“Green” Synthesis and Antioxidant Activity of Thermally Stable Gold Nanoparticles Encapsulated in Carbon Nanosheets
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apple Extract Preparation
2.3. Pectin-Capped AuNP Preparation
2.4. Citrate-Capped AuNP Preparation
2.5. Preparation of AuNPs Encapsulated in Carbon Nanosheets
2.6. Characterization
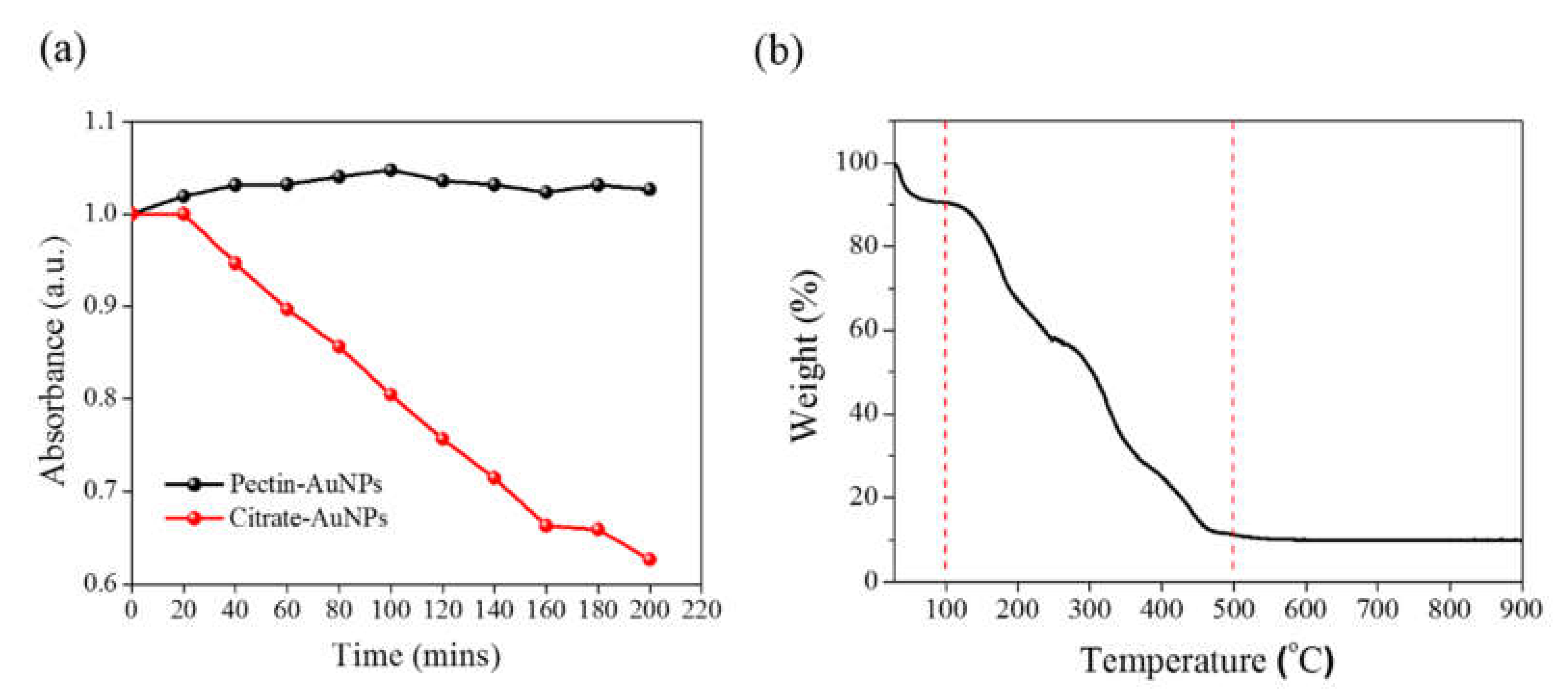
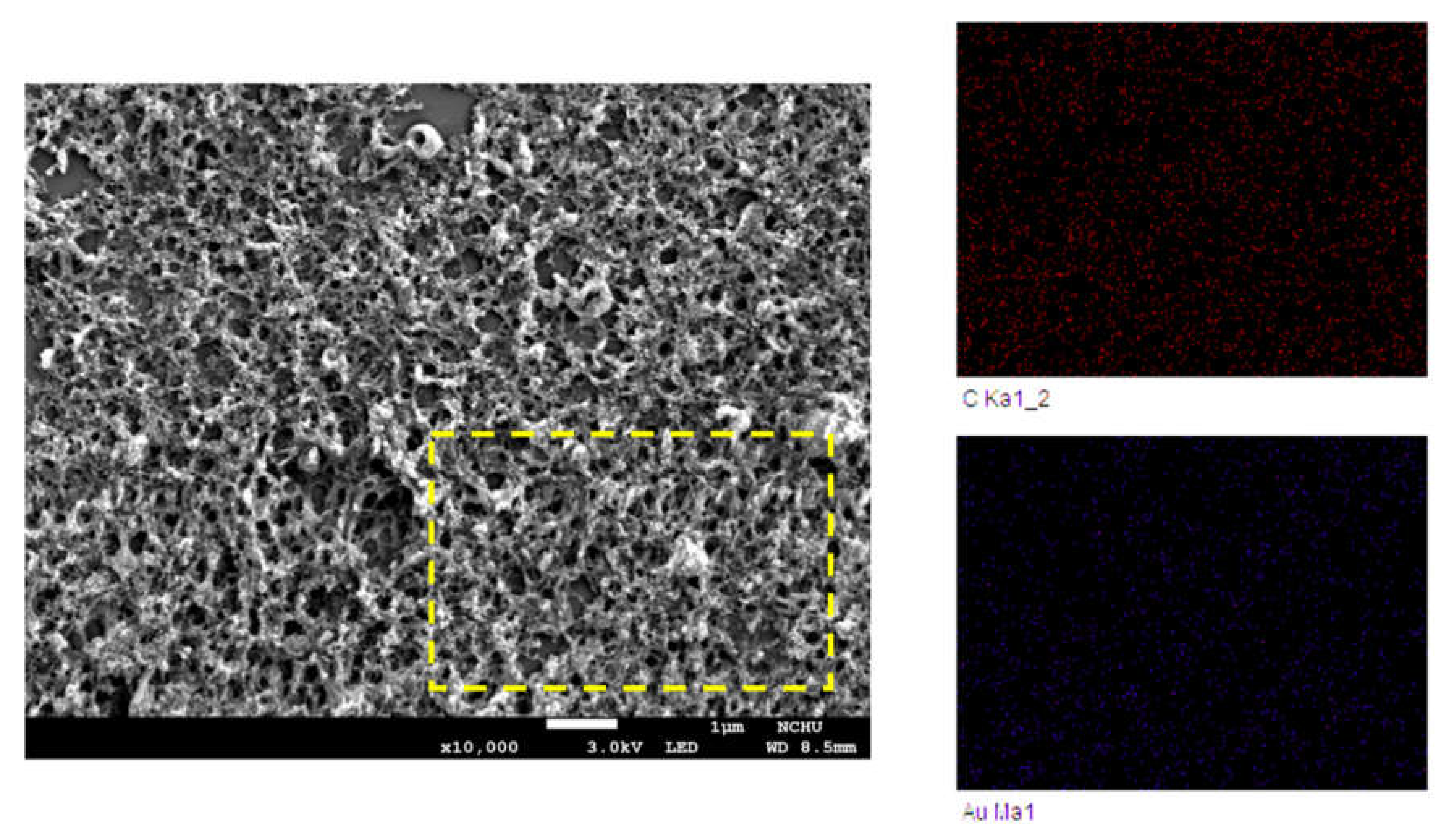
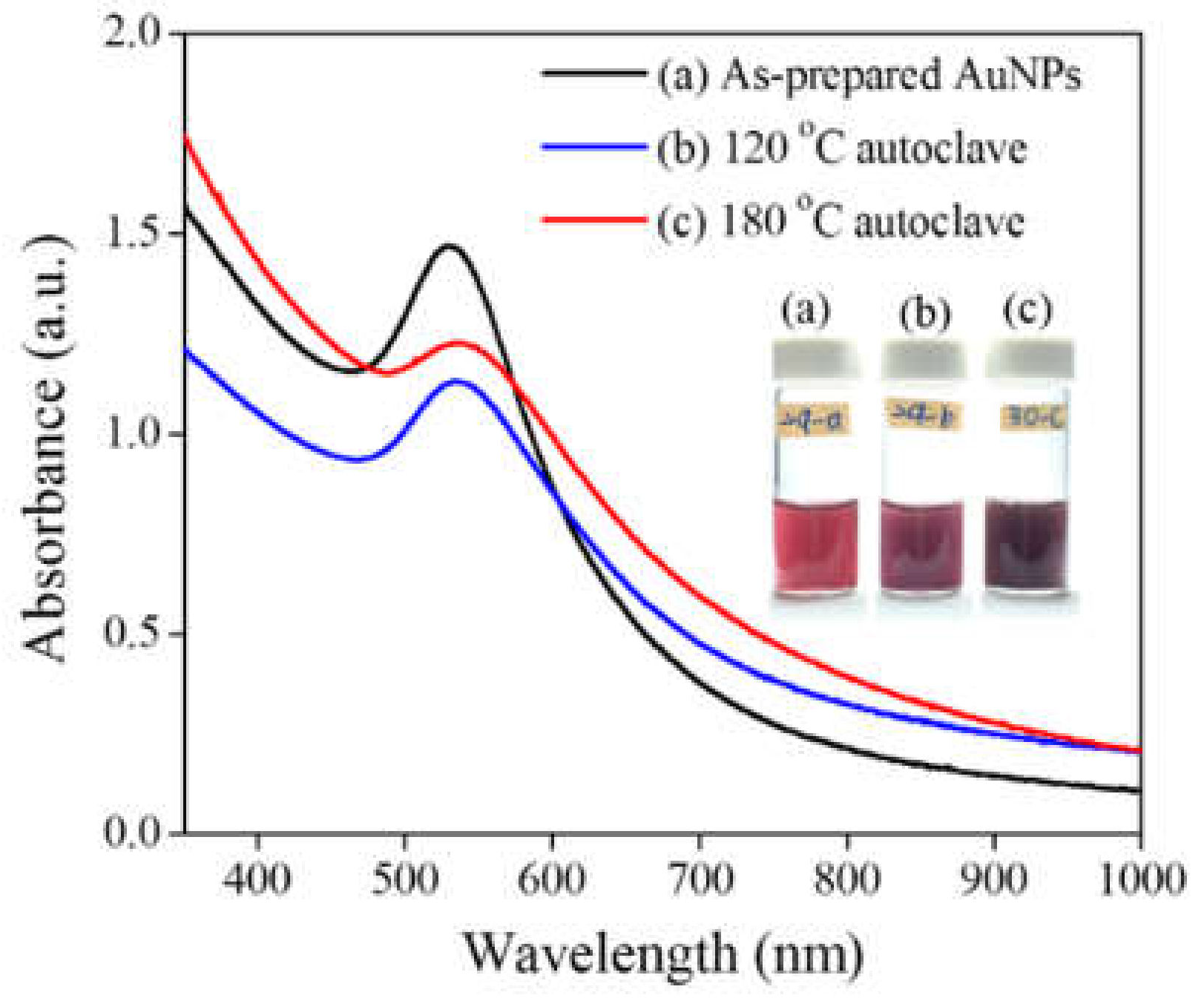
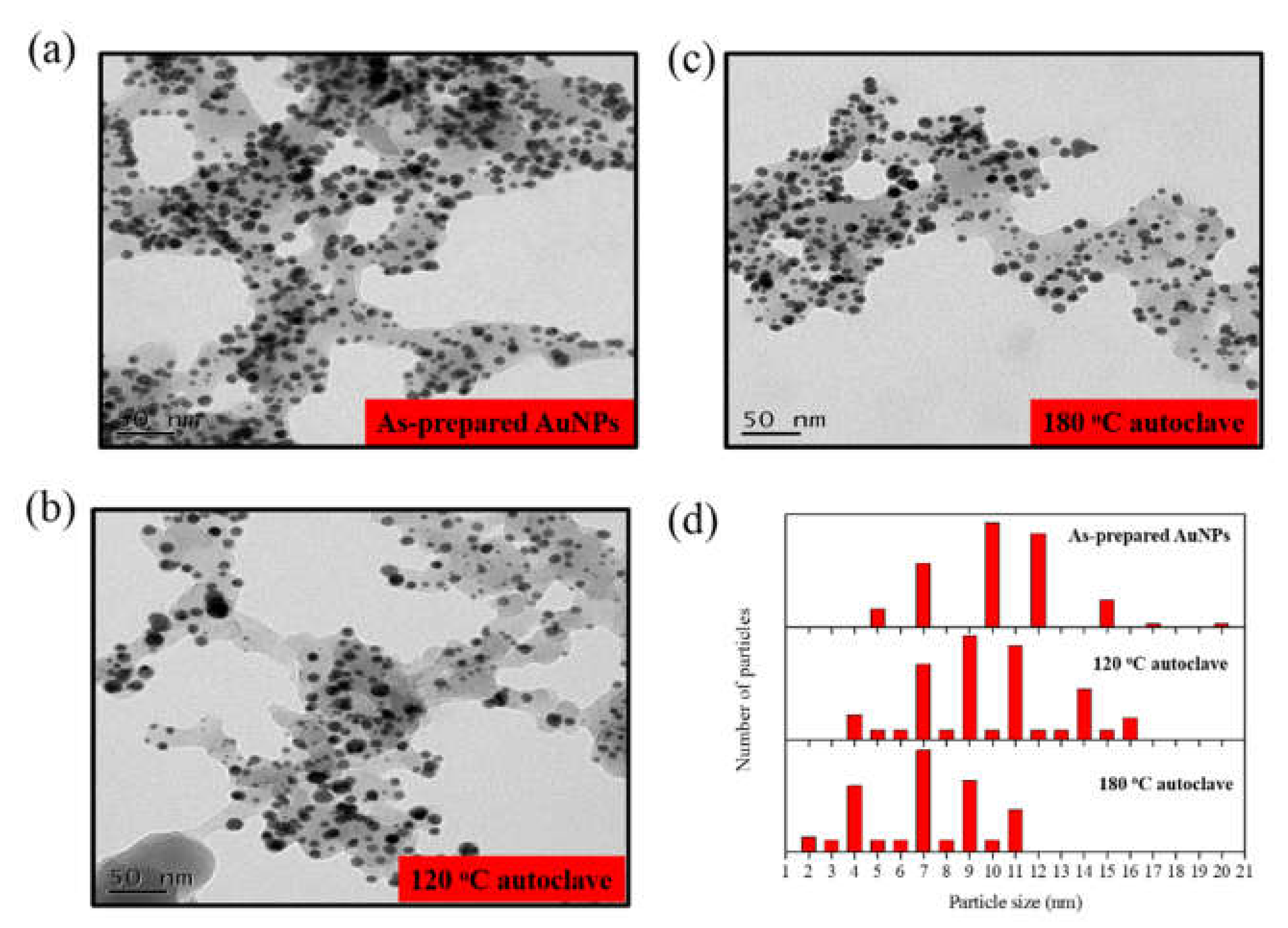
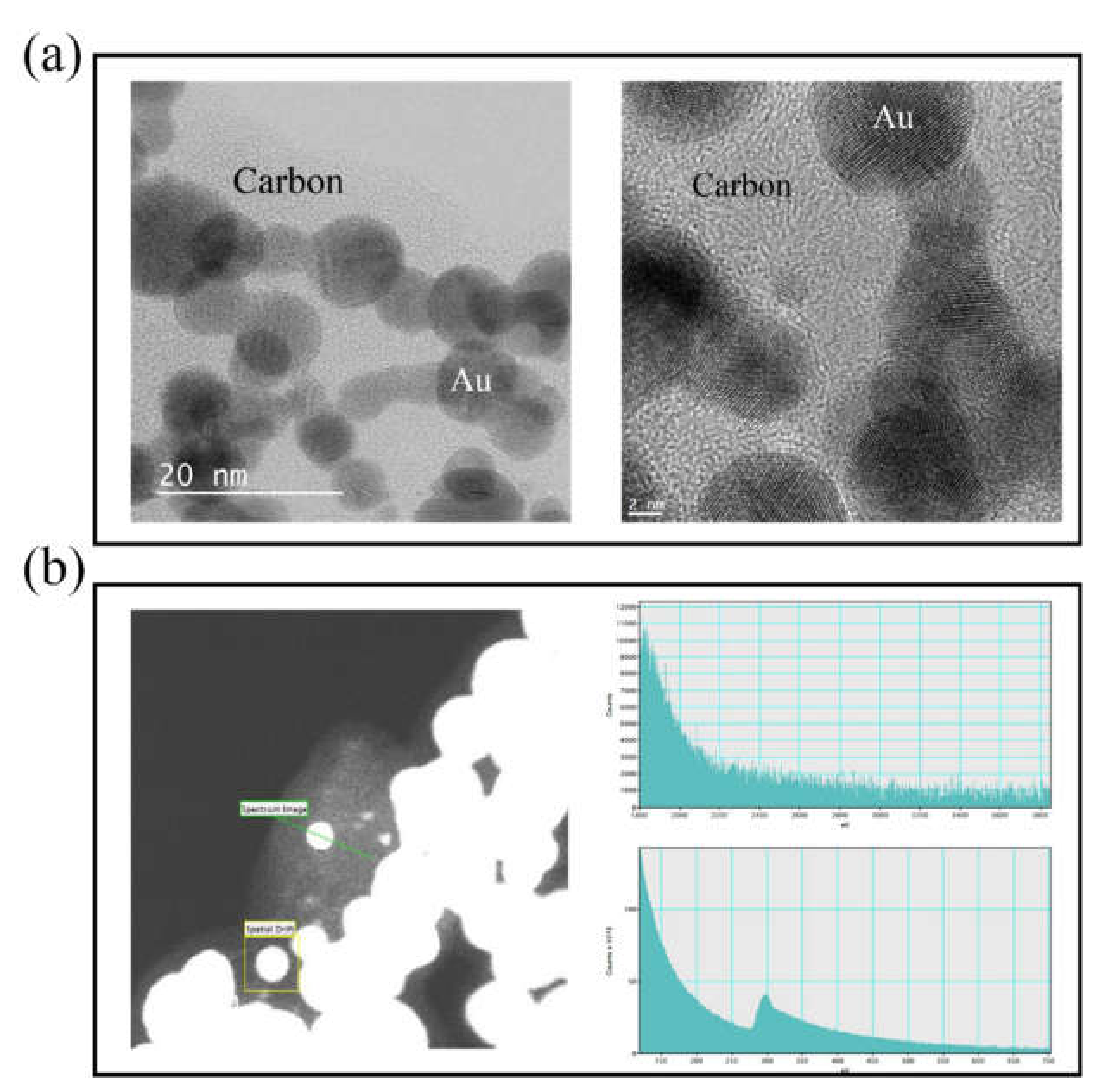
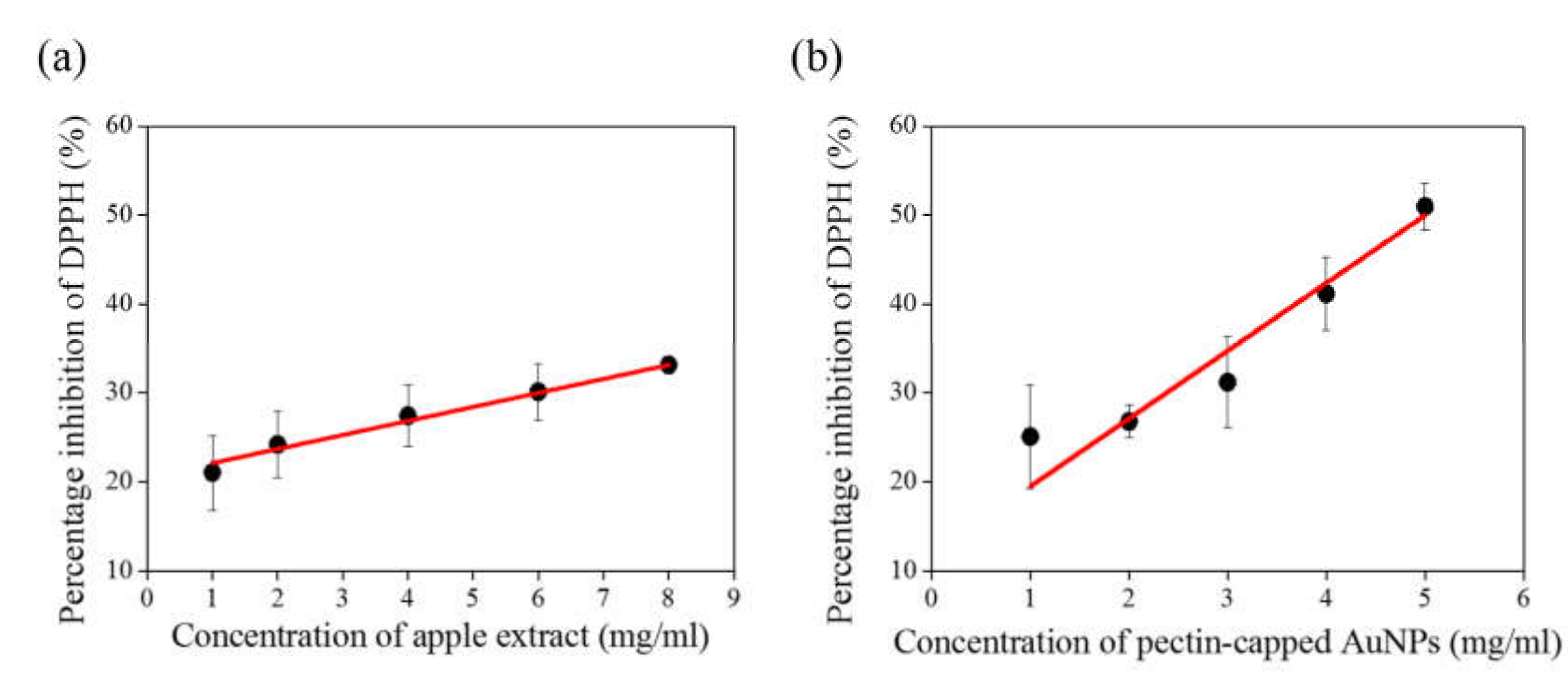
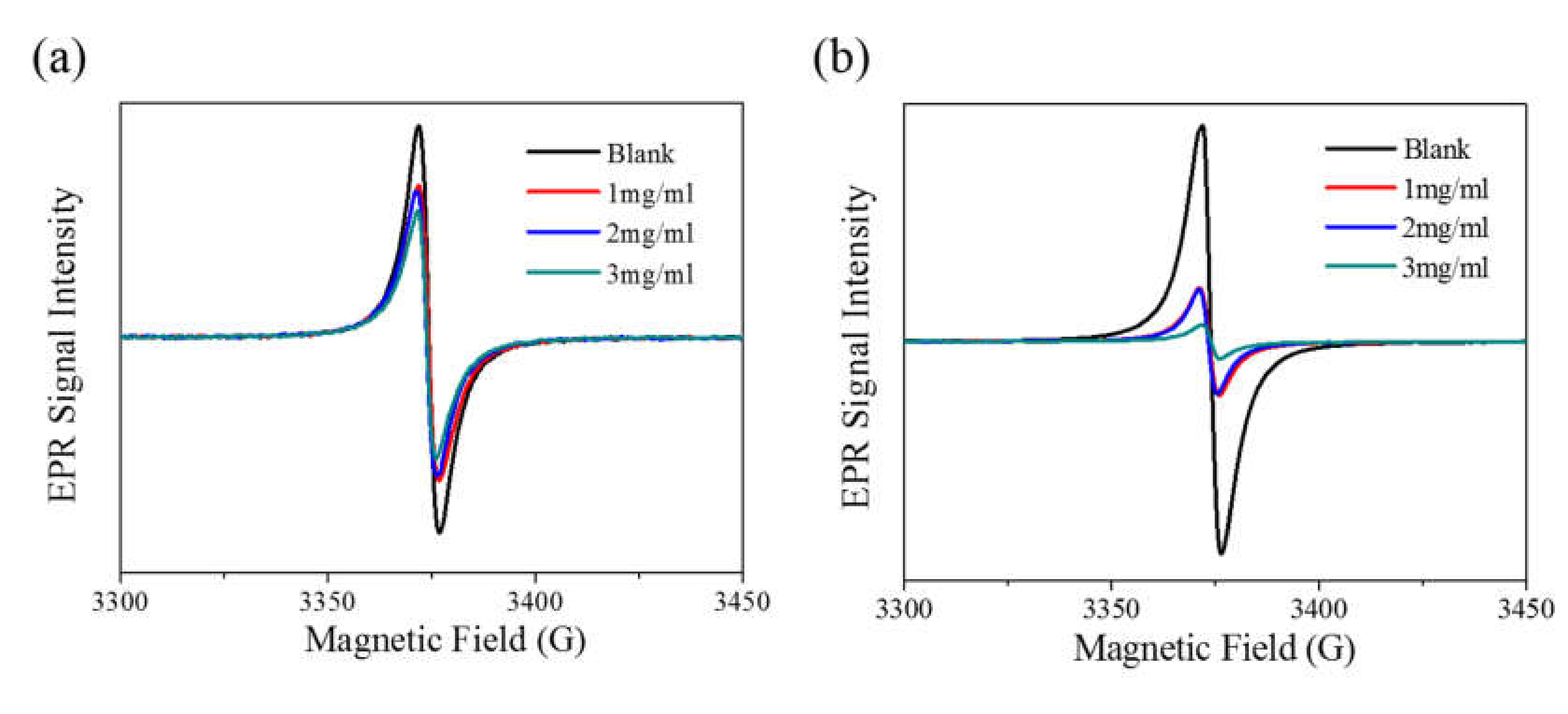
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teimuri-Mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-review. Nanochem. Res 2017, 2, 8–19. [Google Scholar]
- Madhusudhan, A.; Reddy, G.B.; Krishana, I.M. Chapter 4 Green Synthesis of Gold Nanoparticles by Using Natural Gums. Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 111–134. [Google Scholar]
- Vijayakumar, S. Eco-friendly synthesis of gold nanoparticles using fruit extracts and in vitro anticancer studies. J. Saudi Chem. Soc. 2019, 23, 753–761. [Google Scholar] [CrossRef]
- Devendiran, R.M.; Chinnaiyan, S.K.; Yadav, N.K.; Moorthy, G.K.; Ramanathan, G.; Singaravelu, S.; Sivagnanam, U.T.; Perumal, P.T. Green synthesis of folic acid-conjugated gold nanoparticles with pectin as reducing/stabilizing agent for cancer theranostics. RSC Adv. 2016, 6, 29757–29768. [Google Scholar] [CrossRef]
- Rimal Isaac, R.S.; Sakthivel, G.; Murthy, C.H. Green Synthesis of Gold and Silver Nanoparticles Using Averrhoa bilimbi Fruit Extract. J. Nanotechnol. 2013, 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.X.; Shameli, K.; Miyake, M.; Kuwano, N.; Ahmad Khairudin, N.B.B.; Mohamad, S.E.B.; Yew, Y.P. Green Synthesis of Gold Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Peels. J. Nanomater. 2016, 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta A 2013, 102, 15–23. [Google Scholar] [CrossRef]
- Sett, A.; Gadewar, M.; Sharma, P.; Deka, M.; Bora, U. Green synthesis of gold nanoparticles using aqueous extract of Dillenia indica. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025005. [Google Scholar] [CrossRef]
- Ramírez Castro, J.; García Hernández, L.; Ramírez Ortega, P.A.; Arenas Islas, D. Green synthesis of gold nanoparticles (AuNPs) by Cupressus goveniana extract. ECS Transact. 2018, 84, 207–215. [Google Scholar] [CrossRef]
- Vijaya Kumar, P.; Mary Jelastin Kala, S.; Prakash, K.S. Green synthesis of gold nanoparticles using Croton Caudatus Geisel leaf extract and their biological studies. Mater. Lett. 2019, 236, 19–22. [Google Scholar] [CrossRef]
- Shabestariana, H.; Homayouni-Tabrizib, M.; Soltanic, M.; Namvard, F.; Azizif, S.; Mohamadd, R.; Shabestarianb, H. Green Synthesis of Gold Nanoparticles Using Sumac Aqueous Extract and Their Antioxidant Activity. Mater. Res. 2017, 20, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Lal, S.; Nayak, P.L. Green synthesis of gold nanoparticles using various extract of plants and spices. IJSID 2012, 2, 325–350. [Google Scholar]
- Anjana, P.M.; Bindhu, M.R.; Rakhi, R.B. Green synthesized gold nanoparticle dispersed porous carbon composites for electrochemical energy storage. Mater. Sci. Energy Tech. 2019, 2, 389–395. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar]
- Darabdhara, G.; Das, M.R.; Singh, S.P.; Rengan, A.K.; Szunerits, S.; Boukherroub, R. Green one-pot synthesis of gold nanoparticles using Sansevieria roxburghiana leaf extract for the catalytic degradation of toxic organic pollutants. Mater. Res. Bull. 2019, 117, 18–27. [Google Scholar]
- Bogireddy, N.K.R.; Pal, U.; Martinez Gomezc, L.; Agarwal, V. Size controlled green synthesis of gold nanoparticles using Coffea arabica seed extract and their catalytic performance in 4-nitrophenol reduction. RSC Adv. 2018, 8, 24819. [Google Scholar] [CrossRef] [Green Version]
- Aljabali, A.A.A.; Akkam, Y.; Al-Zoubi, M.S.; Al-Batayneh, K.M.; Al-Trad, B.; Alrob, O.A.; Alkilany, A.M.; Benamara, M.; Evans, D.J. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus zizyphus and their Antimicrobial Activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golmoraj, V.E.; Khoshayand, M.R.; Amini, M.; Moghadamd, K.M.; Amin, G.; Shahverdi, A.R. The surface chemistry and stability of gold nanoparticles prepared using methanol extract of Eucalyptus camaldulensis. J. Exp. Nanosci. 2010, 6, 200–208. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.-M.; Palanisamy, S. One-Pot Green Synthesis of Graphene Nanosheets Encapsulated Gold Nanoparticles for Sensitive and Selective Detection of Dopamine. Sci. Rep. 2017, 7, 412213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Mohanta, B. Microwave-assisted green synthesis of Gold nanoparticles and its catalytic activity. Int. J. Nano Dimens. 2019, 10, 359–367. [Google Scholar]
- Liu, Y.; Kim, S.; Kim, Y.J.; Perumalsamy, H.; Lee, S.; Hwang, E.; Yi, T.-H. Green synthesis of gold nanoparticles using Euphrasia officinalis leaf extract to inhibit lipopolysaccharide-induced inflammation through NF-κB and JAK/STAT pathways in RAW 264.7 macrophages. Int. J. Nanomed. 2019, 14, 2945–2959. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Ng, H.P.; Xu, Y.; Li, Y.; Zheng, Y.; Yu, J.; Han, F.; Peng, F.; Fu, L. Gold Nanoparticles: Synthesis, Stability Test, and Application for the Rice Growth. J. Nanomater. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Vanitha Kumari, G.; JothiRajan, M.A.; Mathavan, T. Pectin functionalized gold nanoparticles towards singlet oxygen generation. Mater. Res. Express 2018, 5, 085027. [Google Scholar] [CrossRef]
- Veith, G.M.; Lupini, A.R.; Rashkeev, S.; Pennycook, S.J.; Mullins, D.R.; Schwartz, V.; Bridges, C.A.; Dudney, N.J. Thermal stability and catalytic activity of gold nanoparticles supported on silica. J. Catal. 2009, 262, 92–101. [Google Scholar] [CrossRef]
- Masoud, N.; Partsch, T.; de Jong, K.P.; de Jongh, P.E. Thermal stability of oxide-supported gold nanoparticles. Gold Bull. 2019, 52, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, A.; Yang, X.; Zhang, T.; Mou, C.-Y.; Su, D.-S.; Li, J. Synthesis of Thermally Stable and Highly Active Bimetallic Au-Ag Nanoparticles on Inert Supports. Chem. Mater. 2009, 21, 410–418. [Google Scholar] [CrossRef]
- Kim, M.; Shhn, K.; Na, H.B.; Hyeon, T. Synthesis of Nanorattles Composed of Gold Nanoparticles Encapsulated in Mesoporous Carbon and Polymer Shells. Nano Lett. 2002, 2, 1383–1387. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Gracio, J. Urface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Xu, D.; Lv, H.; Liu, B. Encapsulation of Metal Nanoparticle Catalysts within Mesoporous Zeolites and Their Enhanced Catalytic Performances: A Review. Front. Chem. 2018, 6, 550. [Google Scholar] [CrossRef]
- Xu, C.; Yang, D.; Mei, L.; Lu, B.; Chen, L.; Li, Q.; Zhu, H.; Wang, T. Encapsulating Gold Nanoparticles or Nanorods in Graphene Oxide Shells as a Novel Gene Vector. ACS Appl. Mater. Interfaces 2013, 5, 2715–2724. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Han, L.; Tu, B.; Zhao, D. One-pot synthesis of thermally stable gold@mesoporous silica core–shell nanospheres with catalytic activity. Nano Res. 2013, 6, 871–879. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.; Kim, H.; Hu, X.; Saito, N.; Choi, J.-H.; Lee, M.-H. In-situ one-step synthesis of carbon-encapsulated naked magnetic metal nanoparticles conduced with additional reductants and agents. Sci. Rep. 2016, 6, 38652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gerhauser, C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Med. 2008, 13, 1608–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-W.; Wang, J.-Y.; Hsiao, V.K.S.; Chu, C.-C. “Green” Synthesis and Antioxidant Activity of Thermally Stable Gold Nanoparticles Encapsulated in Carbon Nanosheets. Appl. Sci. 2020, 10, 2272. https://doi.org/10.3390/app10072272
Lin H-W, Wang J-Y, Hsiao VKS, Chu C-C. “Green” Synthesis and Antioxidant Activity of Thermally Stable Gold Nanoparticles Encapsulated in Carbon Nanosheets. Applied Sciences. 2020; 10(7):2272. https://doi.org/10.3390/app10072272
Chicago/Turabian StyleLin, Hui-Wen, Jia-Yi Wang, Vincent K. S. Hsiao, and Chih-Chien Chu. 2020. "“Green” Synthesis and Antioxidant Activity of Thermally Stable Gold Nanoparticles Encapsulated in Carbon Nanosheets" Applied Sciences 10, no. 7: 2272. https://doi.org/10.3390/app10072272
APA StyleLin, H.-W., Wang, J.-Y., Hsiao, V. K. S., & Chu, C.-C. (2020). “Green” Synthesis and Antioxidant Activity of Thermally Stable Gold Nanoparticles Encapsulated in Carbon Nanosheets. Applied Sciences, 10(7), 2272. https://doi.org/10.3390/app10072272





