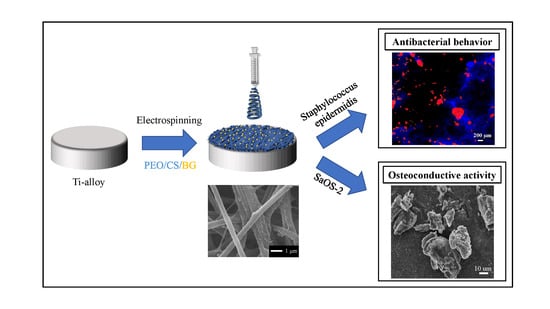
Antibacterial and Osteoconductive Effects of Chitosan/Polyethylene Oxide (PEO)/Bioactive Glass Nanofibers for Orthopedic Applications
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Sample Preparation by Electrospinning
2.3. Pre-Test Characterization
2.4. Bacterial Culture and Characterization
2.5. Osteoblast-Like Cells Culture and Characterization
2.6. Statistical Analysis
3. Results
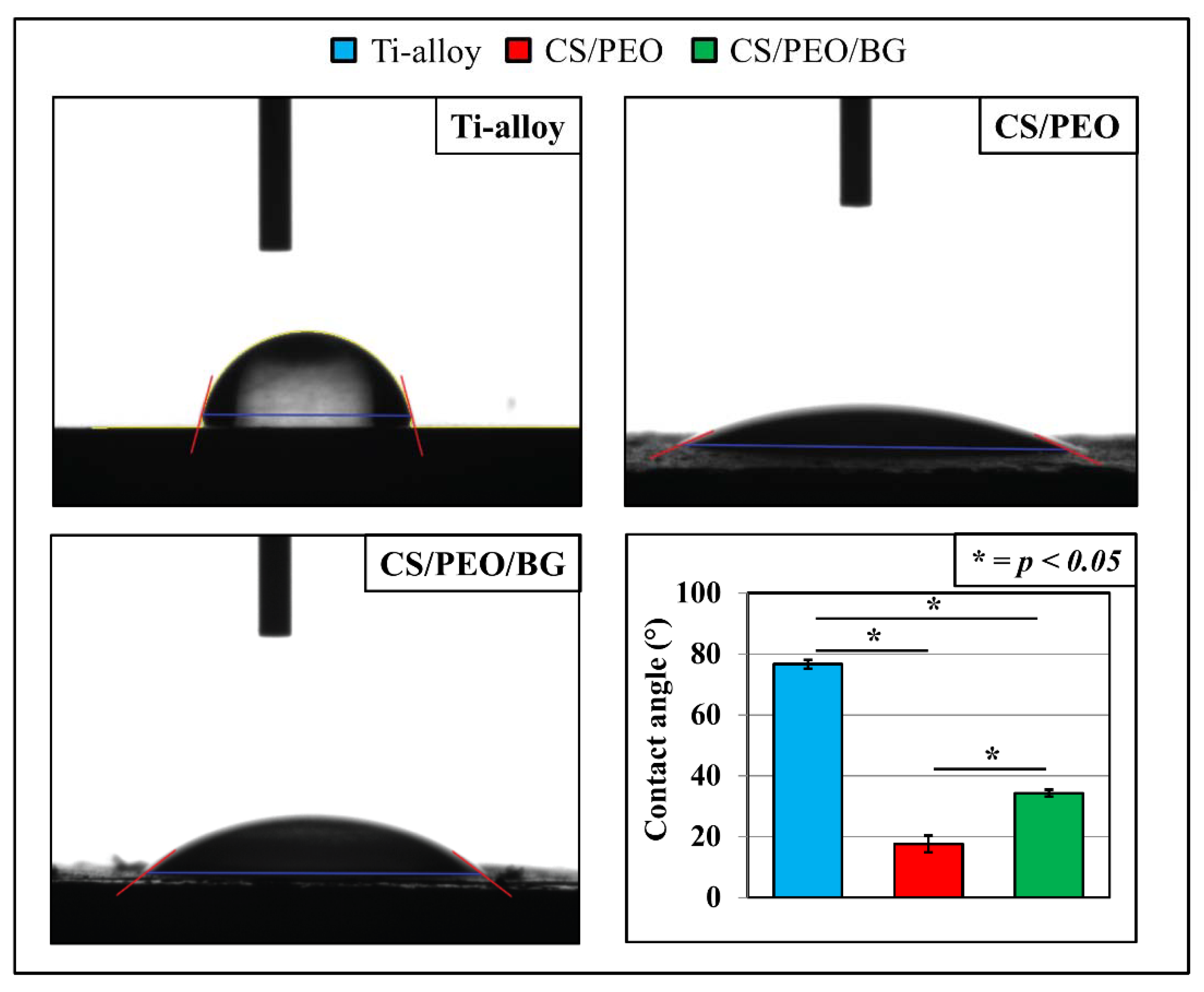
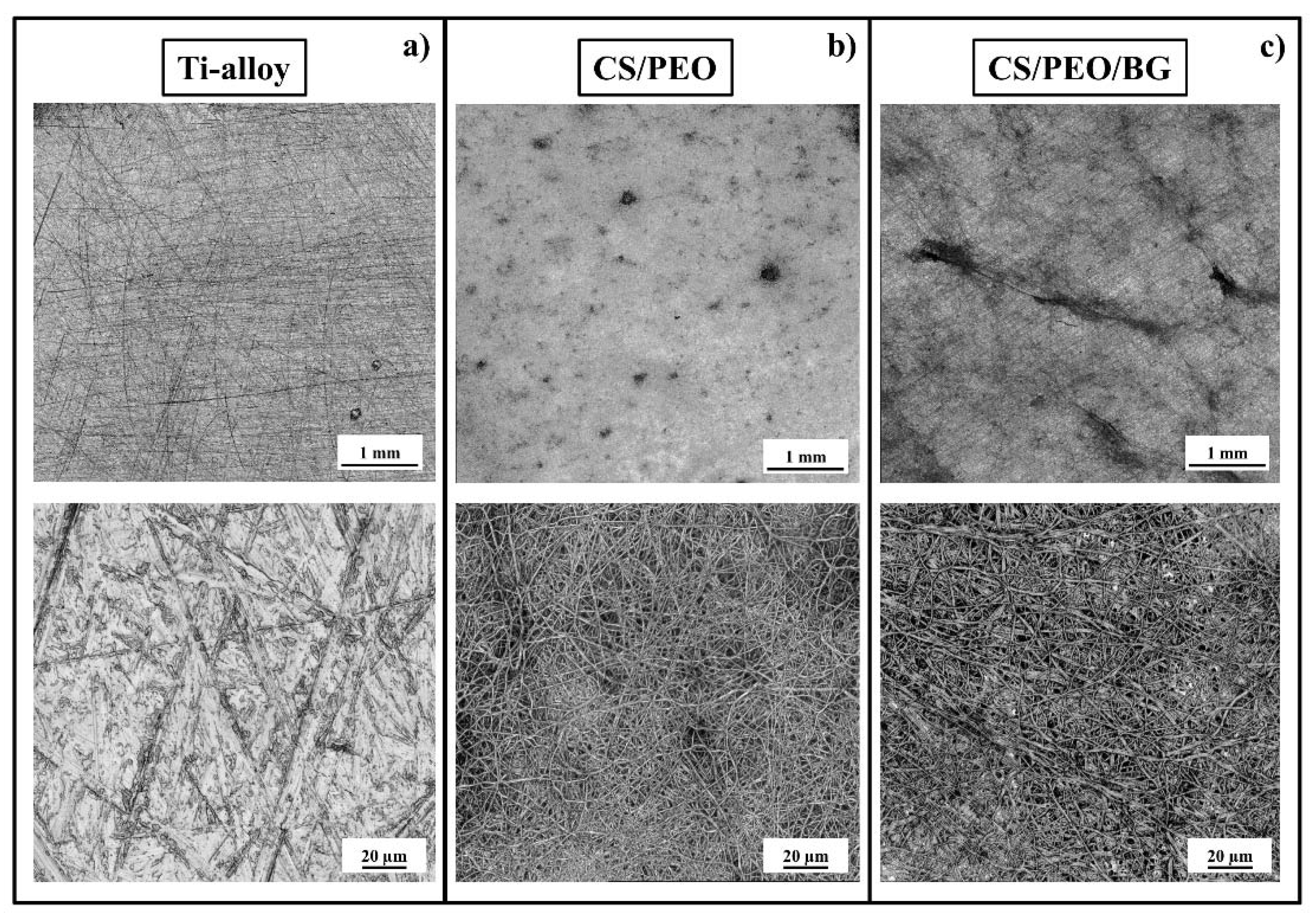
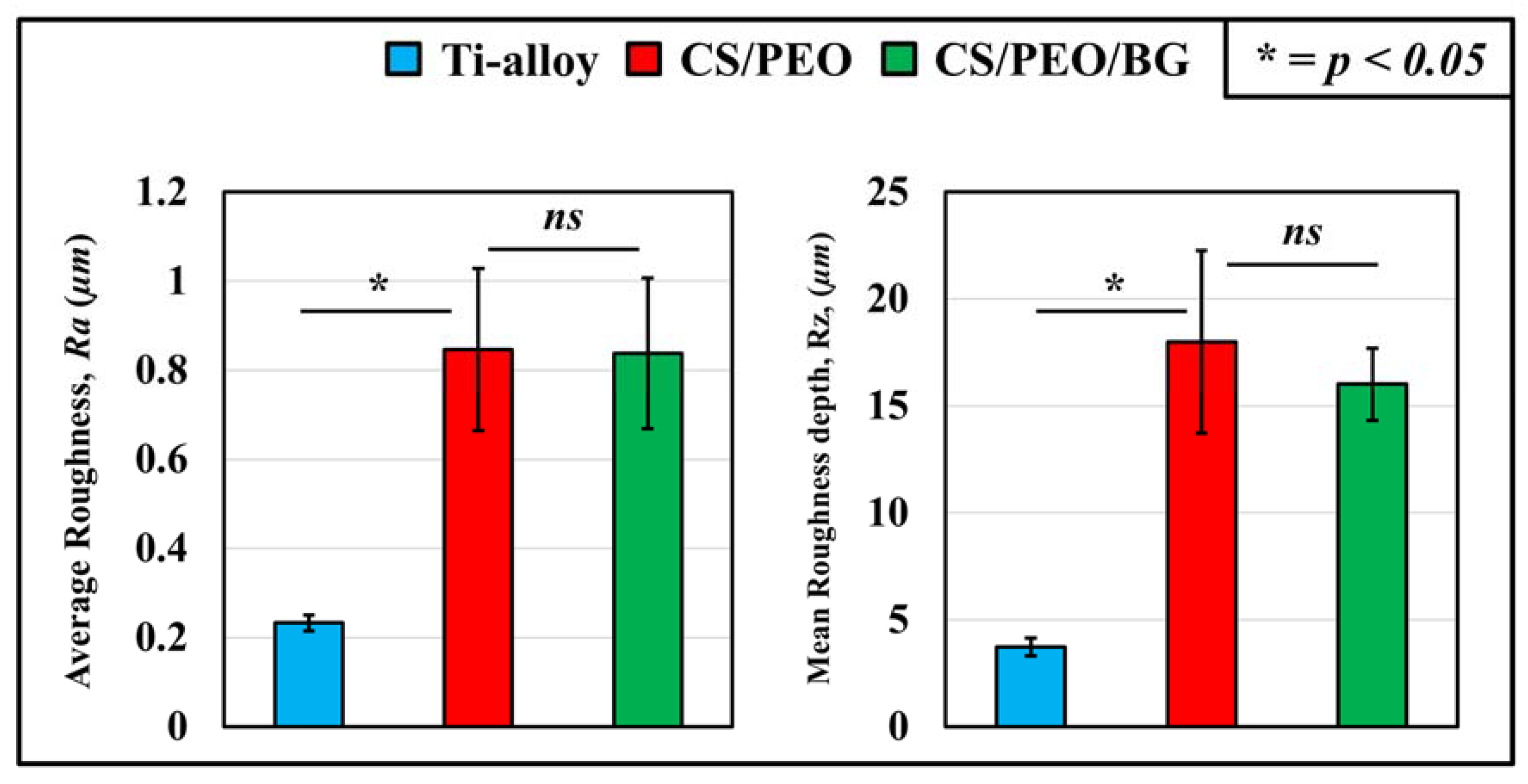
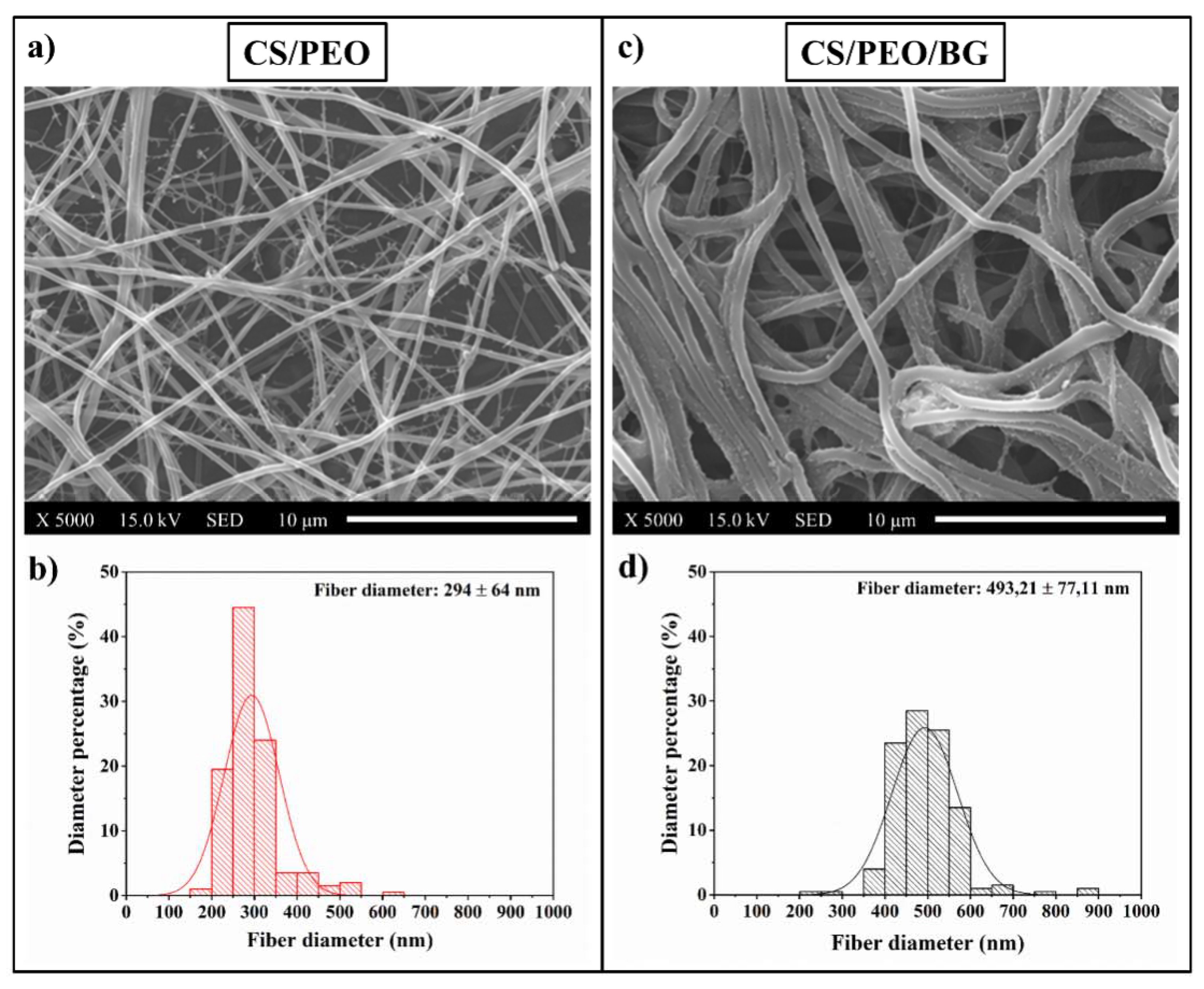
3.1. Pre-Test Characterization
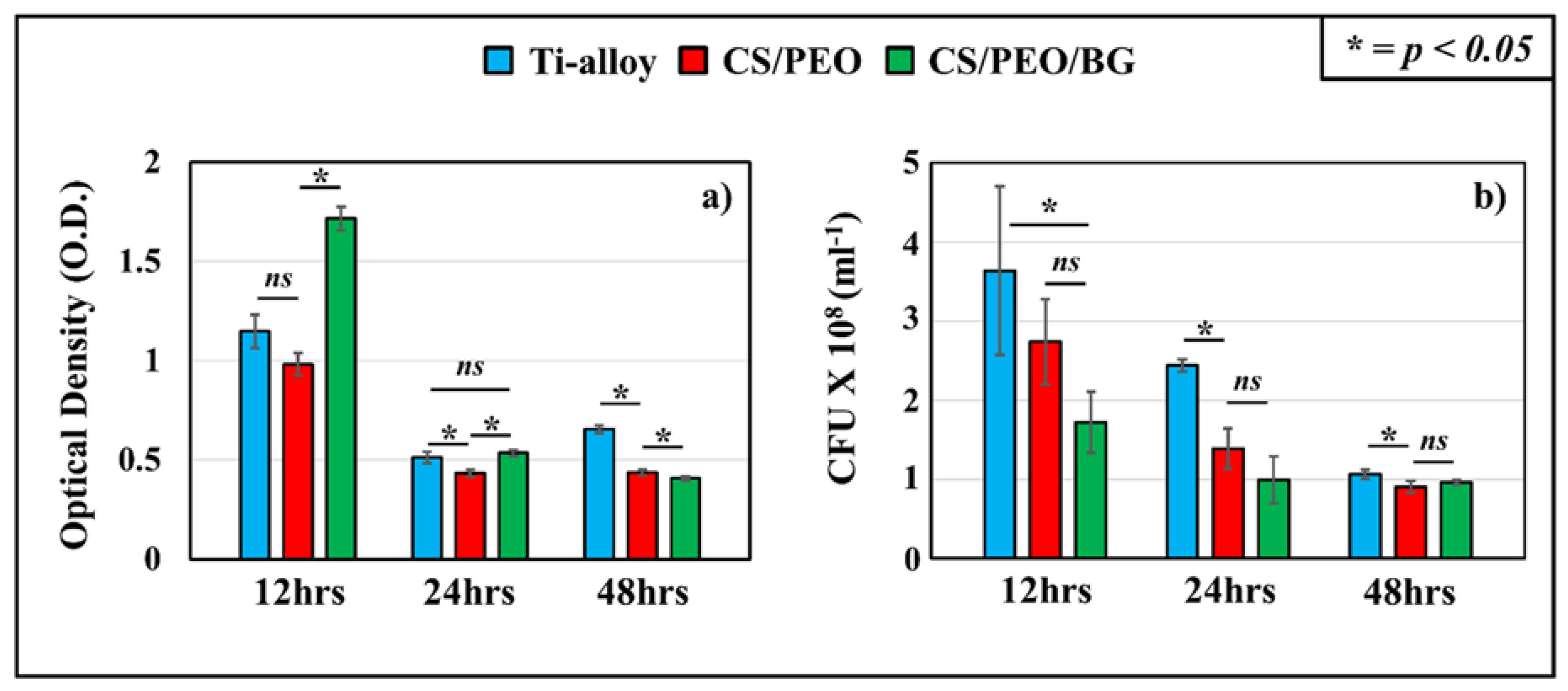
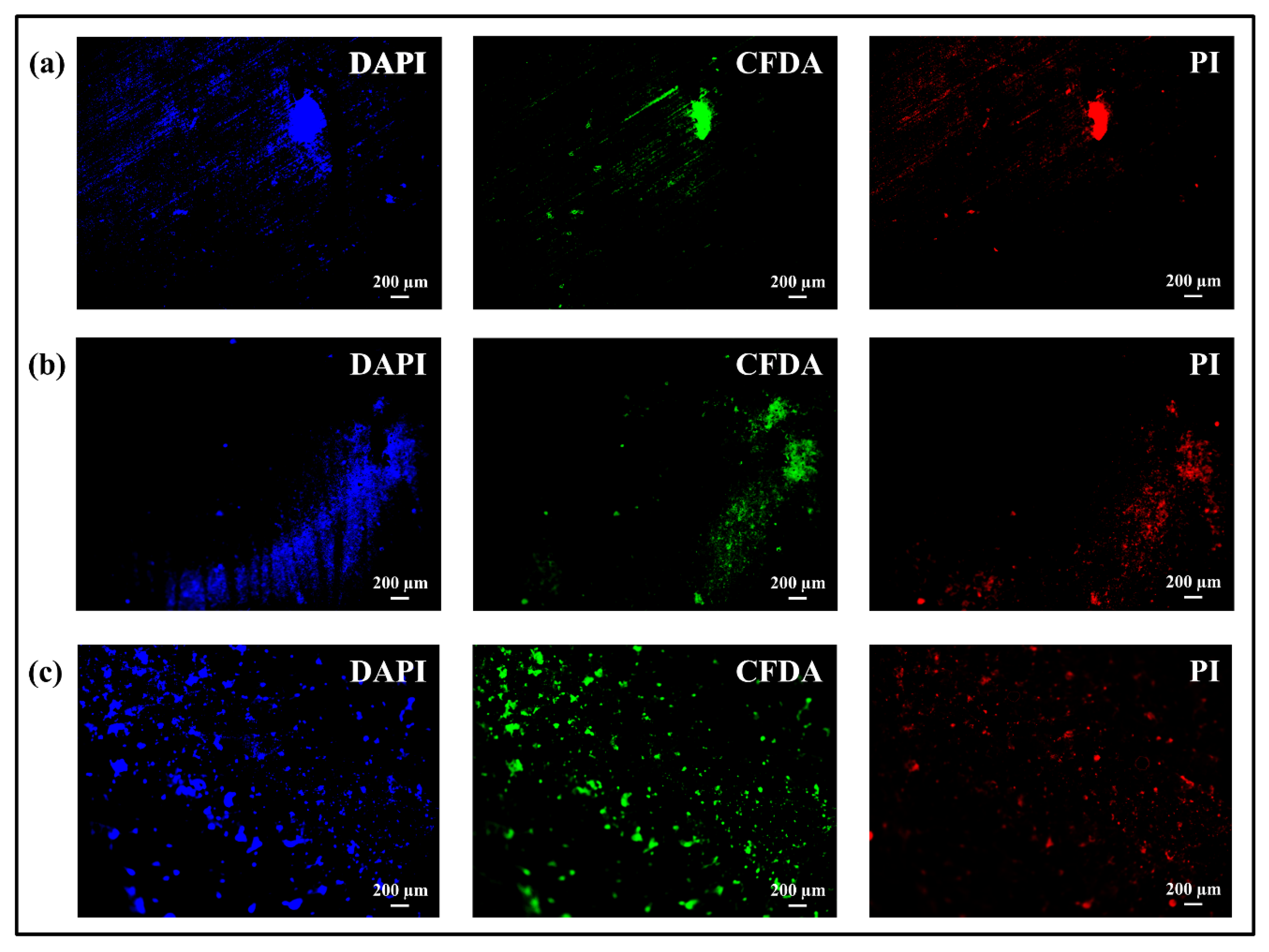
3.2. Bacterial Tests: Microbial Viability Assay, CFU Counting and Fluorescence Microscope
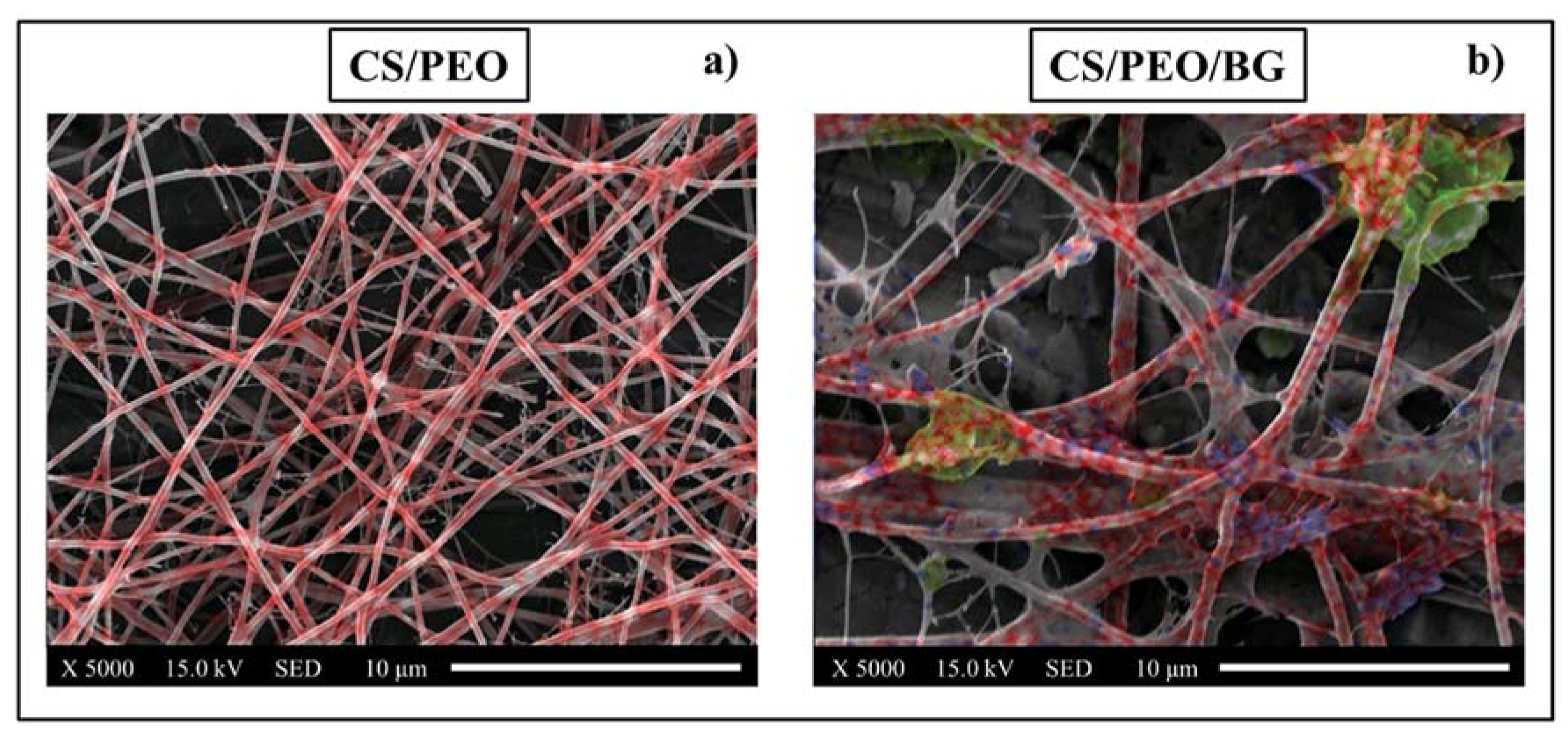
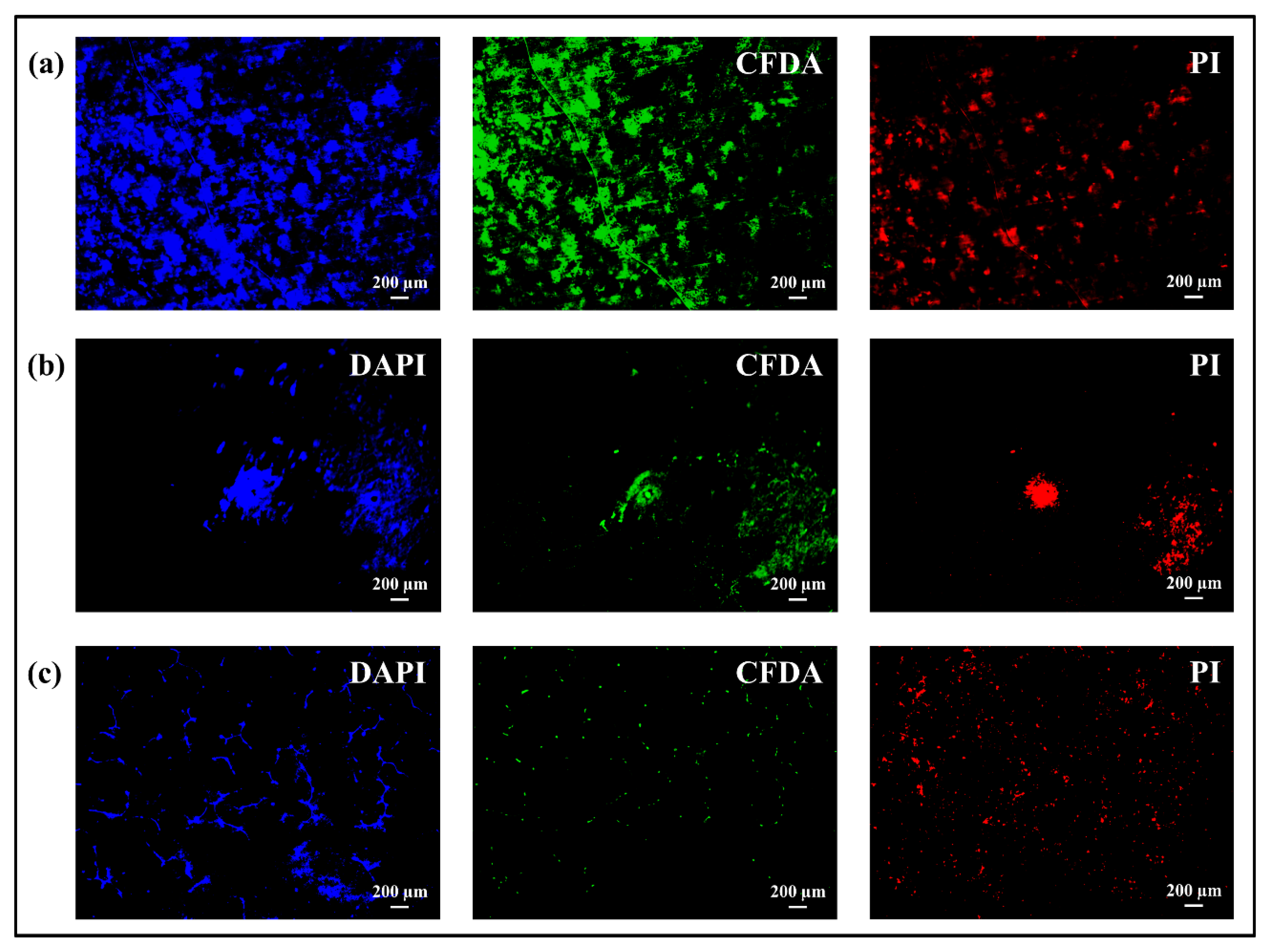
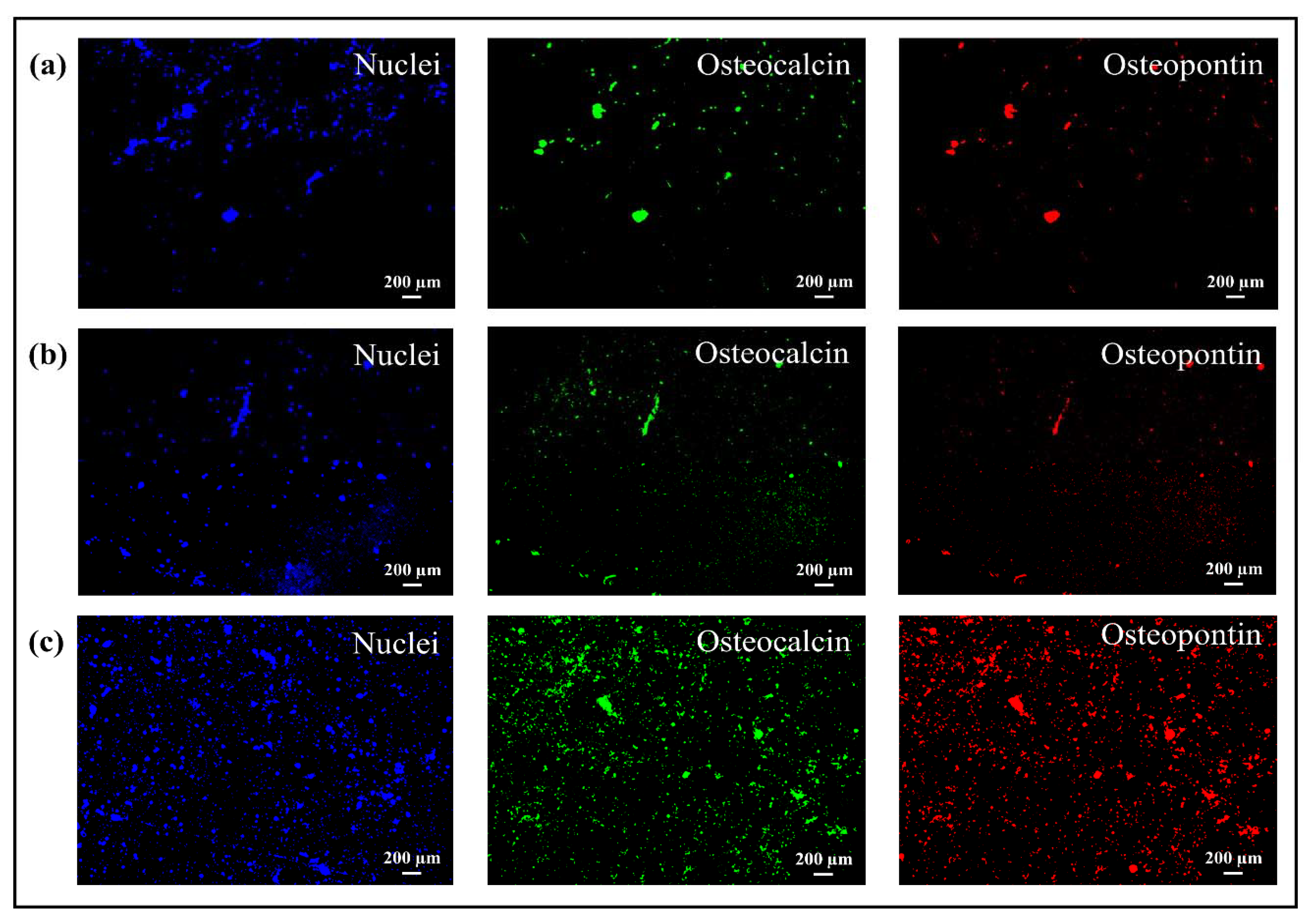
3.3. Osteoconductivity Tests: SEM/EDX and Fluorescence Microscope
4. Discussion
5. Conclusions
- By adding bioglass to the polymeric solution, the viscosity increased significantly and this involved changes in the fibers’ morphological and physiochemical properties (diameter of the fibers and wettability);
- Both coatings showed a more effective action against bacteria over time compared to uncoated Ti-alloy, even if in the case of coating of nanofibers with bioglass there was an initial adhesion and proliferation due to the presence of glass, partly inhibiting the antibacterial effect of chitosan;
- Cells adhered to all substrates, but the coating incorporated with bioglass provided a better performance regarding the formation of mineralized tissue because some areas related to mineralized organic matrix (osteocalcin and osteopontin) were homogeneously distributed on the entire surface.
Author Contributions
Funding
Conflicts of Interest
References
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.; Guo, H.; Li, P.; Hu, T.; Chung, C.Y.; Chu, P.K. Surface nano-architectures and their effects on the mechanical properties and corrosion behavior of Ti-based orthopedic implants. Surf. Coat. Technol. 2013, 233, 13–26. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Lai, B.F.; Ding, C.; Kizhakkedathu, J.N.; Hancock, R.E.; Wang, R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013, 34, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Galed, G.; Miralles, B.; Paños, I.; Santiago, A.; Heras, Á. N-Deacetylation and depolymerization reactions of chitin/chitosan: Influence of the source of chitin. Carbohydr. Polym. 2005, 62, 316–320. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Jung, K.H.; Huh, M.W.; Meng, W.; Yuan, J.; Hyun, S.H.; Bae, J.S.; Hudson, S.M.; Kang, I.K. Preparation and antibacterial activity of PET/chitosan nanofibrous mats using an electrospinning technique. J. Appl. Polym. Sci. 2007, 105, 2816–2823. [Google Scholar] [CrossRef]
- Spasova, M.; Paneva, D.; Manolova, N.; Radenkov, P.; Rashkov, I. Electrospun chitosan-coated fibers of poly (L-lactide) and poly (L-lactide)/poly (ethylene glycol): Preparation and characterization. Macromol. Biosci. 2008, 8, 153–162. [Google Scholar] [CrossRef]
- Son, B.; Yeom, B.Y.; Song, S.H.; Lee, C.S.; Hwang, T.S. Antibacterial electrospun chitosan/poly (vinyl alcohol) nanofibers containing silver nitrate and titanium dioxide. J. Appl. Polym. Sci. 2009, 111, 2892–2899. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of active antimicrobial fiber-based chitosan polysaccharide nanostructures using electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Oudadesse, H.; Bui, X.V.; Le Gal, Y.; Mostafa, A.; Cathelineau, G. Chitosan effects on bioactive glass for application as biocopmosite biomaterial. Int. J. Biol. Biomed. Eng. 2011, 5, 49–56. [Google Scholar]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Gao, W.; Liang, H.; Wang, H.; Li, J. Preparation of chitosan/PLA blend micro/nanofibers by electrospinning. Mater. Lett. 2009, 63, 658–660. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of chitosan-containing nanofibres by electrospinning of chitosan/poly (ethylene oxide) blend solutions. e-Polymers 2004, 4, 1–12. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Li, L.; Hsieh, Y.L. Chitosan bicomponent nanofibers and nanoporous fibers. Carbohydr. Res. 2006, 341, 374–381. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of chitosan solutions in acetic acid with poly (ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef]
- Jones, J.R.; Gentleman, E.; Polak, J. Bioactive glass scaffolds for bone regeneration. Elements 2007, 3, 393–399. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Mohan, S.; Balaji raghavendran, H.R.; Mehrali, M.; Khanlou, H.M.; Kamarul, T.; Afifi, A.M.; Abass, A.A. Chitosan (PEO)/bioactive glass hybrid nanofibers for bone tissue engineering. RSC Adv. 2014, 4, 49144–49152. [Google Scholar] [CrossRef]
- Liverani, L.; Lacina, J.; Roether, J.A.; Boccardi, E.; Killian, M.S.; Schmuki, P.; Schubert, D.W.; Boccaccini, A.R. Incorporation of bioactive glass nanoparticles in electrospun PCL/chitosan fibers by using benign solvents. Bioact. Mater. 2017, 3, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.F.; Akram, M.; Alshemary, A.; Hussain, R. Antibacterial polylactic acid/chitosan nanofibers decorated with bioactive glass. Appl. Surf. Sci. 2016, 387, 1–7. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Hunk, O.; Benkirane-Jessel, N.; et al. Application of chitosan in bone and dental engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Sowmya, S.; Sathish, D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Effect of incorporation of nanoscale bioactive glass and hydroxyapatite in PCL/chitosan nanofibers for bone and periodontal tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 430–440. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef]
- Seo, H.; Matsumoto, H.; Hara, S.; Minagawa, M.; Tanioka, A.; Yako, H.; Inoue, K. Preparation of polysaccharide nanofiber fabrics by electrospray deposition: Additive effects of poly (ethylene oxide). Polym. J. 2005, 37, 391–398. [Google Scholar] [CrossRef]
- Foroughi, M.R.; Karbasi, S.; Khoroushi, M.; Khademi, A.A. Polyhydroxybutyrate/chitosan/bioglass nanocomposite as a novel electrospun scaffold: Fabrication and characterization. J. Porous Mater 2017, 24, 1447–1460. [Google Scholar] [CrossRef]
- Silva, S.M.; Braga, C.R.; Fook, M.V.; Raposo, C.M.; Carvalho, L.H.; Canedo, E.L. Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012; pp. 43–62. [Google Scholar]
- Wen, S.J.; Richardson, T.J.; Ghantous, D.I.; Striebel, K.A.; Ross, P.N.; Cairns, E.J. FTIR characterization of PEO + LiN(CF3SO2)2 electrolytes. J. Electroanal. Chem. 1996, 408, 113–118. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Balakumar, S.; Shajan, X.S. Structural and ionic conductivity studies on nanochitosan incorporated polymer electrolytes for rechargeable magnesium batteries. Chem. Sci. Trans. 2012, 1, 311–316. [Google Scholar]
- Sim, L.H.; Gan, S.N.; Chan, C.H.; Yahya, R. ATR-FTIR studies on ion interaction of lithium perchlorate in polyacrylate/poly (ethylene oxide) blends. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from Parapenaeus longirostris from Moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Turhan, M.; Gunasekaran, S. Selected properties of pH-sensitive, biodegradable chitosan–poly (vinyl alcohol) hydrogel. Polym. Int. 2004, 53, 911–918. [Google Scholar] [CrossRef]
- Bui, X.; Oudadesse, H.; Le Gal, Y.; Mostafa, A.; Cathelineau, G. Microspheres of chitosan-bioactive glass for application in orthopedic surgery. In Vitro Experiment, Recent Researches in Modern Medicine; WSEAS: Cambridge, UK, 2011; pp. 359–367. [Google Scholar]
- Anilkumar, K.M.; Jinisha, B.; Manoj, M.; Jayalekshmi, S. Poly (ethylene oxide) (PEO)–Poly (vinyl pyrrolidone) (PVP) blend polymer based solid electrolyte membranes for developing solid state magnesium ion cells. Eur. Polym. J. 2017, 89, 249–262. [Google Scholar] [CrossRef]
- Theophile, T. Infrared Spectroscopy: Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Kim, S.K. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ouis, M.A.; Abdelghany, A.M.; ElBatal, H.A. Corrosion mechanism and bioactivity of borate glasses analogue to Hench’s bioglass. Process. Appl. Ceram. 2012, 6, 141–149. [Google Scholar] [CrossRef]
- Kouhi, M.; Morshed, M.; Varshosaz, J.; Fathi, M.H. Poly (ε-caprolactone) incorporated bioactive glass nanoparticles and simvastatin nanocomposite nanofibers: Preparation, characterization and in vitro drug release for bone regeneration applications. Chem. Eng. J. 2013, 228, 1057–1065. [Google Scholar] [CrossRef]
- Drew, C.; Wang, X.; Samuelson, L.A.; Kumar, J. The effect of viscosity and filler on electrospun fiber morphology. J. Macromol. Sci. Part A 2003, 40, 1415–1422. [Google Scholar] [CrossRef]
- Huang, F.L.; Wang, Q.Q.; Wei, Q.F.; Gao, W.D.; Shou, H.Y.; Jiang, S.D. Dynamic wettability and contact angles of poly (vinylidene fluoride) nanofiber membranes grafted with acrylic acid. Express Polym. Lett. 2010, 4, 551–558. [Google Scholar] [CrossRef]
- Chen, C.S.; Liau, W.Y.; Tsai, G.J. Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. J. Food Prot. 1998, 61, 1124–1128. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrieres, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Tsai, G.J.; Su, W.H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A.; Kendra, D.G.; Fristensky, B.W.; Wagoner, W. Chitosan both activated genes in plants and inhibits RNA synthesis in fungi. In Chitin in Nature and Technology; Muzzarelli, R.A.A., Jeuniaux, C., Gooday, G.W., Eds.; Plenum: New York, NY, USA, 1981. [Google Scholar]
- Papineau, A.M.; Hoover, D.G.; Knorr, D.; Farkas, D.F. Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol. 1991, 5, 45–57. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Fang, S.W.; Li, C.F.; Shih, D.Y. Antifungal activity of chitosan and its preservative effect on low-sugar candied kumquat. J. Food Prot. 1994, 57, 136–140. [Google Scholar] [CrossRef]
- Xynos, I.D.; Hukkanen, M.V.J.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass® 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
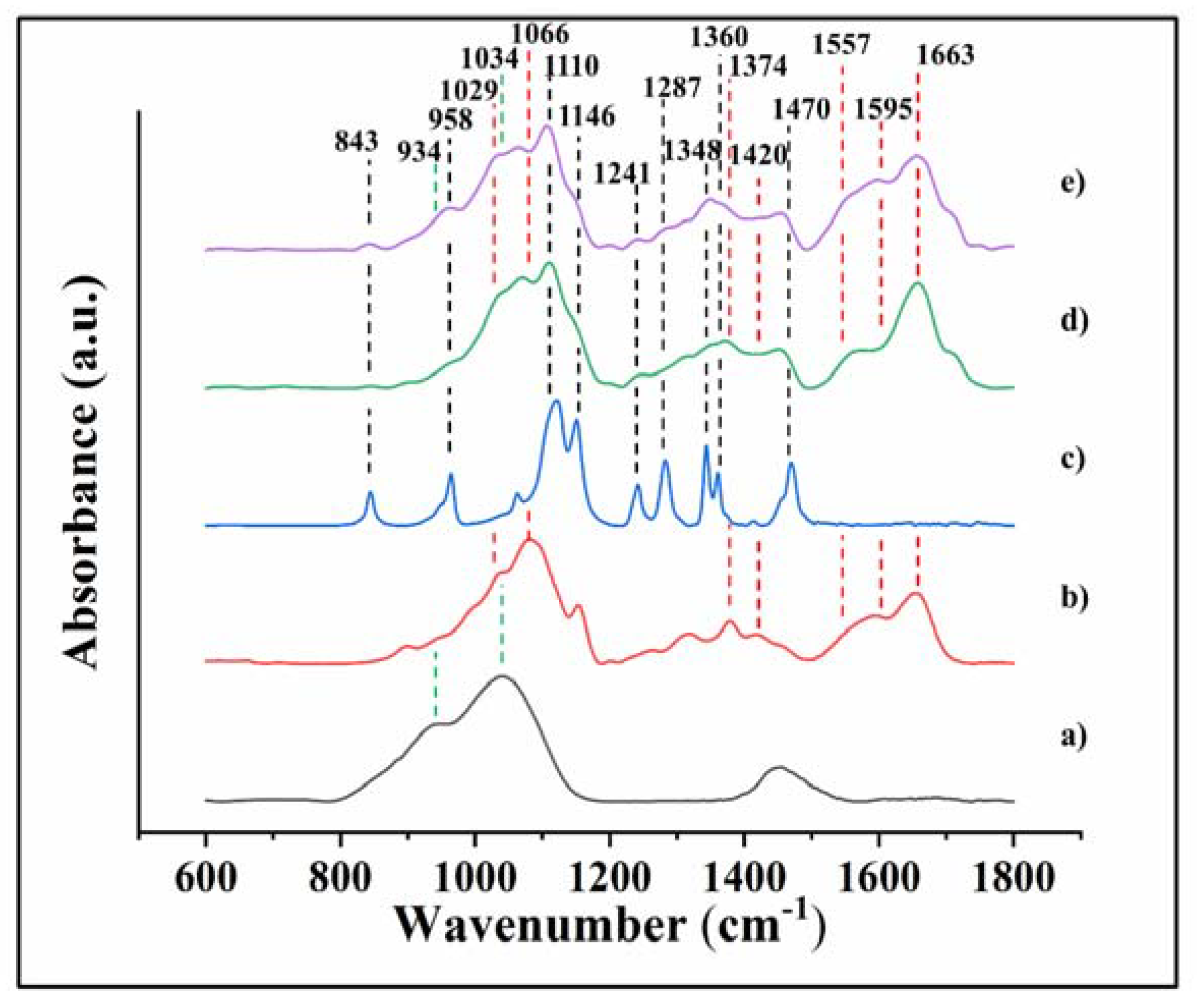


| Number | Position (cm−1) | Assignments | References |
|---|---|---|---|
| 1 | 843 | CH2 rocking | [38] |
| 2 | 900 | wagging (C-H) | [28] |
| 3 | 934 | Si-O stretching | [28] |
| 4 | 958 | CH2 rocking of methylene group | [38] |
| 5 | 999 | CO group | [39] |
| 6 | 1029 (1034) | stretching C-O, (Si-O stretching) | [28,30,(36)] |
| 7 | 1066 | symmetric and asymmetric stretching (C-O) | [28,30] |
| 8 | 1110 | Asymmetric stretching (C-O-C) | [29] |
| 9 | 1146 | Symmetric stretching (C-O-C) | [28] |
| 10 | 1159 | (carbonyl bands) bridge C-O-C | [28,30] |
| 11 | 1241 | Symmetric twisting CH2 | [31] |
| 12 | 1287 | Asymmetric twisting CH2 | [32] |
| 13 | 1315 | C-N stretching, C-H symmetric stretching (CH3) (amide III) | [28] |
| 14 | 1348 | CH2 wagging (C-H) | [31] |
| 15 | 1374 | CH3 bending | [28,30] |
| 16 | 1420 | CH2 bending | [28,30] |
| 17 | 1456 | Asymmetric and symmetric CH2 bending | [29] |
| 18 | 1470 | Asymmetric CH2 bending | [38] |
| 19 | 1518 | N-H deformation and C-N stretching of amide II | [34] |
| 20 | 1557 | N-H bending (amide II) | [35] |
| 21 | 1595 | N-H bending | [33] |
| 22 | 1634 | N-H bending of NH2, C=N stretching (Schiff base) | [40] |
| 23 | 1663 | Stretching C=O (amide I) | [34] |
| 24 | 1713 | Carboxyl band (C=O) | [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschetto, F.; Ngoc Doan, H.; Phong Vo, P.; Zanocco, M.; Zhu, W.; Sakai, W.; Adachi, T.; Ohgitani, E.; Tsutsumi, N.; Mazda, O.; et al. Antibacterial and Osteoconductive Effects of Chitosan/Polyethylene Oxide (PEO)/Bioactive Glass Nanofibers for Orthopedic Applications. Appl. Sci. 2020, 10, 2360. https://doi.org/10.3390/app10072360
Boschetto F, Ngoc Doan H, Phong Vo P, Zanocco M, Zhu W, Sakai W, Adachi T, Ohgitani E, Tsutsumi N, Mazda O, et al. Antibacterial and Osteoconductive Effects of Chitosan/Polyethylene Oxide (PEO)/Bioactive Glass Nanofibers for Orthopedic Applications. Applied Sciences. 2020; 10(7):2360. https://doi.org/10.3390/app10072360
Chicago/Turabian StyleBoschetto, Francesco, Hoan Ngoc Doan, Phu Phong Vo, Matteo Zanocco, Wenliang Zhu, Wataru Sakai, Tetsuya Adachi, Eriko Ohgitani, Naoto Tsutsumi, Osam Mazda, and et al. 2020. "Antibacterial and Osteoconductive Effects of Chitosan/Polyethylene Oxide (PEO)/Bioactive Glass Nanofibers for Orthopedic Applications" Applied Sciences 10, no. 7: 2360. https://doi.org/10.3390/app10072360
APA StyleBoschetto, F., Ngoc Doan, H., Phong Vo, P., Zanocco, M., Zhu, W., Sakai, W., Adachi, T., Ohgitani, E., Tsutsumi, N., Mazda, O., Kinashi, K., Marin, E., & Pezzotti, G. (2020). Antibacterial and Osteoconductive Effects of Chitosan/Polyethylene Oxide (PEO)/Bioactive Glass Nanofibers for Orthopedic Applications. Applied Sciences, 10(7), 2360. https://doi.org/10.3390/app10072360