 | 5.04163 × 107 | 7.77376 × 107 | 7.32277 × 107 | 6.9663 × 107 | 1.06877 × 106 |
| STEARIC-ACID | 4.9358 × 107 | 0 | 4.97674 × 107 | 4.97636 × 107 | 4.99137 × 107 | 7.91572 × 107 | 7.50376 × 107 | 7.14531 × 107 | 1.08363 × 106 |
| OLEIC-ACID | 5.1459 × 107 | 4.97674 × 107 | 0 | 5.0711 × 107 | 5.06511 × 107 | 7.98982 × 107 | 7.50658 × 107 | 7.13841 × 107 | 1.10073 × 106 |
| LINOLEIC-ACID | 5.12412 × 107 | 4.97636 × 107 | 5.0711 × 107 | 0 | 5.06399 × 107 | 7.97126 × 107 | 7.49239 × 107 | 7.12536 × 107 | 1.09782 × 106 |
| LINOLENIC-ACID | 5.04163 × 107 | 4.99137 × 107 | 5.06511 × 107 | 5.06399 × 107 | 0 | 7.92915 × 107 | 7.47387 × 107 | 7.11074 × 107 | 1.08977 × 106 |
| BENZENE | 7.77376 × 107 | 7.91572 × 107 | 7.98982 × 107 | 7.97126 × 107 | 7.92915 × 107 | 0 | 5.54719 × 107 | 5.26227 × 107 | 6.08513 × 107 |
| TOLUENE | 7.32277 × 107 | 7.50376 × 107 | 7.50658 × 107 | 7.49239 × 107 | 7.47387 × 107 | 5.54719 × 107 | 0 | 5.53553 × 107 | 4.84006 × 107 |
| ETHYLBENZENE | 6.9663 × 107 | 7.14531 × 107 | 7.13841 × 107 | 7.12536 × 107 | 7.11074 × 107 | 5.26227 × 107 | 5.53553 × 107 | 0 | 4.33625 × 107 |
| AIR | 1.06877 × 106 | 1.08363 × 106 | 1.10073 × 106 | 1.09782 × 106 | 1.08977 × 106 | 6.08513 × 107 | 4.84006 × 107 | 4.33625 × 107 | 0 |
| Heat Transfer Coefficients for Liquid (Watt/m2 K) | Heat Transfer Coefficients for Vapor (Watt/m2 K) |
|---|---|
| 4512.399 | 48.685 |
| Acids | Soybean Oil |
|---|---|
| Palmitic acid (16:0) | 9% |
| Steric acid (18:0) | 4.4% |
| Oleic acid (18:1) | 26.4% |
| Linoleic acid (18:2) | 51.6% |
| Linolenic acid (18:3) | 6.8% |
| MAPE at Flow Rates of 53 mL/min, and Bed Height of 0.5 m | ||||||
|---|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | ||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 1.0% | 6.7% | 0.1% | 4.2% | 1.9% | 1.9% |
| 40 °C | 2.4% | 8.7% | 0.1% | 5.5% | 1.6% | 3.0% |
| 50 °C | 6.2% | 4.3% | 1.0% | 6.3% | 1.6% | 3.8% |
| MAPE at flow rates of 63 mL/min, and bed height of 0.5 m | ||||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 0.7% | 4.7% | 0.2% | 3.0% | 1.6% | 1.3% |
| 40 °C | 1.3% | 7.1% | 0.1% | 4.1% | 1.5% | 2.0% |
| 50 °C | 2.9% | 14.6% | 2.5% | 8.3% | 0.2% | 4.6% |
| MAPE at flow rates of 73 mL/min, and bed height of 0.5m | ||||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 0.1% | 4.2% | 0.9% | 3.4% | 1.0% | 1.3% |
| 40 °C | 2.0% | 8.4% | 1.0% | 4.3% | 1.1% | 1.8% |
| 50 °C | 1.4% | 11.1% | 1.5 5 | 6.0% | 0.0% | 3.5% |
| MAPE at Flow Rates of 53 mL/min, and Bed Height of 0.8 m | ||||||
|---|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | ||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 0.9% | 1.8% | 2.9% | 3.8% | 1.2% | 2.0% |
| 40 °C | 1.5% | 3.9% | 3.5% | 5.0% | 2.0% | 3.1% |
| 50 °C | 6.3% | 0.8% | 3.5% | 6.2% | 2.8% | 4.3% |
| MAPE at flow rates of 63 mL/min, and bed height of 0.8 m | ||||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 0.4% | 1.1% | 2.4% | 3.0% | 0.8% | 1.3% |
| 40 °C | 1.0% | 4.5% | 3.1% | 4.1% | 2.1% | 2.9% |
| 50 °C | 1.4% | 4.4% | 6.6% | 8.3% | 2.8% | 3.8% |
| MAPE at flow rates of 73 mL/min, and bed height of 0.8 m | ||||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 0.9% | 1.8% | 3.4% | 3.8% | 1.7% | 2.0% |
| 40 °C | 3.5% | 5.8% | 4.9% | 5.6% | 2.6% | 3.1% |
| 50 °C | 1.3% | 5.9% | 4.5% | 5.6% | 3.1% | 3.8% |
| MAPE at Flow Rates of 53 mL/min, and a 0.5 Bed Height of 1.1 m | ||||||
|---|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | ||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 0.0% | 1.0% | 3.0% | 3.2% | 1.3% | 1.4% |
| 40 °C | 1.4% | 1.3% | 3.3% | 3.7% | 1.5% | 1.7% |
| 50 °C | 5.5% | 2.6% | 5.1% | 6.0% | 4.2% | 4.6% |
| MAPE at flow rates of 63 mL/min, and bed height of 1.1 m | ||||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 0.7% | 1.1% | 3.4% | 3.5% | 2.1% | 2.1% |
| 40 °C | 1.8% | 3.2% | 4.7% | 4.9% | 2.7% | 2.9% |
| 50 °C | 0.9% | 2.2% | 5.9% | 6.4% | 4.6% | 4.8% |
| MAPE at flow rates of 73 mL/min, and bed height of 1.1 m | ||||||
| Temperatures °C | RB | EQ | RB | EQ | RB | EQ |
| 30 °C | 1.4% | 1.6% | 4.0% | 4.1% | 2.1% | 2.1% |
| 40 °C | 2.3% | 3.1% | 4.9% | 5.0% | 3.2% | 3.3% |
| 50 °C | 0.5% | 1.7% | 3.8% | 4.1% | 2.2% | 2.4% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almoslh, A.; Alobaid, F.; Heinze, C.; Epple, B. Comparison of Equilibrium-Stage and Rate-Based Models of a Packed Column for Tar Absorption Using Vegetable Oil. Appl. Sci. 2020, 10, 2362. https://doi.org/10.3390/app10072362
Almoslh A, Alobaid F, Heinze C, Epple B. Comparison of Equilibrium-Stage and Rate-Based Models of a Packed Column for Tar Absorption Using Vegetable Oil. Applied Sciences. 2020; 10(7):2362. https://doi.org/10.3390/app10072362
Chicago/Turabian StyleAlmoslh, Adel, Falah Alobaid, Christian Heinze, and Bernd Epple. 2020. "Comparison of Equilibrium-Stage and Rate-Based Models of a Packed Column for Tar Absorption Using Vegetable Oil" Applied Sciences 10, no. 7: 2362. https://doi.org/10.3390/app10072362
APA StyleAlmoslh, A., Alobaid, F., Heinze, C., & Epple, B. (2020). Comparison of Equilibrium-Stage and Rate-Based Models of a Packed Column for Tar Absorption Using Vegetable Oil. Applied Sciences, 10(7), 2362. https://doi.org/10.3390/app10072362





