Abstract
The green synthesis of silica has been extensively explored over the last few decades, as silica compounds found in commercial products can cause negative effects on human health. This calls for alternative ways to produce silica that are safer, cheaper and more environmentally friendly. Some of the agricultural wastes proven to contain silica include rice husk, sugarcane bagasse, coconut shells and coconut husk. This paper describes the synthesis of silica from coconut husk waste, and its physical and optical properties for potential utilization in optical applications. Coconut husk was subjected to fire at 500–700 °C so as to form coconut husk ash (CHA), and was then treated with sulfuric acid to extract silica from the ash. Most of the weight degradation subsequently occurred at temperatures from 221 to 360 °C. X-ray fluorescence (XRF) analysis proved that 91.76% of the silica was obtained, while major peaks on the X-ray diffraction (XRD) spectrum were observed after the acid treatment. Chemical bonds such as Si-O-Si, CH2, -OH and Si-OH were found in the spectrum of the Fourier transform infrared spectroscopy (FTIR). Furthermore, the particles displayed rod-like shapes and irregular sizes, but the particle with sizes ranging from 200–750 nm decreased after the acid treatment. The relationship between the absorption coefficient and photon energy was obtained by finding the optical energy gap, which was found to be 4.3 eV. These data points provide critical information when used in optical applications. The overall studies show that synthesized silica has great potential for use in optical field applications.
1. Introduction
Green synthesis is defined as an alternative synthesis method for substances that offers a reduced negative impact on the environment. Plants are known as nature’s “chemical factories”. Therefore, out of all of the biological alternatives present, plants and plant extracts seem to be the best option. They are cost efficient and low maintenance. Materials such as silver [1,2,3,4], gold [5,6], titanium [7] and silica [8,9,10,11,12,13,14,15,16,17] can be extracted from agricultural products. Furthermore, plant-mediated nanoparticles are preferred, as they are cost effective, environmentally friendly and safe for therapeutic use in humans [1,6]. The extraction of raw materials from agricultural wastes is considered to be green synthesis, as these waste products are otherwise left unused and cause pollution if not treated properly.
Silica is one of the most abundant elements found in the world. It can exist in gel, crystalline or even in amorphous forms in nature, depending on the way it is produced [13]. The standard form of crystalline silica found in nature is quartz. Silica has been found to be a versatile material, and is heavily utilized in various fields and for various applications, such as in petroleum refining, ceramics, anticorrosion agents, cosmetics products and as a catalyst in chemical reactions [10,12,18]. The use of commercialized high purity silica is quite costly, and is unsuitable in most industrial applications because of its high melting point [19,20]. Therefore, lots of methods have been explored to synthesize the raw materials of SiO2, as they are suitable for various applications and are cost-efficient at the same time.
Several past studies have proven that silica can be extracted from various agricultural wastes, such as rice husk, rice straw, sugarcane bagasse, corn cob and palm ash [8,11,12,13,15,16,17,21,22,23,24,25,26,27,28]. However, information regarding its synthesis from coconut husk waste is limited. This work can be considered novel, because there are only a few published analyses related to coconut husk ash (CHA) being used as a source of silica. Therefore, it is sensible to add a more extensive analysis to this work in order to realize the full potential of CHA when used as source of silica.
Generally, coconuts are consumed worldwide as food, drink or as other products. The husks are typically discarded as a waste material, either by burning or disposal in waste disposal sites. Without proper treatment or disposal method, these waste materials will result in various environmental problems that can lead to a multitude of health issues. In this study, the silica extracted from CHA was originally planned to be used as a silica substitute for the preparation of a ZnO-SiO2 composite. The ZnO-SiO2 composite is a versatile material and is often used in optical applications. The physical and optical properties of these materials were explored accordingly.
2. Materials and Methods
Coconut husk waste collected from local stores was cleaned with normal tap water and dried in an electrical oven to remove impurities and moisture. The fibers of the husks were manually separated from the coconut fruit, and approximately 10 g of the fibers were placed in an alumina crucible. After that, they were subjected to fire at 500–700 °C for 2 h under a constant heating rate of 10 °C. The coconut husk ash (CHA) obtained was cooled down inside an electrical furnace and collected for acid treatment. The CHA was added to 20 mL of 5 N (normality) sulfuric acid in a beaker. After that, the mixture was heated up to 50 °C, and continuously stirred for 1 h at a speed of 300 r.p.m. using a hotplate magnetic stirrer under a fume hood. After that, the filtration process was carried out using Whatmann No. 41 ashless filter paper, whereby distilled water was used three times to remove excess acid in the residue. The treated CHA was then characterized to determine its properties for potential glass-ceramics applications. A quantitative analysis of the sample’s chemical compositions was carried out using an X-ray fluorescence (XRF) XRF-EDX-720/7000 (Shimadzu, Kyoto, Japan) machine. Thermographic analysis (TGA) and difference thermogravimetry (DTG) were implemented using a Mettler Toledo TGA/DSC 1HT (Mettler Toledo, Küsnacht, Switzerland) in order to measure the rate of mass loss over the temperature of the coconut husk. The TGA was carried out in the presence of oxygen gas, with a constant heating rate of 10 °C/min. The FTIR analysis was conducted using a Thermo Nicolet Nexus 47 FT-IR (ThemoFisher Scientific, Waltham, MA, USA) in a common range of 280 to 4000 cm−1. Then, the phase identification of the samples was carried by using an X-ray diffraction (XRD) Phillips X’Pert High Pro PANanalytical Diffractometer (Malvern Pananalytical, Almelo (The Netherlands) and Malvern (UK)), with a data range of 2θ from 20° to 80°, while a field emission scanning electron microscopy (FESEM) Nova NanoSEM 30 (FEI, Hillsboro, OR, USA) was used to analyze the morphological structure of the samples. Meanwhile, the particle size of the samples was determined using a particle size analyzer (PSA) Malvern Instrument Zetasizer Nano S (Malvern Pananalytical, Almelo (The Netherlands) and Malvern (UK)). In this study, UV-3600 Shimadzu (Shimadzu, Kyoto, Japan) was used in the UV-VIS analysis, and the absorption edge observed in the spectrum was used to calculate the optical band gap for the materials. The overall methodology described is shown in the schematic diagram in Figure 1.
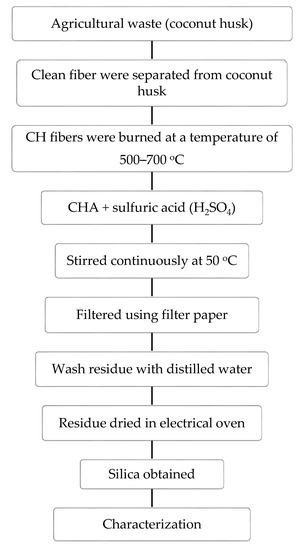
Figure 1.
Schematic diagram of methodology of silica extraction from coconut husk. CHA—coconut husk ash.
3. Results
TGA was used to determine the thermal stability of the coconut husk. The thermographic curve was plotted as shown in Figure 2, which indicates the weight loss percentage of the coconut husk against the temperature ranged between 25 to 700 °C. The obtained curves show three stages of coconut husk weight decomposition, whereby most of the weight degradation occurred at temperatures between 221 and 360 °C. The first stage of weight loss occurred below a temperature of 200 °C, which was mainly due to the evaporation of the moisture present in the coconut husk. About 7.77% of the coconut husk total weight was lost during the first stage. Then, the second stage of rapid mass degradation occurred at temperatures between 200 and 280 °C. The weight loss percentage was about 38.44%, which was due to the decomposition of organic compounds (e.g., hemicellulose). Finally, the third stage of decomposition depicted a weight loss of 47.54% at temperatures between 280 and 365 °C, due to rapid cellulose decomposition. The maximum weight loss rate was observed at a temperature of 365 °C. Following the third stage, occurring between temperatures ranging from 365 to 700 °C, no obvious degradation in weight was seen, as most of the volatiles were already pyrolyzed, while the remaining materials were converted to char and gases. The data obtained using TGA/DTG show a good agreement with previous studies [29,30,31,32,33,34]. The remaining 5.67% was the product’s ash, also known as coconut husk ash (CHA).
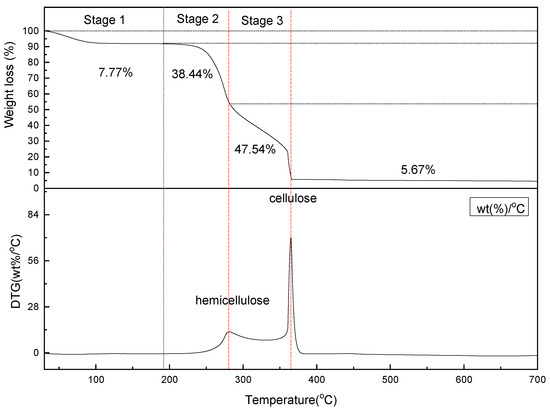
Figure 2.
Thermographic analysis curve of coconut husk. DTG—difference thermogravimetry.
A quantitative analysis of the elements was carried out by XRF analysis. The elemental compositions of the CHA were found to contain several elements, such as SiO2, K2O, Al2O3, SO3, Fe2O3, P2O5, Cl and other elements, which are naturally formed in the soil. The value of silica in CHA showed no consistency alongside the increased temperature, as silica deposition in agricultural waste is totally dependent on plants and the soil itself. For example, the amount of silica found in rice husk was around ~90% [13,16], while its content in CHA ranged from ~8% to 11% [9]. When produced in large quantities, CHA can be a potential source of silica production. The percentage of other elements, except for SiO2, decreased after the acid treatment process with sulfuric acid, which was due to the reaction with the acid, which dissolved the other elements. The metal impurities were eliminated from the ash via a chelate reaction between the sulfuric acid and the metal ions, turning them into water-soluble compounds [35]. SiO2 is a very weakly acidic oxide capable of reacting with strong bases (e.g., NaOH), and it does not react with water. Because of this behavior of SiO2, the acid treatment method was used to extract the silica from the CHA [9]. The composition of CHA is shown in Table 1.

Table 1.
Elemental composition of CHA at different conditions.
Based on the TGA and XRF analyses, the yield of SiO2 can be calculated. For every 1000 g of coconut husk used, about 5.67% ash was obtained. Theoretically, about 56.7 g of CHA can be obtained for every 1000 g of coconut husk after pyrolysis. From the XRF analysis, about 8–11% SiO2 was found in the CHA. Therefore, about 4.5–6.2 g of SiO2 can be obtained from CHA, with 91.76% purity, after acid treatment.
The crystalline or amorphous nature of the samples was analyzed using XRD. Figure 3 depicts the XRD spectra of the CHA plotted from the data obtained using X’Pert Highscore Plus (Malvern Pananalytical, Almelo (The Netherlands) and Malvern (UK)). From the spectra, the samples were likely to be in a crystalline form, as a result of the presence of many diffraction peaks in the spectra. Several peaks were found, and were identified as P2O5, Cl, SO3 and SiO2, on CHA at 500–700 °C. The peaks that corresponded to silica (SiO2) could be found on each spectrum (Figure 3a–c), but were not the major peaks found in the sample, according to the spectra. This was due to the low percentage of SiO2 present in the CHA, agreeing with the XRF analysis in Table 1. However, after acid treatment (Figure 3d), the major diffraction peaks of SiO2 could be clearly observed at 2θ = 20.76°, 23.41°, 35.98° and 43.33°, alongside a few peaks of K2O and Al2O3. All of the peaks indicated that SiO2 was still in a crystalline form after the acid treatment, as there was no hump on the spectrum, which would indicate a glass (amorphous) nature. The silica became more apparent in the spectrum because of the other metallic oxides in the sample that dissolved upon reacting with the sulfuric acid. The SiO2 peaks found were described as tridymite (JCPDS no. 042-1401), with an orthorhombic structure and parameters of a = 17.0839 Å, b = 9.9313 Å and c = 16.3041 Å. These values further validated the data obtained from the XRF analysis, which showed the SiO2 composition to be the major constituent in these samples after the acid treatment.
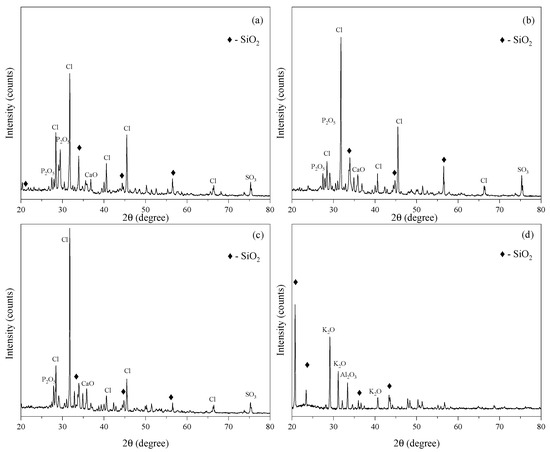
Figure 3.
XRD spectra of CHA at: (a) 500 °C, (b) 600 °C, (c) 700 °C and (d) after acid treatment.
Figure 4 shows the FESEM images of CHA at a magnification of 25,000. The rod-like structure can be observed from the images of the 500, 600 and 700 °C treatment, which signifies that this CHA had a high crystallinity compared with the CHA (in Figure 5d) after the acid treatment. The CHA was observed to have irregular sizes according to the images, thus indicating that in a temperature range of 500–700 °C, the temperature did not affect the size of the elements in the CHA. On the contrary, the CHA became slightly smaller in size after the acid treatment, as shown in Figure 4d, which indicates that the acid treatment decreased the particle sizes of the CHA.
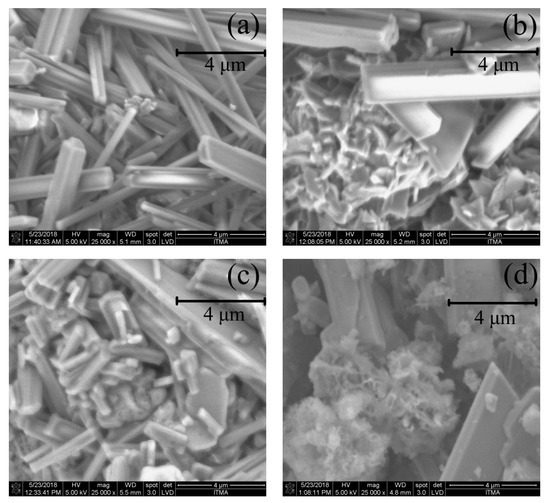
Figure 4.
FESEM images of CHA at: (a) 500, (b) 600 and (c) 700 °C, and (d) after acid.
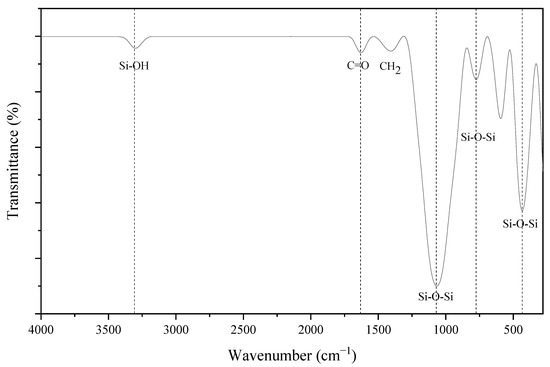
Figure 5.
FTIR spectrum of the CHA after treatment.
The bonding of elements inside the samples was identified by the FTIR analysis, which was operated at room temperature with a wavelength range of 250–4000 cm−1. Figure 5 presents the FTIR spectrum of the CHA after the acid treatment. The band of the silanol group (Si-OH) bonding was found at 3300 cm−1 [11,12,36]. Then, a band located at 1628 cm−1 corresponding to the C=O stretching, which might be attributed to the hemicellulose and lignin aromatic groups [37], and a band located at 1407 cm−1, identified as the CH2 strain of the cellulose in the CHA, were found [38]. Furthermore, a strong band at 1066 cm−1 indicated Si-O-Si stretching vibration [8,11,12,39]. Meanwhile, the band at the wavelength of 788 cm−1 was due to the Si-O-Si bond stretching, while at 432 cm−1, it was due to the bending vibration of the same functional group [8,11]. Hence, the presence of bands at 1066, 788 and 432 cm−1 confirmed the formation of silica following the acid treatment method using CHA, and is tabulated in Table 2.

Table 2.
Functional group in the CHA after treatment.
Nanosize 30 was used in this study to determine the average particle size. The graph of the particle size distribution for CHA is shown in Figure 6. Based on the graph, the average particle size distributions were found to be in the range of 500–750, 270–560, 360–740 and 200–410 nm for 500, 600 and 700 °C, and after acid treatment, respectively. With the increase in temperature, no obvious pattern was observed in the average particle size distributions of the CHA. In contrast, the average particle sizes decreased to 200–410 nm after the acid treatment, which was due to the reaction with sulfuric acid, which caused the particles to become finer and smaller [9,40]. This occurrence may also be caused by the reaction between sulfuric acid and CHA, which dissolved the bigger-sized elements in the ash and caused the distribution of the average particles to become smaller. The data obtained are also in line with the FESEM images, where the size distribution decreased after the acid treatment.
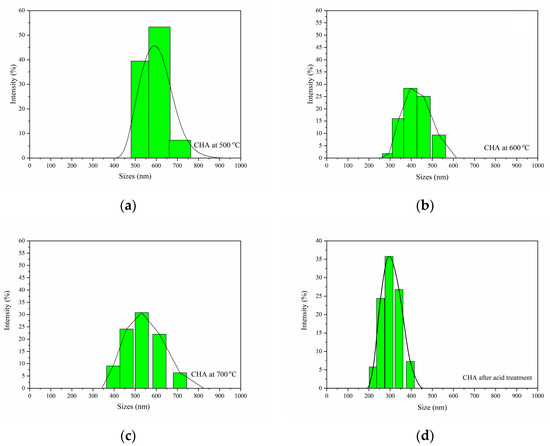
Figure 6.
Particle size distribution of CHA at: (a) 500, (b) 600 and (c) 700 °C, and (d) acid treatment.
The UV-VIS spectroscopy of CHA samples was performed at room temperature in the spectral region of 220–800 nm in order to determine the optical absorbance of the sample. Figure 7 illustrates the absorption curves of the CHA at 500, 600 and 700 °C. The absorption region displayed a broad band in the UV region below 350 nm for all of the spectra, except for 500 °C. Based on the absorbance spectrum, the valence–conduction band transition of the sample could be determined, while its optical band gap could be calculated based on the absorption edge. Basically, the measurement of the optical band gap can be used to describe to the nature of the materials. The principle of the absorbance spectroscopy is based on the Beer–Lambert law, whereby the relation between the absorption coefficient, α and photon energy (hv) is used to plot a graph so as to find the optical band gap [41].
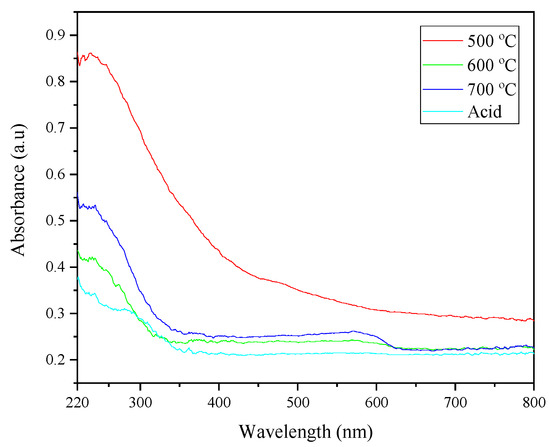
Figure 7.
Absorbance spectra of CHA under different conditions.
Inserting n = ½ allows for a direct transition, as follows:
where ν is the frequency, h is the Planck’s constant, k is proportional constant, exponent n is the type of transition that allowed for the transition (n = 1/2) and E is the optical energy band gap.
The optical band graph can be found by extrapolating the linear portion of the optical absorption curve to the x-intercept. From the plotted graph, as in Figure 8, the optical band gaps of the CHA were 3.50, 4.15 and 4.05 eV for 500, 600 and 700 °C, respectively. After acid treatment, the sample of CHA showed a slightly higher band gap of 4.30 eV in comparison with the other samples. This suggests that the presence of oxide impurities (approximately 50%) in CHA lowers the optical band gap of the samples. Silica is an insulator in nature, with a wide band gap found to be greater than 6 eV by several studies [42,43,44]. However, after the acid treatment, there were still up to 8.24% impurities according to the XRF data, and this caused a disturbance in the treated CHA structure and caused the optical band gap value to become lower when compared with pure silica.
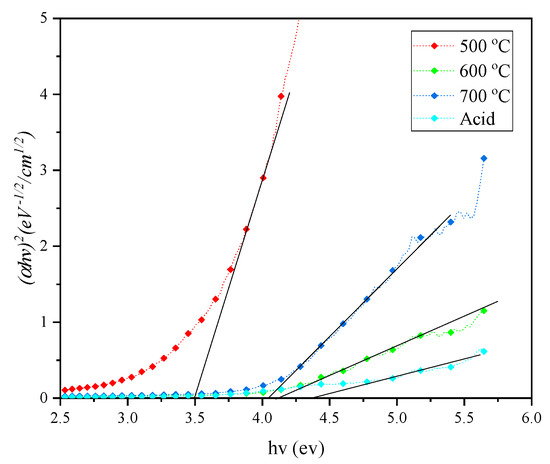
Figure 8.
Optical band gap of CHA under different conditions.
4. Conclusions
The silica particles were successfully synthesized using the green synthesis method. Most of the weight degradation of the coconut husk occurred at a temperature between 221–360 °C. Meanwhile, the major peaks of the orthorhombic tridymite (silica) were found based on the XRD spectra after acid treatment, whereas the bands found on the FTIR spectrum indicated the formation of silica in the samples. Moreover, about 90% of the SiO2 was extracted using the acid treatment method on the CHA. The particle size distribution was around 200–750 nm, which decreased to 200–410 nm after the acid treatment. In contrast, based on the absorption spectrum, the optical band gap of the treated CHA was found to be 4.3 eV after being calculated using the Beer–Lambert law.
Author Contributions
M.F.A. designed this paper and performed the numerical investigations; Y.W.F. helped by supervising the study, and reviewing and editing the manuscript. K.A.M. and M.H.M.Z. helped with the design of the methodology. R.E.M.K. helped with the investigation and the methodology. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by Malaysian Ministry of Education (MOE) and the Universiti Putra Malaysia (UPM) through the Putra Grant 9642800. The fund was used for purchasing materials, apparatus and the characterization process fee.
Acknowledgments
The authors gratefully acknowledge the financial support for this study from the Malaysian Ministry of Education (MOE) and the Universiti Putra Malaysia (UPM) through the Putra Grant. The laboratory facilities provided by the Institute of Advanced Technology and Faculty of Science, Universiti Putra Malaysia, are also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Kumar, R.; Roopan, S.M.; Prabhakarn, A.; Khanna, V.G.; Chakroborty, S. Agricultural waste Annona squamosa peel extract: Biosynthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 173–176. [Google Scholar] [CrossRef]
- Al-Kawaz, H.S.; Al-Mashhedy, L.A.M. Green synthesis of silver nanoparticles using Actinidia deliciosa extracts. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2212–2223. [Google Scholar]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Sci. Iran. 2013, 20, 1049–1054. [Google Scholar]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Abdul, J.; Nuaman, R.; Aham, A. Biological synthesis of Titanium Dioxide nanoparticles by Curcuma longa plant extract and study its biological properties. World Sci. 2016, 49, 204–222. [Google Scholar]
- Okoronkwo, E.A.; Imoisili, P.E.; Olubayode, S.A.; Olusunle, S.O.O. Development of Silica Nanoparticle from Corn Cob Ash. Adv. Nanopart. 2016, 5, 135–139. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and structural properties of coconut husk as potential silica source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Mupa, M.; Hungwe, C.B.; Witzleben, S.; Mahamadi, C.; Muchanyereyi, N. Extraction of silica gel from Sorghum bicolour (L.) moench bagasse ash. Afr. J. Pure Appl. Chem. 2015, 9, 12–17. [Google Scholar] [CrossRef]
- Mor, S.; Manchanda, C.K.; Kansal, S.K.; Ravindra, K. Nanosilica extraction from processed agricultural residue using green technology. J. Clean. Prod. 2017, 143, 1284–1290. [Google Scholar] [CrossRef]
- Vaibhav, V.; Vijayalakshmi, U.; Roopan, S.M. Agricultural waste as a source for the production of silica nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 139, 515–520. [Google Scholar] [CrossRef]
- Todkar, B.S.; Deorukhkar, O.A.; Deshmukh, S.M. Extraction of Silica from Rice Husk. Int. J. Eng. Res. Dev. 2016, 12, 69–74. [Google Scholar]
- Chakraverty, A.; Mishra, P.; Banerjee, H.D. Investigation of combustion of raw and acid-leached rice husk for production of pure amorphous white silica. J. Mater. Sci. 1988, 23, 21–24. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Wilayat Husain, S. Towards tunable size of silica particles from rice husk. J. Non. Cryst. Solids 2015, 429, 61–69. [Google Scholar] [CrossRef]
- Della, V.P.; Kühn, I.; Hotza, D. Rice husk ash as an alternate source for active silica production. Mater. Lett. 2002, 57, 818–821. [Google Scholar] [CrossRef]
- Worathanakul, P.; Payubnop, W.; Muangpet, A. Characterization for Post-treatment Effect of Bagasse Ash for Silica Extraction. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2009, 3, 339–341. [Google Scholar]
- Vadery, V.; Narayanan, B.N.; Ramakrishnan, R.M.; Kochiyil, S.; Sugunan, S.; Narayanan, D.P. Room temperature production of jatropha biodiesel over coconut husk ash. Energy 2014, 70, 588–594. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M.; Norhafizah, M.R.; Nurzilla, M.; Zamratul, M.I.M. Synthesis and optical properties of europium doped zinc silicate prepared using low cost solid state reaction method. J. Mater. Sci. Mater. Electron. 2016, 27, 1092–1099. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A. Photoluminescence properties of Eu3+-doped low cost zinc silicate based glass ceramics. Optik 2016, 127, 3727–3729. [Google Scholar] [CrossRef]
- Pa, F.C.; Chik, A.; Bari, M.F. Palm Ash as an Alternative Source for Silica Production. MATEC Web Conf. 2016, 78, 01062. [Google Scholar] [CrossRef]
- Norsuraya, S.; Fazlena, H.; Norhasyimi, R. Sugarcane bagasse as a renewable source of silica to synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Eng. 2016, 148, 839–846. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Lee, K.J.; Cho, M.; Lim, S.S.; Seo, S.K.; Cho, K.M.; Bang, K.S.; Oh, B.T. Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method. J. Ind. Eng. Chem. 2015, 29, 298–303. [Google Scholar] [CrossRef]
- Matori, K.A.; Haslinawati, M.M.; Wahab, Z.A.; Ban, T.K. Producing Amorphous White Silica from Rice Husk. J. Basic Appl. Sci. 2009, 1, 512–515. [Google Scholar]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Optical band gap and photoluminescence studies of Eu 3+ -doped zinc silicate derived from waste rice husks. Optik 2019, 182, 486–495. [Google Scholar] [CrossRef]
- Wahab, S.A.A.; Matori, K.A.; Aziz, S.H.A.; Zaid, M.H.M.; Kechik, M.M.A.; Azman, A.Z.K.; Khaidir, R.E.M.; Khiri, M.Z.A.; Effendy, N. Synthesis of cobalt oxide Co3O4 doped zinc silicate based glass-ceramic derived for LED applications. Optik 2019, 179, 919–926. [Google Scholar] [CrossRef]
- Agbagla-Dohnani, A.; Nozie, P.; Cle, G. In sacco degradability, chemical and morphological composition of 15 varieties of European rice straw. Anim. Feed Sci. Technol. 2001, 94, 15–27. [Google Scholar] [CrossRef]
- Hessien, M.M.; Rashad, M.M.; Zaky, R.R.; Abdel-aal, E.A.; El-barawy, K.A. Controlling the synthesis conditions for silica nanosphere from semi-burned rice straw. Mater. Sci. Eng. B 2009, 162, 14–21. [Google Scholar] [CrossRef]
- Alias, N.; Ibrahim, N.; Hamid, M.K.A.; Hasbullah, H.; Ali, R.R.; Sadikin, A.N.; Asli, U.A. Thermogravimetric Analysis of Rice Husk and Coconut Pulp for Potential Biofuel Production by Flash Pyrolysis. Malays. J. Anal. Sci. 2014, 18, 705–710. [Google Scholar]
- Kausar, A.; Bhatti, H.N.; MacKinnon, G. Equilibrium, kinetic and thermodynamic studies on the removal of U(VI) by low cost agricultural waste. Colloids Surf. B Biointerfaces 2013, 111, 124–133. [Google Scholar] [CrossRef]
- Khan, G.M.A.; Alam, S.; Arifuzzaman Khan, G.M.; Alam, M.S.; Terano, M. Thermal characterization of chemically treated coconut husk fibre. Indian J. Fibre Text. Res. 2012, 37, 20–26. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Yarmo, M.A.; Sopian, K. Effect of Chemical Treatments on Rice Husk (RH) Water Absorption Property. Int. J. Chem. Eng. Appl. 2015, 6, 273–276. [Google Scholar] [CrossRef]
- Chen, W.H.; Lu, K.M.; Tsai, C.M. An experimental analysis on property and structure variations of agricultural wastes undergoing torrefaction. Appl. Energy 2012, 100, 318–325. [Google Scholar] [CrossRef]
- Umeda, J.; Kondoh, K. High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J. Mater. Sci. 2008, 43, 7084–7090. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N.; Praveen, L. Effect of organic acid treatment on the properties of rice husk silica. J. Mater. Sci. 2005, 40, 6535–6544. [Google Scholar] [CrossRef]
- Bansal, M.; Garg, U.; Singh, D.; Garg, V.K. Removal of Cr(VI) from aqueous solutions using pre-consumer processing agricultural waste: A case study of rice husk. J. Hazard. Mater. 2009, 162, 312–320. [Google Scholar] [CrossRef]
- Marin, D.C.; Vecchio, A.; Ludueña, L.N.; Fasce, D.; Alvarez, V.A.; Stefani, P.M. Revalorization of rice husk waste as a source of cellulose and silica. Fibers Polym. 2015, 16, 285–293. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Characterization of adsorbent developed from rice husk: Effect of surface functional group on phenol adsorption. J. Appl. Sci. 2010, 10, 1060–1067. [Google Scholar] [CrossRef]
- Faizul, C.P.; Chik, A.; Bari, M.F.; Noorina, H.J. Extraction of Silica from Palm Ash Using Organic Acid Leaching Treatment. Key Eng. Mater. 2013, 594–595, 329–333. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–636. [Google Scholar] [CrossRef]
- Calabrese, E.; Fowler, W.B. Electronic energy-band structure of a quartz. Phys. Rev. B 1978, 18, 2888–2896. [Google Scholar] [CrossRef]
- Chelikowsky, R.J.; Schluter, M. Electron states in a~quartz: A self-consistent pseufiopotential calculation. Phys. Rev. B 1977, 15, 4020–4029. [Google Scholar] [CrossRef]
- Ramos, L.E.; Furthmuller, J.; Bechstedt, F. Quasiparticle band structures and optical spectra of B-cristobalite SiO2. Phys. Rev. B 2004, 69, 1–8. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).