A Soft, Biocompatible Magnetic Field Enabled Wireless Surgical Lighting Patty for Neurosurgery
Abstract
1. Introduction
2. Materials and Methods
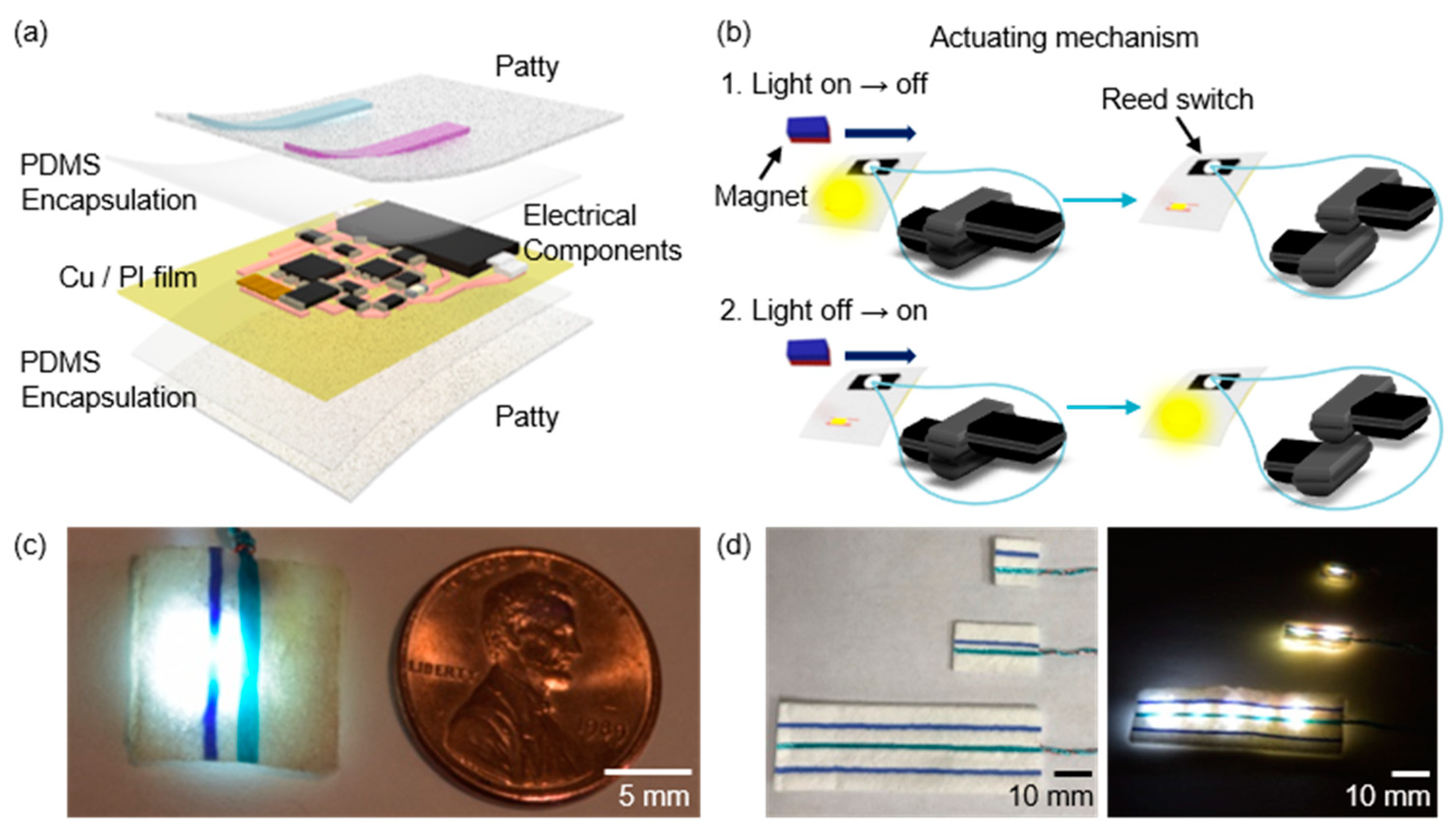
2.1. Fabrication for Surgical Lighting Patty
2.2. Finite Element Analysis
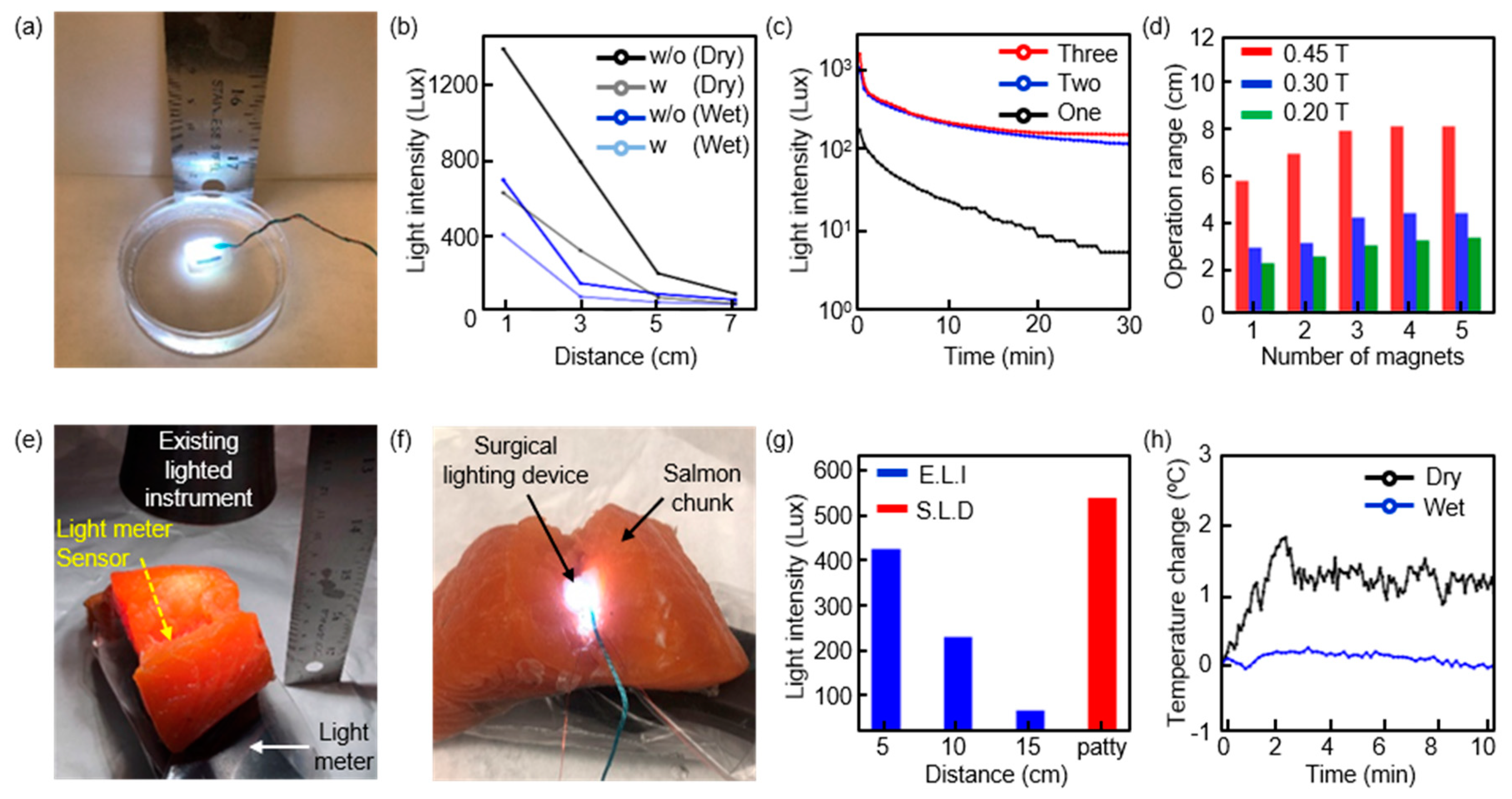
2.3. Measurements of Surgical Lighting Devices
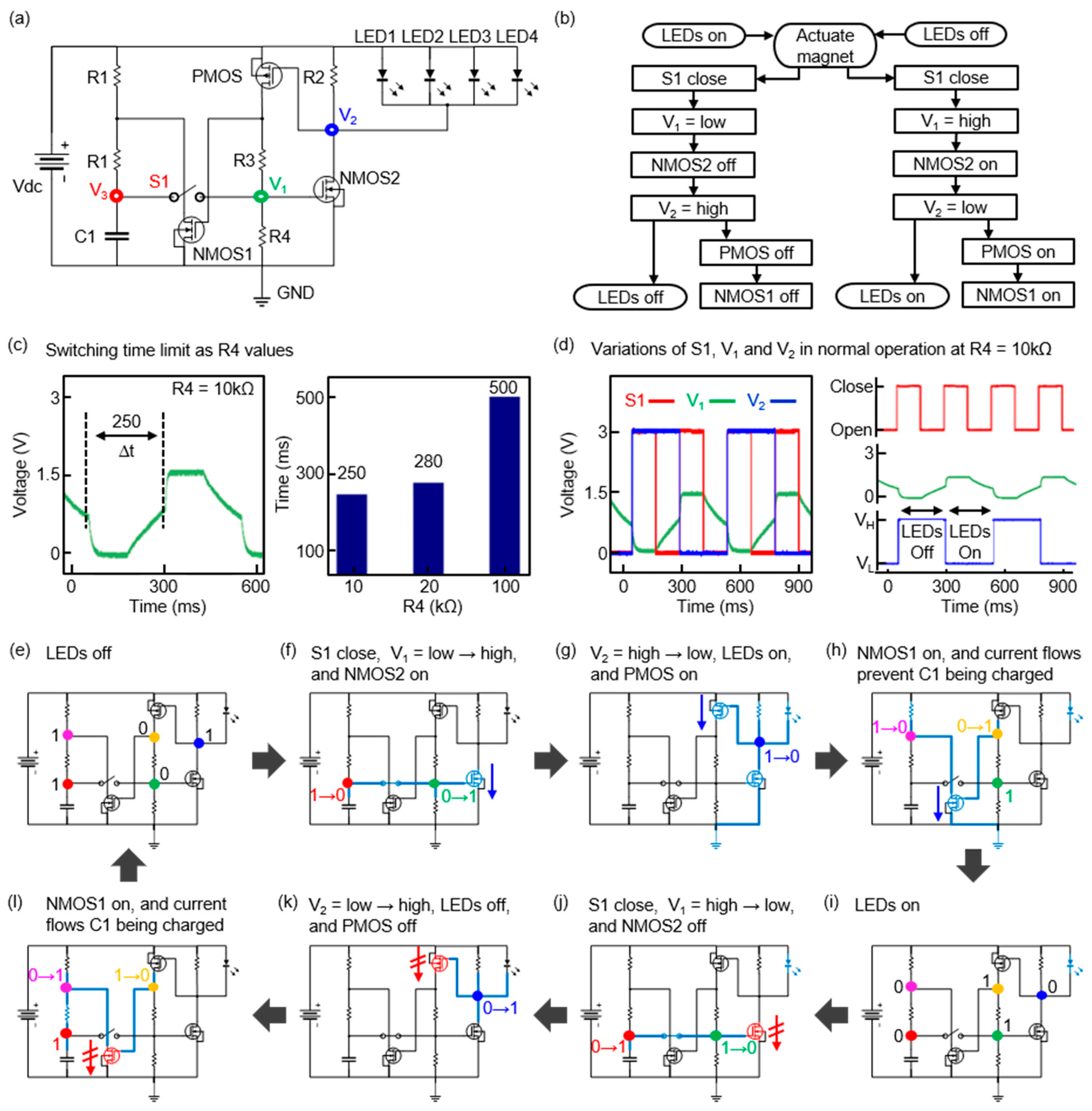
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Obholzer, R.J.; Graham, J.M. A novel retractor for use in cochlear implantation. Otol. Neurotol. 2003, 24, 749–750. [Google Scholar] [CrossRef]
- Steele, P.R.C.; Curran, J.F.; Mountain, R.E. Current and future practices in surgical retraction. Surgeon 2013, 11, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Riskalla, A.; Wall, K.; O’Connor, A.F.; Jiang, D. An illuminated retractor for minimal access surgery in cochlear implantation: How we do it. Acta Otolaryngol. 2010, 130, 1199–1200. [Google Scholar] [CrossRef] [PubMed]
- Clancy, N.T.; Li, R.; Rogers, K.; Driscoll, P.; Excel, P.; Yandle, R.; Elson, D.S. Development and evaluation of a light-emitting diode endoscopic light source. Adv. Biomed. Clin. Diagnostic Syst. X 2012, 8214, 82140R. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Gogoi, A.; Lee, S.Y.; Tsai-Lin, Y.; Yi, P.W.; Lu, M.K.; Kao, F.J. Coherent Narrow-Band Light Source for Miniature Endoscopes. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Knulst, A.J.; Mooijweer, R.; Jansen, F.W.; Stassen, L.P.S.; Dankelman, J. Indicating shortcomings in surgical lighting systems. Minim. Invasive Ther. Allied Technol. 2011, 20, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Khanicheh, A.; Leiner, D.; Shafer, D.; Zobel, J. Endoscope field of view measurement. Biomed. Opt. Express 2017, 8, 1441. [Google Scholar] [CrossRef]
- Kalani, M.Y.S.; Wanebo, J.E.; Martirosyan, N.L.; Nakaji, P.; Zabramski, J.M.; Spetzler, R.F. A raised bar for aneurysm surgery in the endovascular era. J. Neurosurg. 2017, 126, 1731–1739. [Google Scholar] [CrossRef]
- Killory, B.D.; Nakaji, P.; Gonzales, L.F.; Ponce, F.A.; Wait, S.D.; Spetzler, R.F. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery 2009, 65, 456–462. [Google Scholar] [CrossRef]
- Melles, G.R.J.; Remeijer, L.; Geerards, A.J.M.; Beekhuis, W.H. A quick surgical technique for deep, anterior lamellar keratoplasty using visco-dissection. Cornea 2000, 19, 427–432. [Google Scholar] [CrossRef]
- Yadav, Y.R.; Yadav, S.; Sherekar, S.; Parihar, V. A new minimally invasive tubular brain retractor system for surgery of deep intracerebral hematoma. Neurol. India 2011, 59, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Hernesniemi, J.; Niemelä, M.; Karatas, A.; Kivipelto, L.; Ishii, K.; Rinne, J.; Lehecka, M. Some collected principles of microneurosurgery: Simple and fast, while preserving normal anatomy—A review. Surg. Neurol. 2005, 64, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; McCall, J.G.; Jung, Y.H.; Huang, X.; Siuda, E.R.; Li, Y.; Lu, C. Injectable, Cellular-Scale Optoelectronics with Applications for Wireless Optogenetics. Science (80-) 2013, 340, 211–216. [Google Scholar] [CrossRef]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Yoon, J. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015, 33. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Gomez, A.M.; Al-Hasani, R.; Jeong, Y.R.; Kim, J.; Xie, Z.; Lee, J.L. Flexible Near-Field Wireless Optoelectronics as Subdermal Implants for Broad Applications in Optogenetics. Neuron 2017, 93, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Shin, G.; McCall, J.G.; Al-Hasani, R.; Norris, A.; Xia, L.; Jang, K.I. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proc. Natl. Acad. Sci. USA 2016, 113, E8169–E8177. [Google Scholar] [CrossRef]
- Mickle, A.D.; Won, S.M.; Noh, K.N.; Yoon, J.; Meacham, K.W.; Xue, Y.; Kim, D.H. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 2019, 565, 361–365. [Google Scholar] [CrossRef]
- Yao, K.; Lee, K.; Xu, M.; Lee, F.C. Optimal design of the active droop control method for the transient response. In Proceedings of the IEEE Eighteenth Annual IEEE Applied Power Electronics Conference and Exposition (APEC), Miami Beach, FL, USA, 9–13 February 2003; Volume 2, pp. 718–723. [Google Scholar] [CrossRef]
- Lee, S.H.; Bang, J.S.; Yoon, K.S.; Hong, S.W.; Shin, C.S.; Jung, M.Y.; Cho, G.H. A 0.518 mm2 quasi-current-mode hysteretic buck DC-DC converter with 3 μs load transient response in 0.35 μm BCDMOS. In Proceedings of the IEEE International Solid-State Circuits Conference—(ISSCC) Digest of Technical Papers, San Francisco, CA, USA, 22–26 February 2015; Volume 58, pp. 214–215. [Google Scholar] [CrossRef]
- Wu, J.; Yan, L.; Xu, H.; Tang, W.C.; Zeng, F.G. A curvature-controlled 3D micro-electrode array for cochlear implants. In Proceedings of the IEEE 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Digest of Technical Papers. TRANSDUCERS ’05, Seoul, Korea, 5–9 June 2005; Volume 2, pp. 1636–1639. [Google Scholar] [CrossRef]
- Jones, G.M. The Functional Significance of Semicircular Canal Size. In Vestibular System Part 1: Basic Mechanisms; Springer: Berlin/Heidelberg, Germany, 1974; pp. 171–184. [Google Scholar]
- Im, K.; Lee, J.M.; Lyttelton, O.; Kim, S.H.; Evans, A.C.; Kim, S.I. Brain size and cortical structure in the adult human brain. Cereb. Cortex 2008, 18, 2181–2191. [Google Scholar] [CrossRef]
- Sato, T.; Adachi, Y.; Tomori, M.; Ishii, S.; Kawabata, S.; Sekihara, K. Functional Imaging of Spinal Cord Electrical Activity from Its Evoked Magnetic Field. IEEE Trans. Biomed. Eng. 2009, 56, 2452–2460. [Google Scholar] [CrossRef]
- Wei, H.Y.; Soleimani, M. Hardware and software design for a National Instrument-based magnetic induction tomography system for prospective biomedical applications. Physiol. Meas. 2012, 33, 863–879. [Google Scholar] [CrossRef]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.H.; Mahoney, B.J.; Lee, G.; Smith, J.R. Optimal coil size ratios for wireless power transfer applications. In Proceedings of the 2014 IEEE International Symposium on Circuits and Systems (ISCAS), Melbourne, VIC, Australia, 1–5 June 2014; Volume 1, pp. 2045–2048. [Google Scholar] [CrossRef]
- Rossi, M.A.; Go, V.; Murphy, T.; Fu, Q.; Morizio, J.; Yin, H.H. A wirelessly controlled implantable LED system for deep brain optogenetic stimulation. Front. Integr. Neurosci. 2015, 9, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaertner, A.; Belloni, P. Analysis and simulation of the illumination optics of rigid medical endoscopes. Curr. Dir. Biomed. Eng. 2018, 4, 169–172. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Vignoni, M. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Jurczyszyn, K.; Woźniak, M.; Symonowicz, K.; Sprutta, N.; Latos-Grażyński, L.; Ziółkowski, P.; Trzeciakowski, W. Assessment of in vivo experiments: The newly synthesized porphyrin with proper light source enhanced effectiveness of PDT comparing to 5-ALA-mediated PDT. Photodiagnosis Photodyn. Ther. 2017, 18, 179–184. [Google Scholar] [CrossRef]
- Marra, K.; LaRochelle, E.P.; Chapman, M.S.; Hoopes, P.J.; Lukovits, K.; Maytin, E.V.; Pogue, B.W. Comparison of Blue and White Lamp Light with Sunlight for Daylight-Mediated, 5-ALA Photodynamic Therapy, in vivo. Photochem. Photobiol. 2018, 94, 1049–1057. [Google Scholar] [CrossRef]
- van Manen, L.; Handgraaf, H.J.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef]
- Blau, R.; Epshtein, Y.; Pisarevsky, E.; Tiram, G.; Dangoor, S.I.; Yeini, E.; Scomparin, A. Image-guided surgery using near-infrared Turn-ON fluorescent nanoprobes for precise detection of tumor margins. Theranostics 2018, 8, 3437–3460. [Google Scholar] [CrossRef]
- López-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Pérez-Plasencia, C.; Álvarez-Sánchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef]
- Kulms, D.; Schwarz, T. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed. 2000, 16, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, M.; Randers-Pehrson, G.; Bigelow, A.W.; Trivedi, S.; Lowy, F.D.; Spotnitz, H.M.; Brenner, D.J. 207-nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. I: In Vitro Studies. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, M.; Stanislauskas, M.; Ponnaiya, B.; Bigelow, A.W.; Randers-Pehrson, G.; Xu, Y.; Brenner, D.J. 207-nm UV light—A promising tool for safe low-cost reduction of surgical site infections. II: In-vivo safety studies. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Asano, K.; Morimoto, Y.; Igarashi, T.; Hamblin, M.R.; Dai, T.; Nakane, A. Disinfection and healing effects of 222-nm UVC light on methicillin-resistant Staphylococcus aureus infection in mouse wounds. J. Photochem. Photobiol. B Biol. 2018, 178, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, P.J.; Gallagher, M.P. Light as a broad-spectrum antimicrobial. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Process | Purpose | Required Time for 10 Devices | Equipment/Tools | Progress Level (%) ‡ |
|---|---|---|---|---|
| 1. Preparation of Copper/Polyimide film on the glass | Sampling for preparation of flexible circuits | 0.5 h | Scissors Kapton tape | 6% |
| 2. UV photo-lithography | Define patterns including copper traces and contact pads | 2 h | Cleanroom Spin-coater Mask aligner | 30% |
| 3. Components transfer | Mount electrical components on a substrate by soldering | 2 h | Microscope (Aven, SPZ-50) Ultrasonic cleaner (VEVOR) Solder Enameled wire | 54% |
| 4. Functional testing | Verify that devices functions as designed | 1 h | Magnets | 66% |
| 5. PDMS encapsulation | Device packaging-1 | 1 h | Vacuum ovens (AI, Accu Temp 1.9) | 78% |
| 6. Sponge encapsulation | Device packaging-2 | 0.5 h | Patty Tweezers | 84% |
| 7. PDMS encapsulation | Device packaging-3 | 1 h | Vacuum ovensv (AI, Accu Temp 1.9) | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.S.; Hong, S.; Morgan, C.; Nakaji, P.; Lawton, M.T.; Park, S.I. A Soft, Biocompatible Magnetic Field Enabled Wireless Surgical Lighting Patty for Neurosurgery. Appl. Sci. 2020, 10, 2001. https://doi.org/10.3390/app10062001
Kim WS, Hong S, Morgan C, Nakaji P, Lawton MT, Park SI. A Soft, Biocompatible Magnetic Field Enabled Wireless Surgical Lighting Patty for Neurosurgery. Applied Sciences. 2020; 10(6):2001. https://doi.org/10.3390/app10062001
Chicago/Turabian StyleKim, Woo Seok, Sungcheol Hong, Clinton Morgan, Peter Nakaji, Michael T. Lawton, and Sung Il Park. 2020. "A Soft, Biocompatible Magnetic Field Enabled Wireless Surgical Lighting Patty for Neurosurgery" Applied Sciences 10, no. 6: 2001. https://doi.org/10.3390/app10062001
APA StyleKim, W. S., Hong, S., Morgan, C., Nakaji, P., Lawton, M. T., & Park, S. I. (2020). A Soft, Biocompatible Magnetic Field Enabled Wireless Surgical Lighting Patty for Neurosurgery. Applied Sciences, 10(6), 2001. https://doi.org/10.3390/app10062001






