Selection of the Appropriate Phase Change Material for Two Innovative Compact Energy Storage Systems in Residential Buildings
Abstract
Featured Application
Abstract
1. Introduction
2. Methodology
2.1. Description of the Application
2.2. PCM Selection Methodology
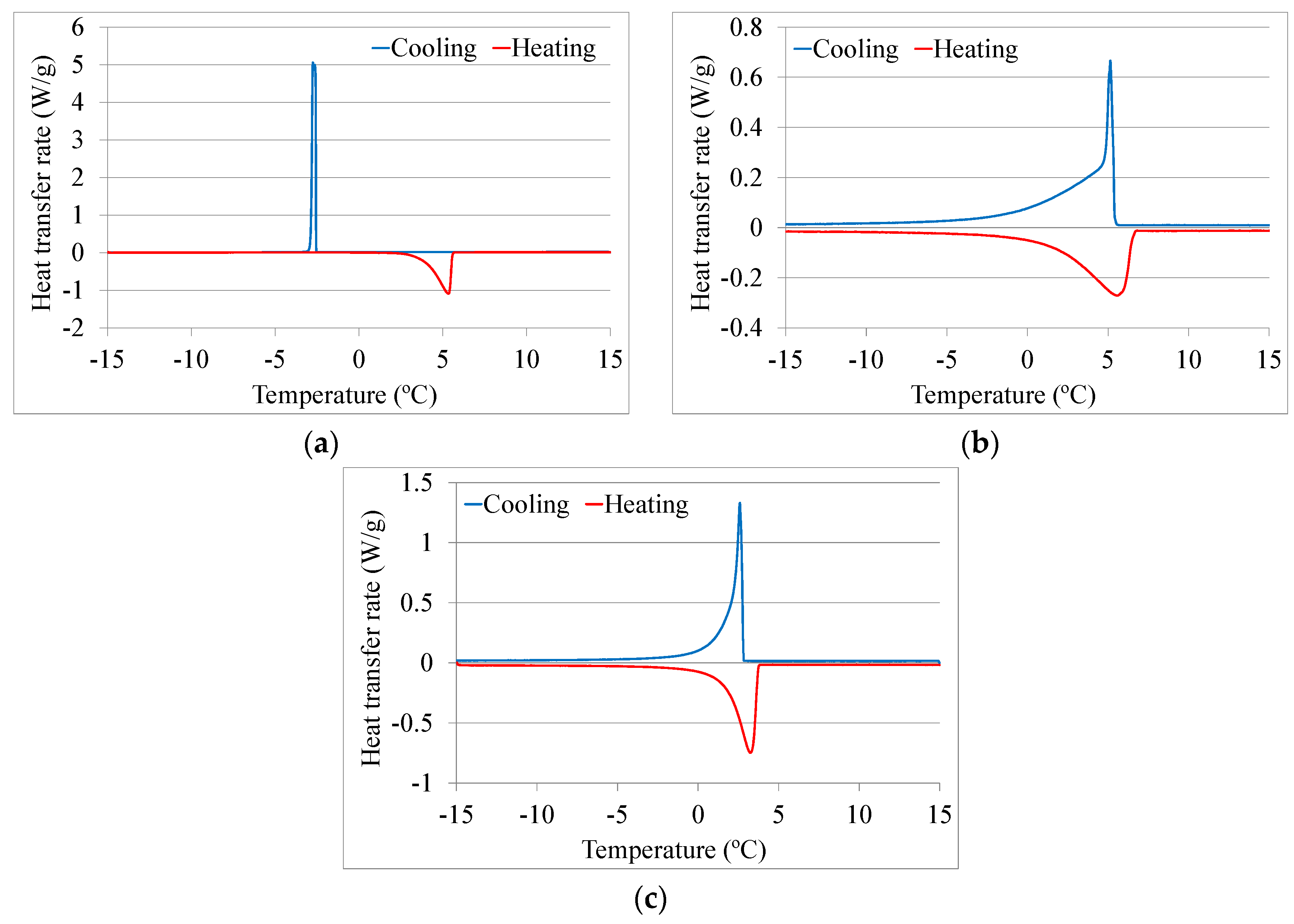
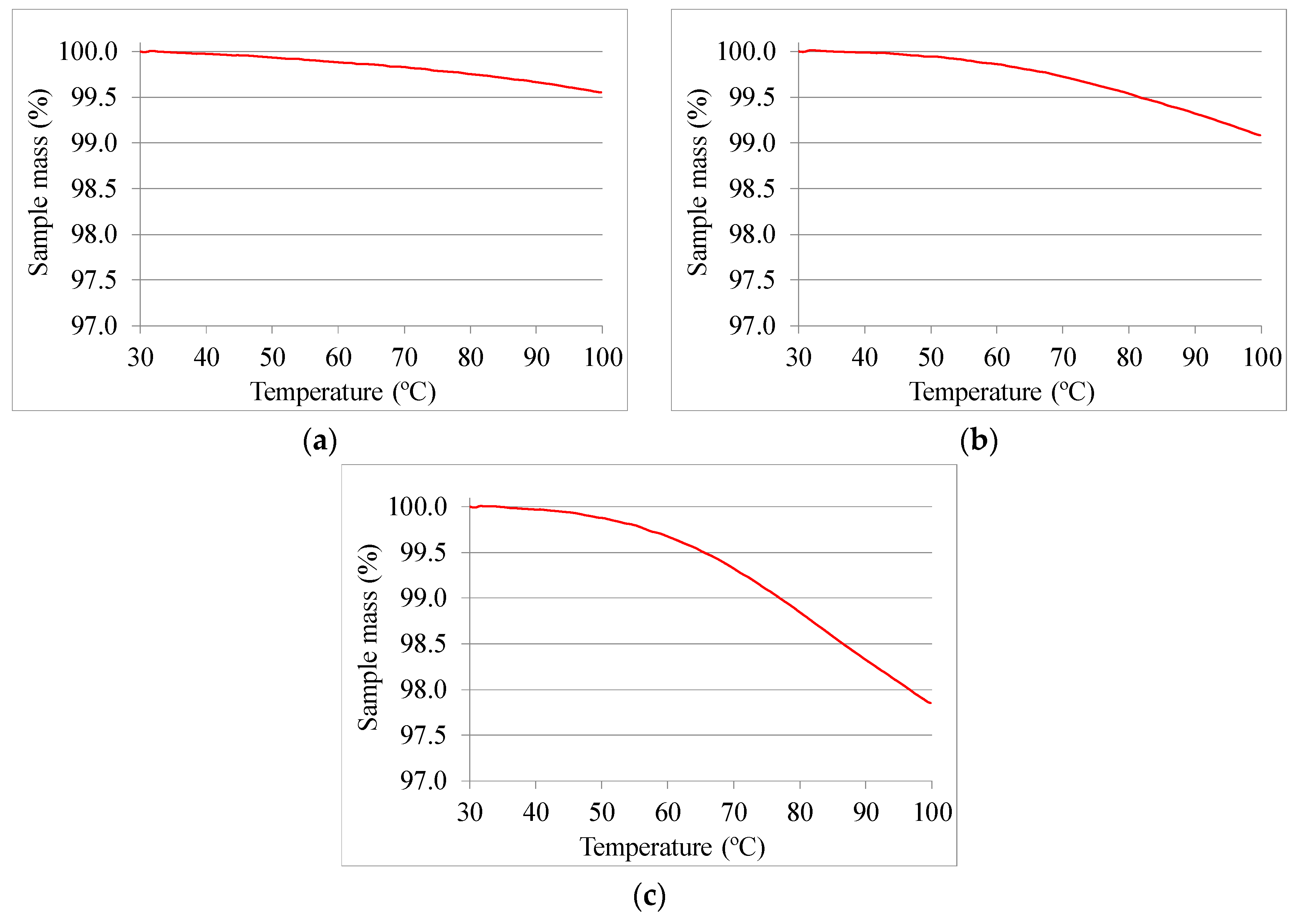
2.3. Thermophysical Characterization
3. Results and Discussion
3.1. Mediterranean System (Low Temperature)
3.2. Continental System
3.3. Sensitivity Analysis of the Selection Criteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dincer, I.; Rosen, M.A. Thermal Energy Storage: Systems and Applications; John Wiley and Sons: Chichester, West Sussex, UK, 2002; ISBN 978-0471495734. [Google Scholar]
- European Commission. European Commission Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions and the European Investment Bank; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Oró, E.; de Gracia, A.; Castell, A.; Farid, M.M.; Cabeza, L.F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef]
- Veerakumar, C.; Sreekumar, A. Phase change material based cold thermal energy storage: Materials, techniques and applications—A review. Int. J. Refrig. 2016, 67, 271–289. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Lin, K.; Zhang, Q.; Di, H. Application of latent heat thermal energy storage in buildings: State-of-the-art and outlook. Build. Environ. 2007, 42, 2197–2209. [Google Scholar] [CrossRef]
- Sharif, M.K.A.; Al-Abidi, A.A.; Mat, S.; Sopian, K.; Ruslan, M.H.; Sulaiman, M.Y.; Rosli, M.A.M. Review of the application of phase change material for heating and domestic hot water systems. Renew. Sustain. Energy Rev. 2015, 42, 557–568. [Google Scholar] [CrossRef]
- de Gracia, A.; Oró, E.; Farid, M.M.; Cabeza, L.F. Thermal analysis of including phase change material in a domestic hot water cylinder. Appl. Therm. Eng. 2011, 31, 3938–3945. [Google Scholar] [CrossRef]
- Mazman, M.; Cabeza, L.F.; Mehling, H.; Nogues, M.; Evliya, H.; Paksoy, H.Ö. Utilization of phase change materials in solar domestic hot water systems. Renew. Energy 2009, 34, 1639–1643. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Prieto, C.; Cabeza, L.F. Thermal energy storage (TES) with phase change materials (PCM) in solar power plants (CSP). Concept and plant performance. Appl. Energy 2019, 254, 113646. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM. An up to Date Introduction into Basics and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-68556-2. [Google Scholar]
- Miró, L.; Barreneche, C.; Ferrer, G.; Solé, A.; Martorell, I.; Cabeza, L.F. Health hazard, cycling and thermal stability as key parameters when selecting a suitable phase change material (PCM). Thermochim. Acta 2016, 627–629, 39–47. [Google Scholar] [CrossRef]
- Gasia, J.; Martin, M.; Solé, A.; Barreneche, C.; Cabeza, L.F. Phase Change Material Selection for Thermal Processes Working under Partial Load Operating Conditions in the Temperature Range between 120 and 200 °C. Appl. Sci. 2017, 7, 722. [Google Scholar] [CrossRef]
- HYBUILD. Available online: http://www.hybuild.eu/ (accessed on 4 February 2020).
- Frazzica, A.; Palomba, V.; Sergi, F.; Ferraro, M.; Cabeza, L.F.; Zsembinszki, G.; Oró, E.; Karellas, S.; Varvagiannis, S.; Emhofer, J.; et al. Dynamic Modelling of a Hybrid Solar Thermal/electric Energy Storage System for Application in Residential Buildings. In Proceedings of the ISES EuroSun 2018 Conference—12th International Conference on Solar Energy for Buildings and Industry, Rapperswil, Switzerland, 10–13 September 2018; p. 12. [Google Scholar]
- Rubitherm RT-PCM. Available online: https://www.rubitherm.eu/en/index.php/productcategory/organische-pcm-rt (accessed on 2 October 2019).
- PCM Products. Available online: http://www.pcmproducts.net/ (accessed on 15 January 2019).
- PCES Phase Change Energy Solutions. Available online: https://phasechange.com/ (accessed on 15 January 2019).
- RGEES LLC. Available online: http://www.rgees.com/products.php (accessed on 15 January 2019).
- PLUSS ®. Available online: http://pluss.co.in/ (accessed on 15 January 2019).
- Shengli, T.; Dong, Z.; Deyan, X. Experimental study of caprylic acid/lauric acid molecular alloys used as low-temperature phase change materials in energy storage. Energy Conserv. 2005, 6, 45–47. [Google Scholar]
- PureTemp LLC. Available online: http://www.puretemp.com/ (accessed on 15 January 2019).
- Jankowski, N.R.; McCluskey, F.P. A review of phase change materials for vehicle component thermal buffering. Appl. Energy 2014, 113, 1525–1561. [Google Scholar] [CrossRef]
- Pereira da Cunha, J.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Climator. Available online: http://climatoribiza.com/ (accessed on 15 January 2019).
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials: Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2016, 145, 255–286. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- TER HELL & Co. GmbH. Available online: https://www.terchemicals.com (accessed on 13 January 2020).
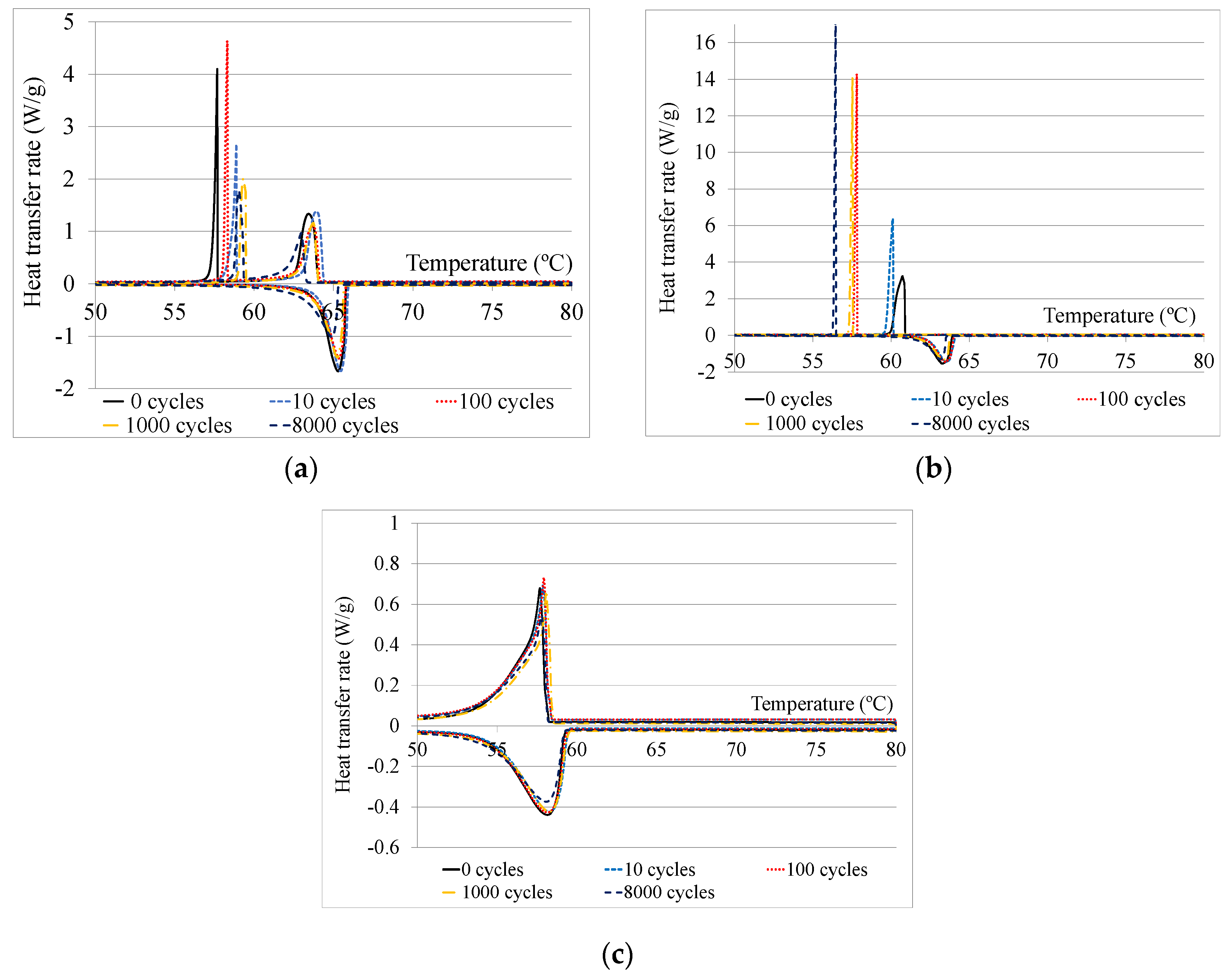
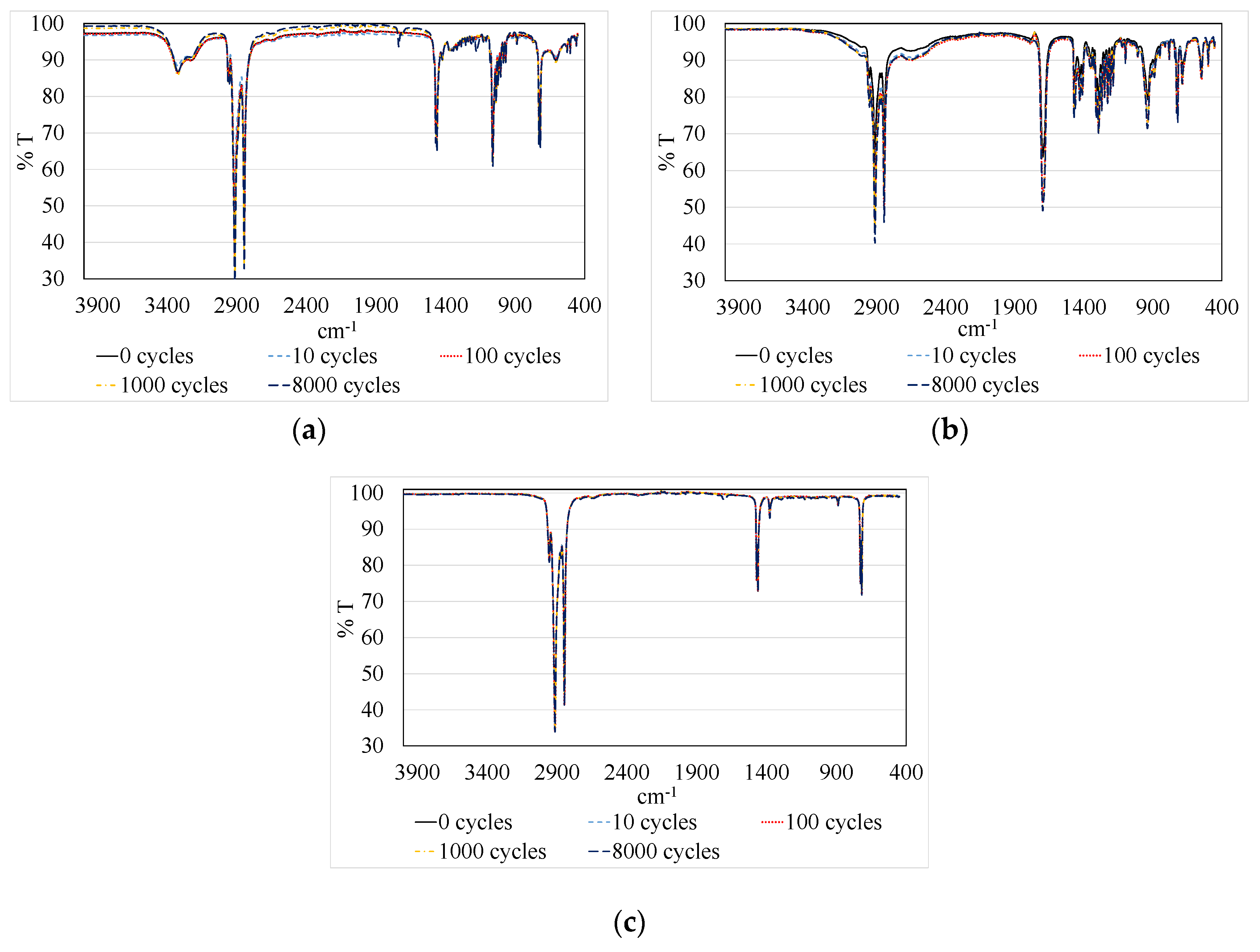
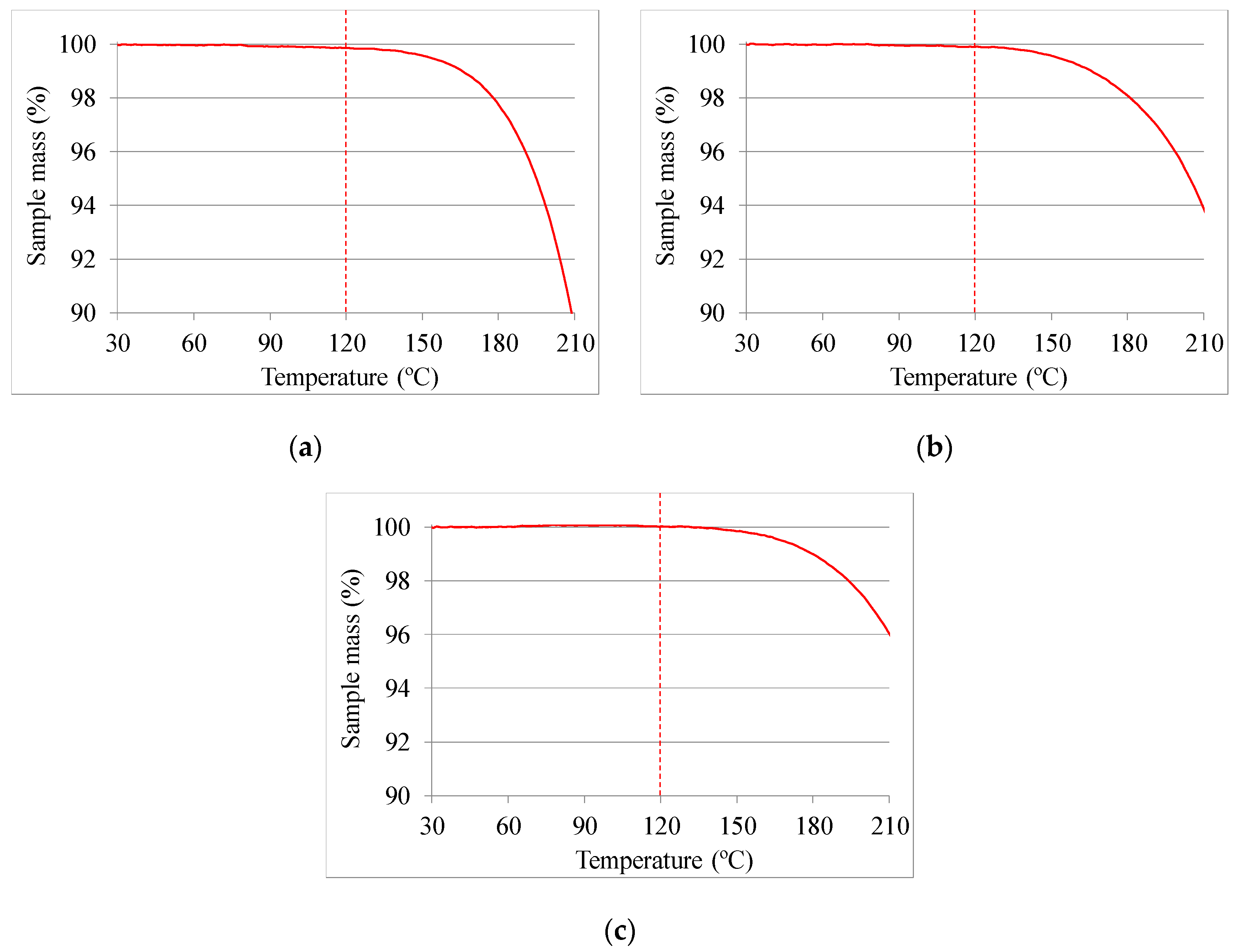
| Temperature Range (T, in °C) | Enthalpy (h, in kJ/kg) | Availability (-) | Price (P, in €/kg) | Maximum Temperature (Tmax, in °C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| T < 2 | 3 | h > 250 | 3 | Yes | 3 | P < 2.5 | 3 | Tmax > 120 | 3 |
| 2 < T <3 | 2 | 200 < h < 250 | 2 | No | 0 | 2.5 < P < 5 | 2 | Tmax < 120 or n.a. | 0 |
| 3 < T <4 | 1 | 150 < h < 200 | 1 | - | - | 5 < P < 10 | 1 | - | - |
| T > 4 or n.a. | 0 | h < 150 or n.a. | 0 | - | - | P > 10 or n.a. | 0 | - | - |
| Decision Parameter | Weight (%) | |||
|---|---|---|---|---|
| MED System | CON System | |||
| Scenario 1 | Scenario 2 | Scenario 1 | Scenario 2 | |
| Phase change range (Cp-T curve) | 25 | 25 | 20 | 20 |
| Enthalpy | 30 | 25 | 25 | 20 |
| Availability | 15 | 25 | 10 | 20 |
| Price | 30 | 25 | 25 | 20 |
| Maximum working temperature | - | - | 20 | 20 |
| Total | 100 | 100 | 100 | 100 |
| Commercial Name/Composition | Type | Melting Temperature (°C) | Phase Change Enthalpy (kJ/kg) | Thermal Conductivity (W/m·K) | Density (kg/m3) | Reference |
|---|---|---|---|---|---|---|
| RT3HC_1 | Organic (paraffin) | 1–3 | 190 | 0.20 (l) 0.20 (s) | 770 (l) 880 (s) | [19] |
| A3 | Organic (n.a.) | 3 | 200 | 0.210 | 765 | [20] |
| 0200- Q2 BioPCM | Organic (bioPCM) | 2 | 200–230 | 0.2–0.7 (l) 0.25–2.5 (s) | 850–1300 (l) 900–1250 (s) | [21] |
| PCM-PDR03P | Organic (n.a.) | 3.5 | 185 | n.a. | 570 | [22] |
| savE OM 03 | Organic (n.a.) | 3.5 | 229 | 0.224 (l) 0.146 (l) | 835 (l) 912 (s) | [23] |
| Caprylic acid + lauric acid (9:1 by mol) | Organic eutectic (fatty acid) | 3.8 | 151.5 | n.a. | n.a. | [24] |
| RT4 | Organic (paraffin) | 2–4 | 175 | 0.20 (l) 0.20 (s) | 770 (l) 880 (s) | [19] |
| 0200- Q4 BioPCM | Organic (bioPCM) | 4 | 200–230 | 0.2–0.7 (l) 0.25–2.5 (s) | 850–1300 (l) 900–1250 (s) | [21] |
| PureTemp 4 | Organic (bio-based) | 4 | 195 | n.a. | n.a. | [25] |
| Tetrahydrofuran clathrate hydrate | Inorganic (clathrate hydrate) | 4.4 | 255 | n.a. | n.a. | [26] |
| Commercial Name/Composition | Type | Melting Temperature (°C.) | Phase Change Enthalpy (kJ/kg) | Thermal Conductivity (W/m·K) | Density (kg/m3) | Reference |
|---|---|---|---|---|---|---|
| A50 | Organic (n.a.) | 50 | 218 | 0.18 | 810 | [20] |
| 0500- Q50 BioPCM | Organic (bioPCM) | 50 | 200–230 | 0.2–0.7 (l) 0.25–2.5 (s) | 850–1300 (l) 900–1250 (s) | [21] |
| savE OM50 | Organic (fatty acids mixture) | 50–51 | 223 | 0.14 (l) 0.21 (s) | 859 (l) 961 (s) | [23] |
| RT54HC | Organic (paraffin) | 53–54 | 200 | 0.2 | 800 (l) 850 (s) | [19] |
| Stearic acid (CH3(CH2)16-COOH) | Organic (fatty acid) | 54 | 157 | 0.17 (l) 0.29 (s) | 940 (s) | [27] |
| Cetyl stearate | Organic (ester) | 54.6 | 212.1–216.3 | n.a. | n.a. | [26] |
| savE OM 55 | Organic (mixture of fatty acids) | 55 | 208 | 0.16 (l) 0.1 (s) | 841 (l) 935 (s) | [23] |
| 0500- Q56 BioPCM | Organic (bioPCM) | 56 | 200–230 | 0.2–0.7 (l) 0.25–2.5 (s) | 850–1300 (l) 900–1250 (s) | [21] |
| Tristearin ((C17H35COO)3C3H5) | Organic | 56 | 190.8 | n.a. | 862 (l) | [26] |
| PureTemp 58 | Organic (bio-based) | 58 | 225 | 0.15 (l) 0.25 (s) | 810 (l) 890 (s) | [25] |
| A58H | Organic (n.a.) | 58 | 243 | 0.18 | 820 | [20] |
| 66.7% Polyethylene oxide 10000 + 33.3% Myristic acid | Organic (plastic + fatty acid) | 58.7 | 191 | n.a. | n.a. | [28] |
| Climsel C58 | Inorganic (salt hydrate) | 58 | 259 | 1.46 | n.a. | [12,14] |
| 55–58 | 260 | 0.47 (l) 0.57 (s) | 1400 | [29] | ||
| 58 | 80 | 0.5–0.7 | 1460 | [30] | ||
| THP 5860 | Organic (paraffin) | 55–60 | 153 | n.a. | n.a. | Own measurements |
| Paraffin C27 | Organic (paraffin) | 58.8 | 236 | n.a. | n.a. | [31] |
| RT60 | Organic (paraffin) | 58–60 | 214 | 0.2 | n.a. | [30] |
| Stearyl stearate | Organic (ester) | 59.2 | 214.75–214.93 | n.a. | n.a. | [26] |
| PureTemp 63 | Organic (bio-based) | 63 | 206 | 0.15 (l) 0.25 (s) | 840 (l) 920 (s) | [25] |
| RT64HC | Organic (n.a.) | 63–65 | 250 | 0.2 | 780 (l) 880 (s) | [19] |
| Stearyl arachidate (C38H76O2) | Organic (ester) | 64.96 | 226 | n.a. | 2350 (l) 1930 (s) | [26] |
| 50% CH3CONH2 + 50% C17H35COOH | Organic (eutectic) | 65 | 218 | n.a. | n.a. | [31] |
| 0500- Q65 BioPCM | Organic (bioPCM) | 65 | 200–230 | 0.2–0.7 (l) 0.25–2.5 (s) | 850–1300 (l) 900–1250 (s) | [21] |
| savE FS 65 | Organic (blend of organic material in polymer matrix) | 66–68 | 218 | 0.25 (s) | 842 (s) | [23] |
| PureTemp 68 | Organic (bio-based) | 68 | 213 | 0.15 (l) 0.25 (s) | 870 (l) 960 (s) | [25] |
| 0500- Q68 BioPCM | Organic (bioPCM) | 68 | 200-235 | 0.2–0.7 (l) 0.25–2.5 (s) | 850–1300 (l) 900–1250 (s) | [21] |
| No. | Scenario 1 | Scenario 2 | ||||
|---|---|---|---|---|---|---|
| Commercial Name/Composition | Average Score (%) | Standard Deviation (%) | Commercial Name/Composition | Average Score (%) | Standard Deviation (%) | |
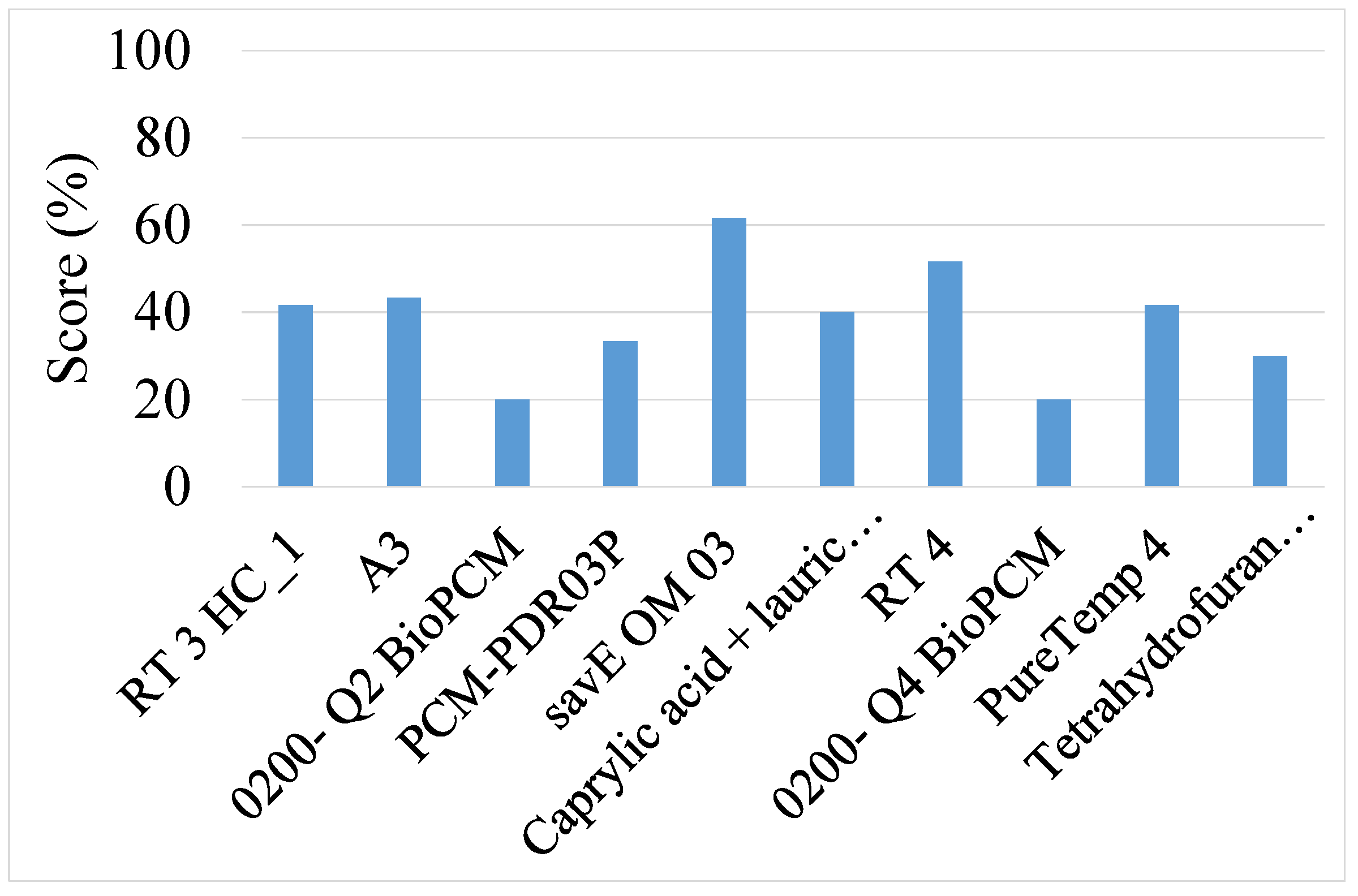
| 1 | savE OM 03 | 61.7 | 4.6 | savE OM 03 | 66.7 | 4.6 |
| 2 | RT4 | 51.7 | 5.4 | RT4 | 58.4 | 5.4 |
| 3 | A3 | 43.3 | 7.2 | Caprylic acid + lauric acid (9:1 by mol) | 50.2 | 9.6 |
| 4 | RT3HC_1 | 41.7 | 7.2 | RT3HC_1 | 50.1 | 7.2 |
| 5 | PureTemp 4 | 41.7 | 7.2 | PureTemp 4 | 50.1 | 7.2 |
| 6 | Caprylic acid + lauric acid (9:1 by mol) | 40.2 | 9.6 | A3 | 49.9 | 7.2 |
| 7 | PCM-PDR03P | 33.3 | 7.1 | PCM-PDR03P | 41.6 | 7.1 |
| 8 | Tetrahydrofuran clathrate hydrate | 29.9 | 8.4 | Tetrahydrofuran clathrate hydrate | 24.9 | 8.4 |
| 9 | 0200- Q2 BioPCM | 19.9 | 5.6 | 0200- Q2 BioPCM | 16.6 | 5.6 |
| 10 | 0200- Q4 BioPCM | 19.9 | 5.6 | 0200- Q4 BioPCM | 16.6 | 5.6 |
| No. | Scenario 1 | Scenario 2 | ||||
|---|---|---|---|---|---|---|
| Commercial Name/Composition | Average Score (%) | Standard Deviation (%) | Commercial Name/Composition | Average Score (%) | Standard Deviation (%) | |
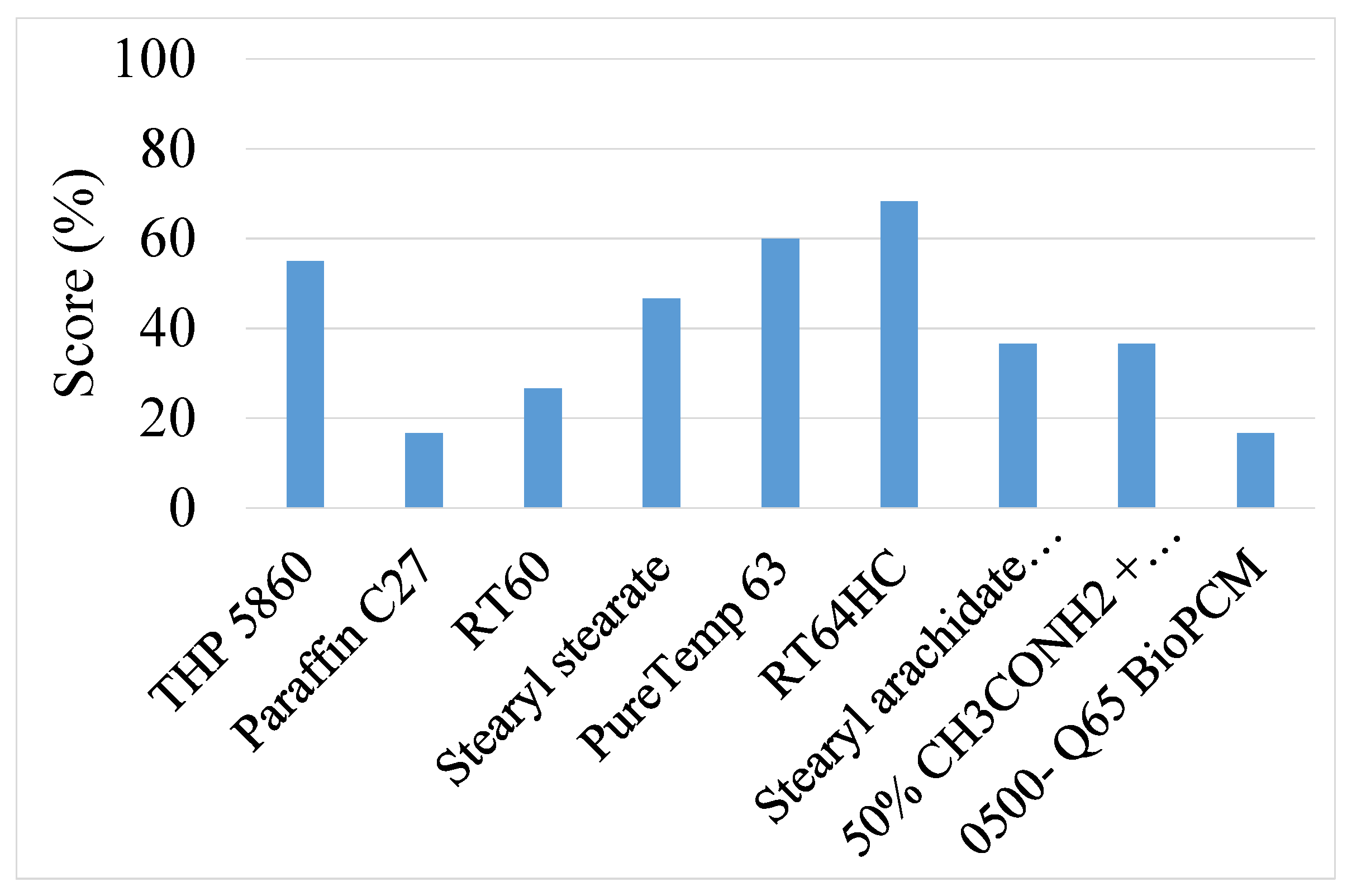
| 1 | RT64HC | 68.4 | 7.2 | RT64HC | 73.3 | 7.2 |
| 2 | PureTemp 63 | 60.1 | 6.7 | PureTemp 63 | 66.7 | 6.8 |
| 3 | THP 5860 | 55.1 | 7.2 | THP 5860 | 60.0 | 7.2 |
| 4 | Stearyl stearate | 46.8 | 8.3 | Stearyl stearate | 53.3 | 8.4 |
| 5 | Stearyl arachidate (C38H76O2) | 36.6 | 7.8 | Stearyl arachidate (C38H76O2) | 33.3 | 7.9 |
| 6 | 50% CH3CONH2 + 50% C17H35 COOH | 36.6 | 7.8 | 50% CH3CONH2 + 50% C17H35 COOH | 33.3 | 7.9 |
| 7 | RT60 | 26.8 | 6.6 | RT60 | 33.3 | 6.8 |
| 8 | Paraffin C27 | 16.6 | 5.0 | Paraffin C27 | 13.3 | 5.0 |
| 9 | 0500- Q65 BioPCM | 16.6 | 5.0 | 0500- Q65 BioPCM | 13.3 | 5.0 |
| Position | Scenario 1 | Scenario 2 | ||
|---|---|---|---|---|
| Commercial Name/Composition | Frequency | Commercial Name/Composition | Frequency | |
| 1st | savE OM 03 | 49/49 (100%) | savE OM 03 | 49/49 (100%) |
| 2nd | RT4 | 47/49 (95.9%) | RT4 | 45/49 (91.8%) |
| 3rd | A3 | 23/49 (46.9%) | Caprylic acid + lauric acid (9:1 by mol) | 28/49 (57.1%) |
| Position | Scenario 1 | Scenario 2 | ||
|---|---|---|---|---|
| Commercial Name/Composition | Frequency | Commercial Name/Composition | Frequency | |
| 1st | RT64HC | 172/180 (95.6%) | RT64HC | 172/180 (95.6%) |
| 2nd | PureTemp 63 | 135/180 (75.0%) | PureTemp 63 | 150/180 (83.3%) |
| 3rd | THP5860 | 109/180 (60.6%) | THP5860 | 111/180 (61.7%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsembinszki, G.; Fernández, A.G.; Cabeza, L.F. Selection of the Appropriate Phase Change Material for Two Innovative Compact Energy Storage Systems in Residential Buildings. Appl. Sci. 2020, 10, 2116. https://doi.org/10.3390/app10062116
Zsembinszki G, Fernández AG, Cabeza LF. Selection of the Appropriate Phase Change Material for Two Innovative Compact Energy Storage Systems in Residential Buildings. Applied Sciences. 2020; 10(6):2116. https://doi.org/10.3390/app10062116
Chicago/Turabian StyleZsembinszki, Gabriel, Angel G. Fernández, and Luisa F. Cabeza. 2020. "Selection of the Appropriate Phase Change Material for Two Innovative Compact Energy Storage Systems in Residential Buildings" Applied Sciences 10, no. 6: 2116. https://doi.org/10.3390/app10062116
APA StyleZsembinszki, G., Fernández, A. G., & Cabeza, L. F. (2020). Selection of the Appropriate Phase Change Material for Two Innovative Compact Energy Storage Systems in Residential Buildings. Applied Sciences, 10(6), 2116. https://doi.org/10.3390/app10062116







