Assessment of Cytotoxicity of Magnesium Oxide and Magnesium Hydroxide Nanoparticles using the Electric Cell-Substrate Impedance Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Impedance Measurement
2.4. Fluorescence Microscope
2.5. The pH Measurement
2.6. Statistical Analysis
3. Results and Discussion
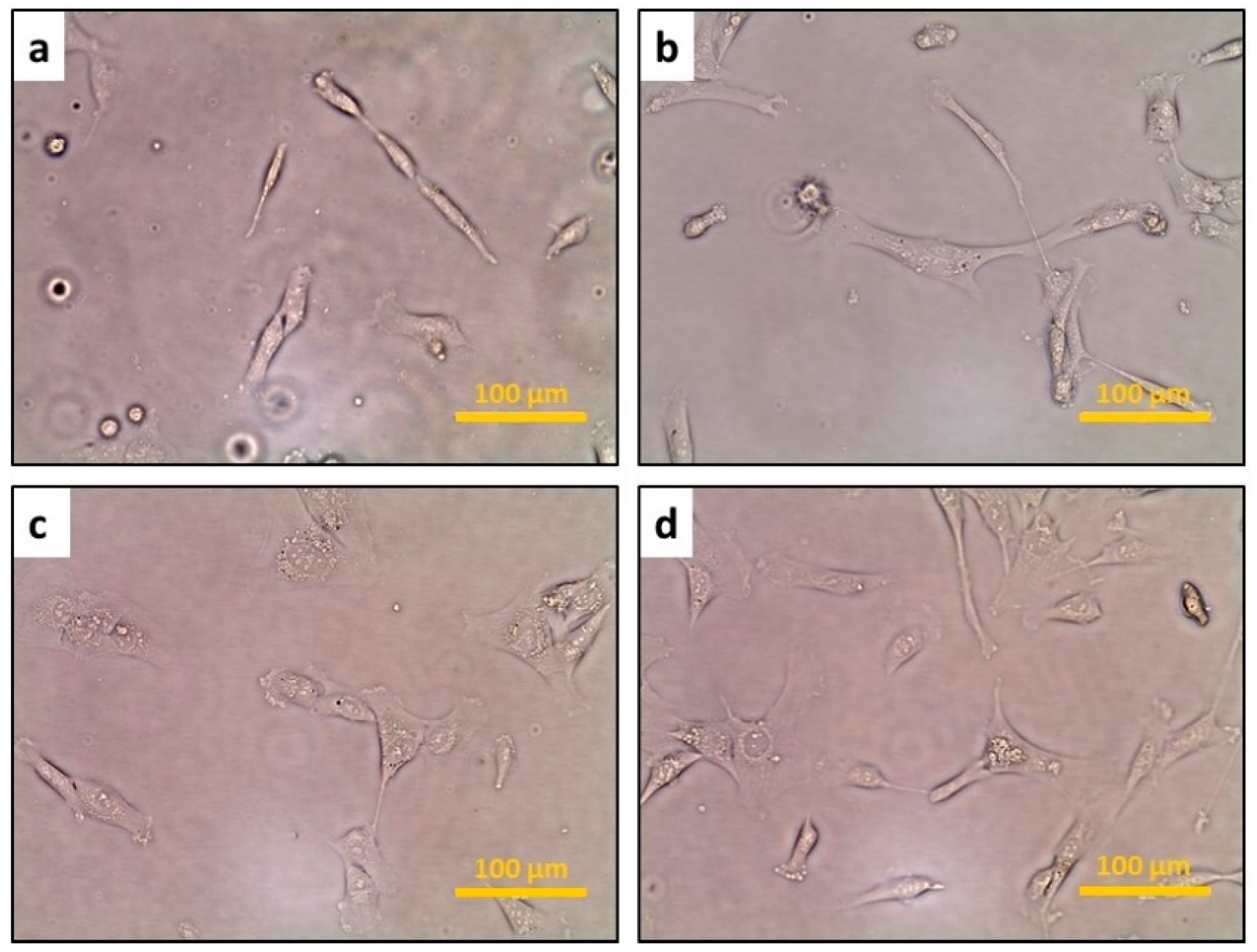
3.1. The Confluence of hFOB Osteoblast Cells
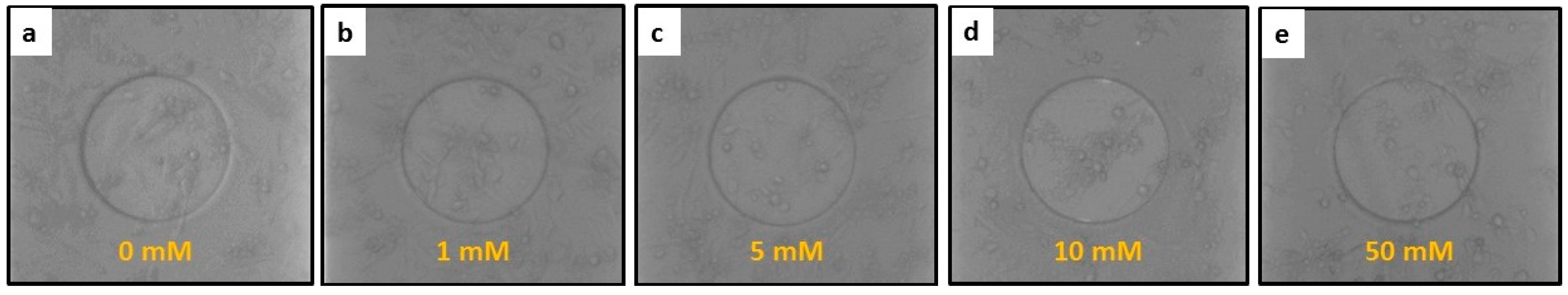
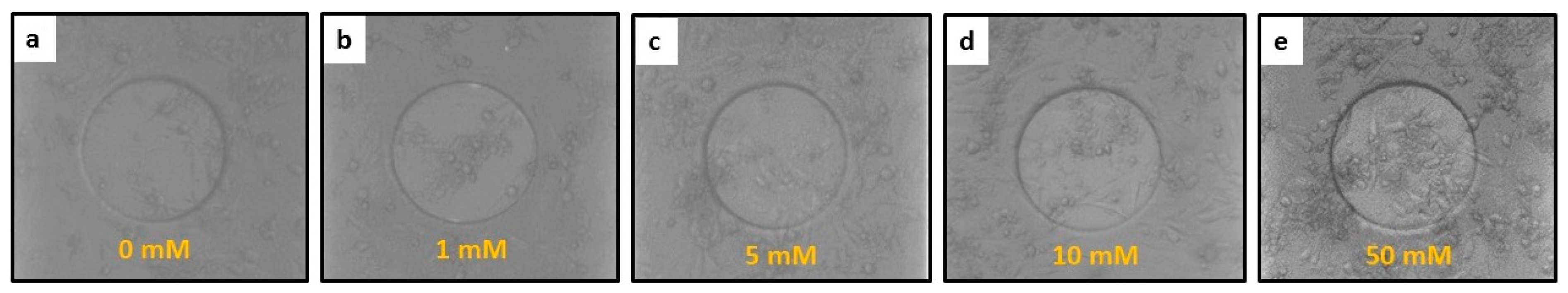
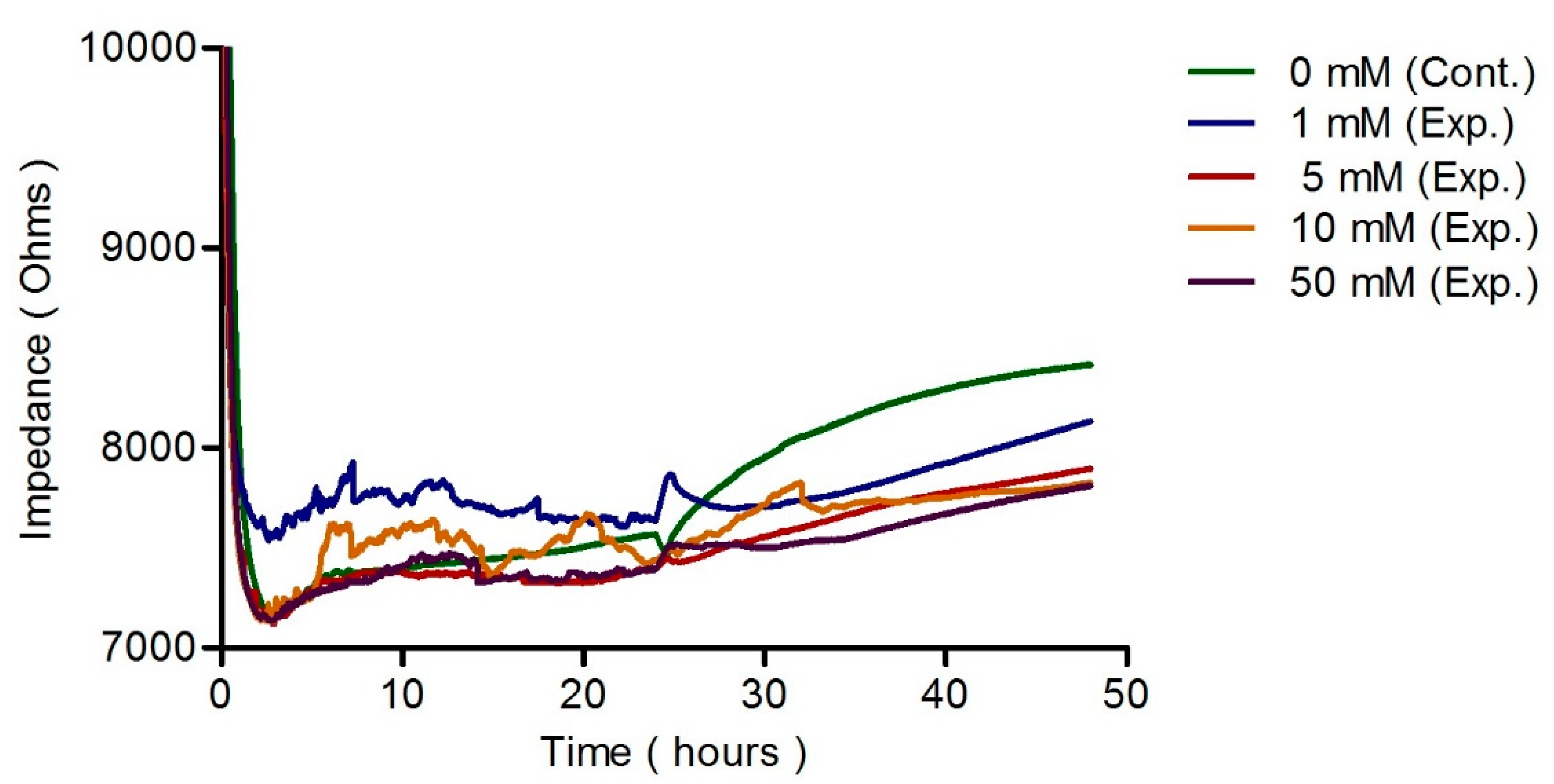
3.2. The Response of hFOB Osteoblast Cells on ECIS after Treatment
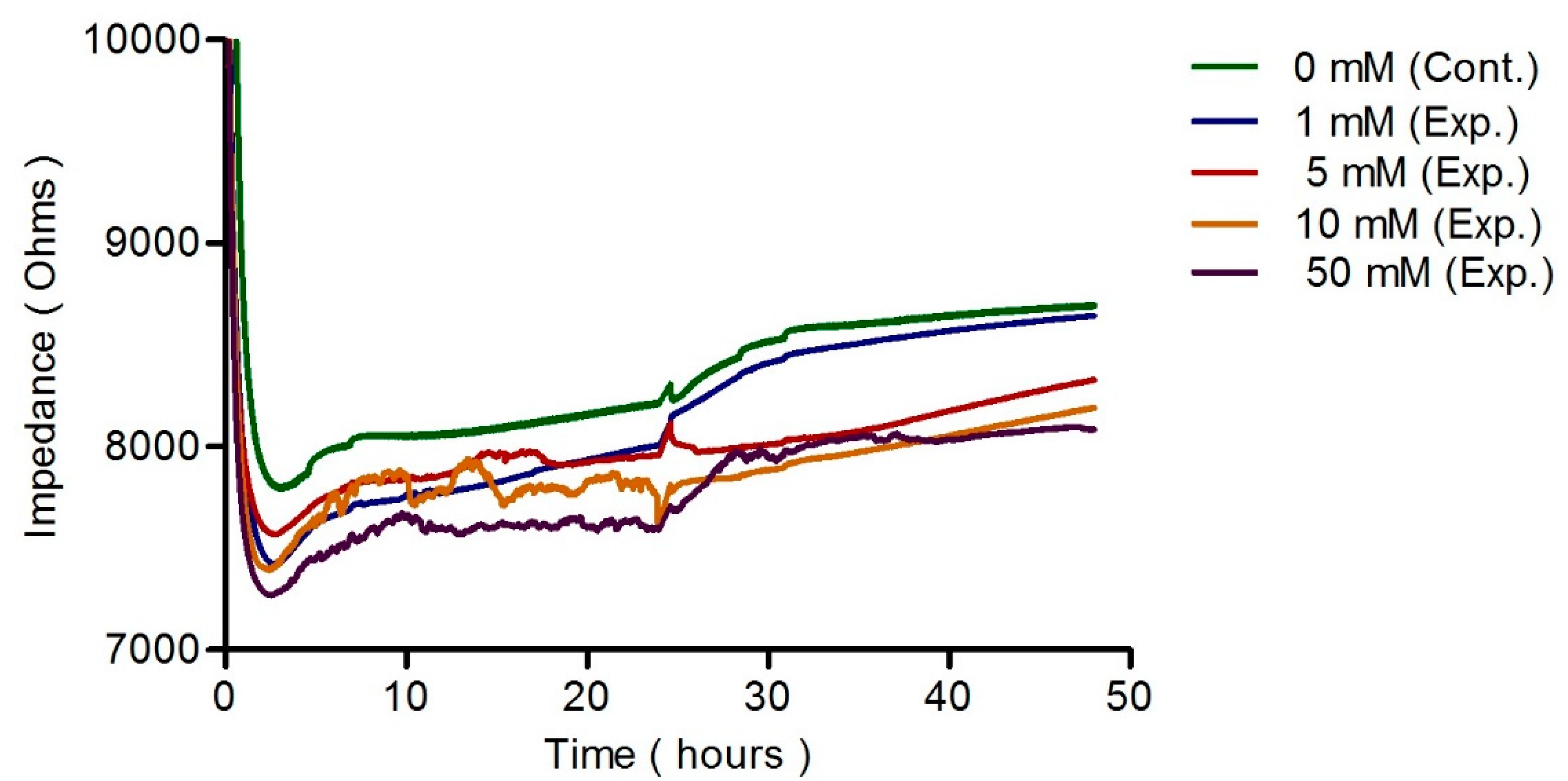
3.3. ECIS Measurement
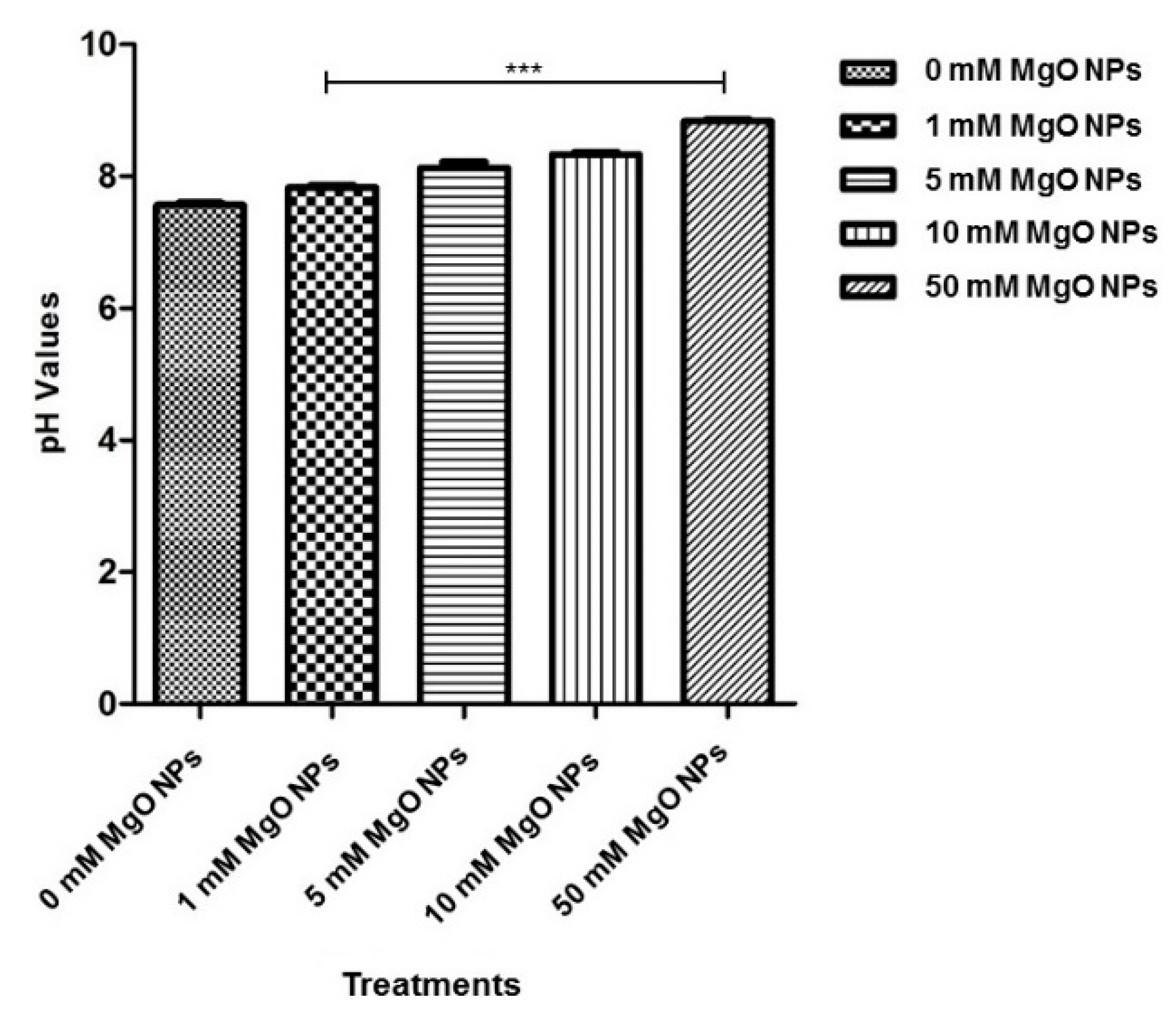
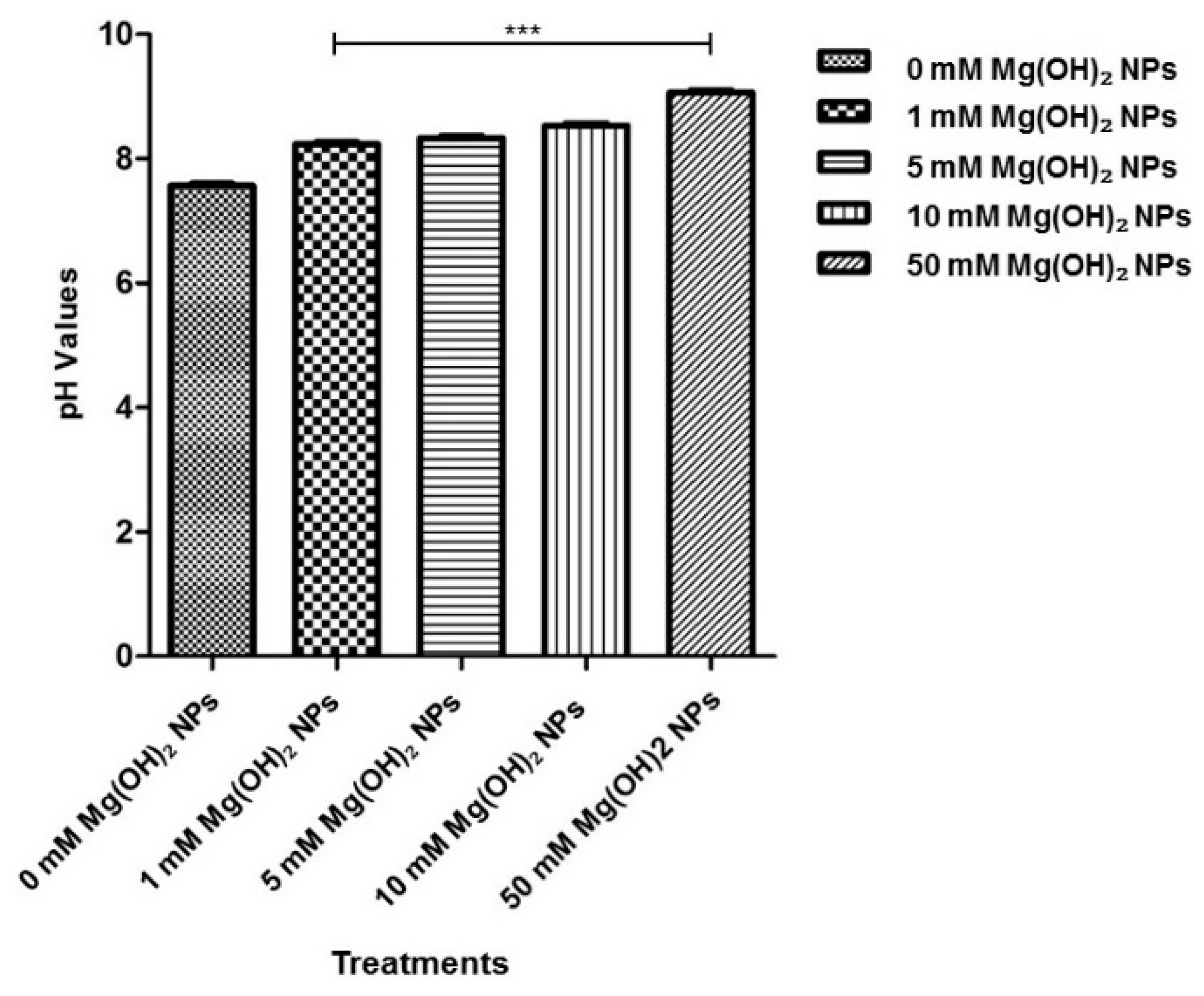
3.4. Analysis of the pH Changes
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- McBride, E.D. Absorbable metal in bone surgery. J. Am. Med. Assoc. 1938, 111, 2464–2467. [Google Scholar] [CrossRef]
- Verbrugge, J. Le Mate’ riel Me´tallique Re´sorbable En Chirurgie Osseuse. La Press Med. 1934, 23, 460–465. [Google Scholar]
- Williams, D.F. Effects of the environment on materials. Biomed. Eng. 1971, 6, 106–113. [Google Scholar] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, M.; Waterman, J.; Koo, Y.; Sankar, J.; Yun, Y. In Vitro Cytotoxicity of Possible Corrosion Products from Mg-Based Biodegradable Metals: Magnesium Oxide and Magnesium Hydroxide Nanoparticles. Appl. Sci. 2019, 9, 4304. [Google Scholar] [CrossRef]
- Günther, T. Comment on the number of magnesium activated enzymes. Magnes. Res. 2008, 1, 185–187. [Google Scholar]
- Rubin, H. Magnesium: The missing element in molecular views of cell proliferation control. Bioessays. 2005, 27, 311–320. [Google Scholar] [CrossRef]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- McCarthy, T.J.; Kumar, R. Divalent Cation Metabolism: Magnesium. In Atlas of Diseases of the Kidney; Schrier, R.W., Ed.; Blackwell Science: Malden, MA, USA, 1999; pp. 4.1–4.8. [Google Scholar]
- Al-Ghamdi, S.M.G.; Cameron, E.C.; Sutton, R.A.L. Magnesium deficiency: Pathophysiologic and clinical overview. Am. J. Kidney Dis. 1994, 24, 737–752. [Google Scholar] [CrossRef]
- De Rouffignac, C.; Quamme, G. Renal magnesium handling and its hormonal control. Physiol. Rev. 1994, 74, 305–322. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.M. Magnesium and its therapeutic uses: A review. Am. J. Med. 1994, 96, 63–76. [Google Scholar] [CrossRef]
- Quamme, G.A. Magnesium homeostasis and renal magnesium handling. Miner. Electrolyte Metab. 1993, 19, 218–225. [Google Scholar]
- Nadler, J.L.; Rude, R.K. Disorders of magnesium metabolism. Endocrin. Metab. Clin. 1995, 24, 623–641. [Google Scholar] [CrossRef]
- Whang, R.; Hampton, E.M.; Whang, D.D. Magnesium homeostasis and clinical disorders of magnesium deficiency. Ann. Pharmacother. 1994, 28, 220–226. [Google Scholar] [CrossRef]
- Abed, E.; Moreau, R. Importance of melastatin-like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet-derived growth factor. Am. J. Physiol. Cell Phys. 2009, 297, C360–C368. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Diener, A.; Nebe, B.; Lüthen, F.; Becker, P.; Beck, U.; Neumann, H.G.; Rychly, J. Control of focal adhesion dynamics by material surface characteristics. Biomaterials 2005, 26, 383–392. [Google Scholar] [CrossRef]
- Hornby, J.E. Measurement of cell adhesion. II. Quantitative study of the effect of divalent ions on cell adhesion. J. Embryol. Exp. Morph. 1973, 30, 511–518. [Google Scholar]
- Li, C.Y.; Gao, S.Y.; Terashita, T.; Shimokawa, T.; Kawahara, H.; Matsuda, S.; Kobayashi, N. In vitro assays for adhesion and migration of osteoblastic cells (Saos-2) on titanium surfaces. Cell Tissue Res. 2006, 324, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta. 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Moomaw, A.S.; Maguire, M.E. The unique nature of mg2+ channels. Physiology 2008, 23, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Rouahi, M.; Champion, E.; Hardouin, P.; Anselme, K. Quantitative kinetic analysis of gene expression during human osteoblastic adhesion on orthopaedic materials. Biomaterials 2006, 27, 2829–2844. [Google Scholar] [CrossRef]
- Spillmann, C.; Osorio, D.; Waugh, R. Integrin activation by divalent ions affects neutrophil homotypic adhesion. Ann. Biomed. Eng. 2002, 30, 1002–1011. [Google Scholar] [CrossRef]
- Turner, D.; Flier, L.; Carbonetto, S. Magnesium-dependent attachment and neurite outgrowth by PC12 cells on collagen and laminin substrata. Dev. Biol. 1987, 121, 510–525. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH Regulates Bone Cell Function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Developmental and Hormone-Regulated Expression of Genes in Osteoblasts: An Integrated Relationship of Cell Growth and Differentiation. In Cellular Molecular Biology of Bone; Noda, M., Ed.; Academic Press: Tokyo, Japan, 1993; pp. 47–95. [Google Scholar]
- Kasperk, C.; Wergedal, J.; Strong, D.; Farley, J.; Wangerin, K.; Gropp, H.; Ziegler, R.; Baylink, D.J. Human bone cell phenotypes differ depending on their skeletal site of origin. J. Clin. Endocr. Metab. 1995, 80, 2511–2517. [Google Scholar]
- Wolf, F.; Trapani, V. Cell (patho) physiology of magnesium. Clin. Sci. 2008, 114, 27–35. [Google Scholar] [CrossRef]
- Arnett, T.R. Acidosis, hypoxia and bone. Arch. Biochem. Biophys. 2010, 503, 103–109. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallavi, M.; Waterman, J.; Koo, Y.; Sankar, J.; Yun, Y. Assessment of Cytotoxicity of Magnesium Oxide and Magnesium Hydroxide Nanoparticles using the Electric Cell-Substrate Impedance Sensing. Appl. Sci. 2020, 10, 2114. https://doi.org/10.3390/app10062114
Pallavi M, Waterman J, Koo Y, Sankar J, Yun Y. Assessment of Cytotoxicity of Magnesium Oxide and Magnesium Hydroxide Nanoparticles using the Electric Cell-Substrate Impedance Sensing. Applied Sciences. 2020; 10(6):2114. https://doi.org/10.3390/app10062114
Chicago/Turabian StylePallavi, Manishi, Jenora Waterman, Youngmi Koo, Jagannathan Sankar, and Yeoheung Yun. 2020. "Assessment of Cytotoxicity of Magnesium Oxide and Magnesium Hydroxide Nanoparticles using the Electric Cell-Substrate Impedance Sensing" Applied Sciences 10, no. 6: 2114. https://doi.org/10.3390/app10062114
APA StylePallavi, M., Waterman, J., Koo, Y., Sankar, J., & Yun, Y. (2020). Assessment of Cytotoxicity of Magnesium Oxide and Magnesium Hydroxide Nanoparticles using the Electric Cell-Substrate Impedance Sensing. Applied Sciences, 10(6), 2114. https://doi.org/10.3390/app10062114




