Abstract
Zinc oxide and zinc oxide doped with tin oxide (ZnO-SnO2) particles were synthesized and successfully incorporated into a polymeric matrix by the photopolymerization reaction in the presence of Irg819 as the photoinitiator. The obtained samples were investigated by means of XRD, ESEM/EDX, TEM, FTIR, and Raman spectroscopy. The ZnO particles were obtained in the form of rods agglomerated in flower (or star) structures with lengths of 2–4 μm and widths between 30 and 100 nm, while ZnO-SnO2 samples evolved in the form of cubes, with sides of 350 nm. The prepared composite films with ZnO and ZnO-SnO2 particles were tested in the photocatalytic degradation of malachite green (MG) dye. While the ZnO-based composite film showed a fairly high photocatalytic activity, the hybrid film containing ZnO doped with SnO2 displayed 100% photocatalytic activity after only 45 min of irradiation, being among the most efficient photocatalysts known for MG degradation. In addition, the recycling tests demonstrated that this film displayed high stability during the photocatalysis reaction since no decrease in the photocatalytic performance was noticed after the first three cycles, indicating its suitability for dyes removal and wastewater purification.
1. Introduction
The increasing amount of pigments and dyes existing in the wastewater coming from the dye manufacturing and textile finishing industry significantly affects the water quality and reduces the photosynthetic reaction of aquatic flora by preventing the sunlight to reach it. Also, some of these dyes are known to be toxic with carcinogenic effects [,]. The dyes that accidentally arrive in the wastewater are very difficult to destroy using regular purification methods due to their high stability to oxidizing agents, light, and even to aerobic digestion [,]. In recent years, various processes based on both chemical and physical principles have been used to treat the wastewater effluents containing different dyes, among which electrochemical methods, hypochlorite, or ozone oxidation could be mentioned. These methods are usually costly, non-damaging, and ineffective since secondary waste products may result in these processes []. Therefore, it is necessary to find new tools and methods for removing dyes from the water.
Recently, fairly extensive research was concentrated on the development of new semiconducting materials for different applications, namely, photocatalysts for water disinfection and cleaning [,,,,,]. Over time, many photocatalysts, such as TiO2, ZnO, SnO2, CdS, etc., have been applied for the decomposition of organic dyes and air pollutants [,,]. Among them, TiO2-based materials have proved to have a very good catalytic activity due to their appropriate properties and large specific surface area that allow reactions to occur [,]. This is the reason why TiO2 is the most suitable semiconductor used in catalytic applications, such as the degradation of organic pollutants or oil spills from wastewater [,]. Apart from TiO2, the second well-known photocatalyst is ZnO, having a significant role in materials research due to its high stability, low price, and nontoxicity. However, both TiO2 and ZnO semiconductors have some limitations, such as agglomeration of the smaller particles into significantly larger ones, poor response to visible-light, or fast recombination rate of the photogenerated holes and electrons [,,]. These issues lead to a decreased photocatalytic efficiency that obstructs the potential practical applications of these photocatalysts in the degradation of contaminants in air and water. Several methods have been applied to overcome these problems; in the case of TiO2, for instance, the photocatalysts can be dispersed in several clay minerals, including montmorillonite, saponite, or nanotubes, to solve the agglomeration problems [,]. To increase the photocatalytic activity of ZnO, different approaches, such as reductive reactions [,], coupling with metal oxides [] or semiconductors, such as LaFeO3 [], metal or non-metal doping, e.g., Mg, Ni, Sn [,,], or surface modification [] have been developed. The photocatalytic performance of ZnO could also be enhanced by modifying the morphology through different synthetic methods, like electrochemical deposition [], electrospinning [], vapor-phase synthesis [], they have been used for the synthesis of ZnO materials with specific nanostructures [,].
In our previous studies [,,], the development of nanosized ZnO with flower and spherical shapes was reported, as well as ZnO coupled with Ag nanoparticles that were obtained in situ during the photopolymerization process. Using this method, a mixture of monomers and nanoparticles was used to achieve flexible films whose photocatalytic activities were evaluated by using Nile red, methylene blue, and rhodamine B as model organic dyes. According to literature data, an improvement of the photocatalytic activity may be obtained by doping ZnO with metals [,,]. Considering this, our current research was focused on the development of ZnO doped with tin dioxide (SnO2), another inorganic material broadly used in photocatalysis due to its photosensitivity, transparency, and chemical stability. SnO2 has excellent properties, such as chemical and thermal stabilities, and found applications in dye-sensitized solar cells [], photocatalyst [], gas sensors [], and so on [,,]. In this study, the doping of ZnO particles with Sn ions was carried out by a chemical process, and their physicochemical characteristics were thoroughly explored; the purpose of this doping was to make structural modifications and the possibility to use these particles in simulated solar light photodegradation experiments. Besides, a new polymer/ZnO-SnO2 hybrid composite material was prepared through a convenient combination of particles and photopolymerization process, designed for efficient photodegradation of malachite green dye.
2. Results and Discussion
2.1. Structural Characterization of the Monomer
The N,N-(diisopropylcarbamoyloxy)ethyl methacrylate (N-MA) monomer was obtained by the reaction of N,N-diisopropylcarbamoyl chloride with 2-hydroxyethyl methacrylate (HEMA), its structure being fully confirmed by FTIR, 1H, and 13C NMR analyses. The 1H and 13C NMR spectra of N-MA monomer are illustrated in Figure 1a,b, respectively. The 1H NMR spectra highlighted the presence of characteristic unsaturated monomer protons at 6.15 and 5.58 ppm due to the trans/cis configuration of methylene protons at 4.37-4.26 ppm, methine protons at 3.85 ppm, and methyl protons at 1.21 ppm. Supplementary confirmation of the correct monomer structure was brought by 13C NMR analysis. In Figure 1b, the peaks at 166.16 and 154.38 ppm are assigned to the ester carbon, while the peaks at 135.08 ppm and 124.88 ppm are attributed to the carbon atoms engaged in the formation of the double bond. The carbon atoms from the methylene group were found at 61.96 ppm and 59.68 ppm; the ones from the methine group arose in the interval between 46.41 and 44.87 ppm, while the methyl carbons appeared at 19.57 ppm and 17.35 ppm.
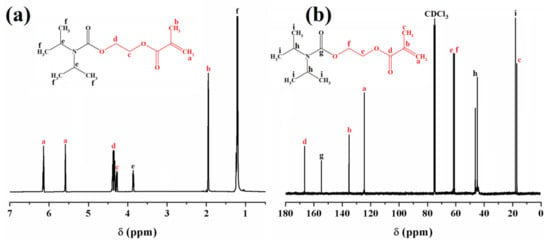
Figure 1.
1H NMR (a) and 13C NMR; (b) spectra of N,N-(diisopropylcarbamoyloxy)ethyl methacrylate (N-MA) in CDCl3.
In the FTIR spectrum shown in Figure S1, Supporting Information (SI), the monomer N-MA displayed absorption bands characteristic for C-H stretching vibrations at 2971-2877 cm−1, carbonyl groups at 1723 cm−1, C=C bonds at 1638 and 815 cm−1, and C-O-C unit at 1291 cm−1. This analysis brought additional proof for the right structure of the synthesized monomer.
2.2. Particle Characterization
One of the main objectives of this study was the development and investigation of some hybrid composites containing ZnO-doped particles intended for photochemical purification of wastewater. Besides the synthesis of the monomer, ZnO and ZnO-SnO2 particles were prepared by the method detailed in the Experimental section. Figure 2a shows typical X-ray diffraction (XRD) patterns for the obtained ZnO and ZnO-SnO2. The pure ZnO sample displayed distinct peaks at 2θ = 31.8°, 34.4°, 36.2°, 47.6°, 56.6°, 62.9°, 68°, 69.2°, 66.4°, 72.6°, 77.1° corresponding to (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202) planes, with no evidence for any secondary phase or possible impurity, which was in line with the standard hexagonal (wurtzite) ZnO powder (standard JCPDS card no. 80-0075) []. The XRD spectrum of ZnO-SnO2 exhibited broad diffraction peaks at 26.1°, 34.3°, and 52.2°, which could be attributed to the (110), (101), and (211) diffraction planes characteristic for the tetragonal structure of SnO2. The characteristic peaks of ZnO could not be accurately distinguished with respect to those of SnO2. Thus, it could be assumed that the ZnO-SnO2 sample was in an amorphous phase [,,].
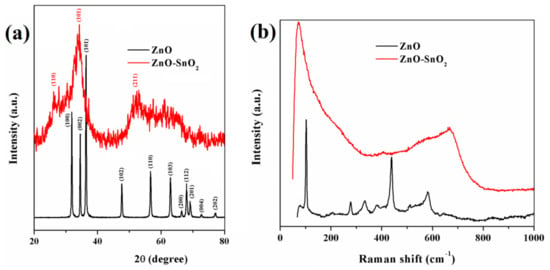
Figure 2.
X-ray diffraction patterns (a) and Raman spectra (b) of ZnO and ZnO-SnO2 powders.
Figure 2b illustrates the Raman spectra of the ZnO and ZnO-SnO2 particles at room temperature. The peaks recorded for the ZnO sample at 101, 277, 331, 379, 438, and 580 cm−1 could be associated with the hexagonal ZnO structure, the spectrum being almost identical to the reference one, reported in the literature [,]. On the other hand, significant peak shifts could be observed for the ZnO–SnO2 sample as compared to the pure ZnO, changes that are probably caused by the appearance of heterostructures between the ZnO and SnO2. For this sample, the peaks were found at 71, 404, 667, and 580 cm−1, the last one characteristic for ZnO being in the form of a small shoulder [,]. The peak at 667 cm−1 could be associated with the oxygen vacancies of SnO2 nanocrystal surface defects [,].
The FTIR analysis of undoped ZnO and ZnO-SnO2 particle samples was also performed (Figure S2a, SI). The results revealed that the peaks registered at 3435 cm−1 and 1637 cm−1 for ZnO and 3400 cm−1 and 1639 cm−1 for ZnO-SnO2 originated from the adsorbed H2O molecules on the particles surfaces. The bands at 516 and 393 cm−1 could be associated with the absorptions of the Zn-O bond []. The incorporation of Sn to ZnO nanostructures led to the appearance of a large band, much intense compared to that of the pure sample at 560 cm−1, which could be attributed, according to the literature data, to the Sn–O bond vibration [].
Figure 3 illustrates the SEM and TEM micrographs of ZnO and ZnO-SnO2 particles. The SEM images (Figure 3a) showed that the ZnO sample was in the form of flowers (or stars) with homogeneous rod shape. TEM analysis (Figure 3b) supported this observation, demonstrating that ZnO particles were in the form of homogeneous rods crowded in floral geometries with lengths of 2–4 μm and widths between 30 and 100 nm. For ZnO-SnO2 particles, the SEM images (Figure 3c) evidenced their form of cubes with sides of approximately 350 nm, as calculated from the TEM analysis (Figure 3d). Compared to the shape of ZnO nanoparticles doped with SnO2, reported in the literature [,,], which have irregular or spherical shapes, our particles displayed well-defined cubic forms, never seen before.
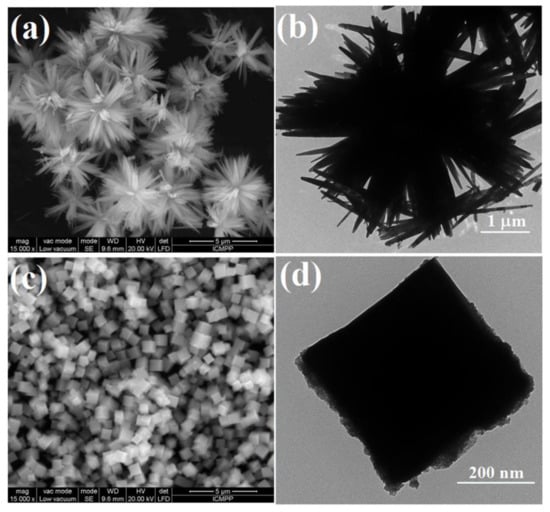
Figure 3.
SEM and TEM images of ZnO (a,b) and ZnO-SnO2 (c,d).
The elemental and spatial distribution of the tin and zinc atoms within the ZnO-SnO2 composite was also explored by using the energy-dispersion X-ray (EDX) spectroscopy. According to Figure 4, the EDX chart clearly evidenced that Sn, Zn, and O atoms were the main components of the sample. The observed peak characteristic for C atoms is supposed to come from the instrument holder. The mapping images indicated that Zn and Sn were uniformly distributed in the analyzed sample.
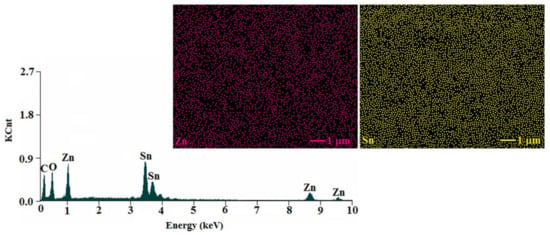
Figure 4.
The spectrum of the cube-like ZnO–SnO2 nanoparticles and mapping images for zinc and tin.
2.3. Synthesis and Characterization of Polymer and Polymer Hybrid Films
Polymer films were prepared by the photopolymerization of poly(propylene glycol) dimethacrylate (PO-UDMA) and N,N-(diisopropylcarbamoyloxy)ethyl methacrylate (N-MA) in a percent weight ratio of 60:40, by using 1.5 wt.% Irg819 as photoinitiator, at room temperature. The same method was applied for the synthesis of hybrid polymer films when the monomers were photopolymerized in the presence of ZnO or ZnO-SnO2 particles (Ps) (Scheme 1), according to the formulations presented in Table 1. The irradiation was carried out with a 365 nm UV lamp at a light intensity of 40 mW/cm2.
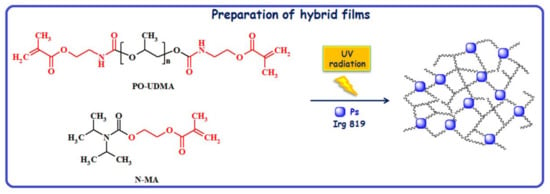
Scheme 1.
Synthesis path towards ZnO or ZnO-SnO2 samples.

Table 1.
Data for L1, L2, and L3 formulations.
The conversion degree of the characteristic methacrylate double bond in the obtained formulations was assessed on the basis of FTIR spectroscopy; the related data is presented in Table 1. As could be seen in Figure 5, the C=C double bond stretching vibration at 1638 cm−1 fell with the enhancement of the UV exposure time, the conversion degree being approx. 98.15% after 60 s of irradiation in the case of sample L1. Under the same regime of lightning, the methacrylate double bonds (C=C) of samples L2 and L3 were transformed into C-C bonds with a conversion degree of 92.94% and 93.10%, respectively []. These results were better compared to our previous reports in which the PO-UDMA monomer was used in combination with another synthesized monomer [] or with a commercially available one [].
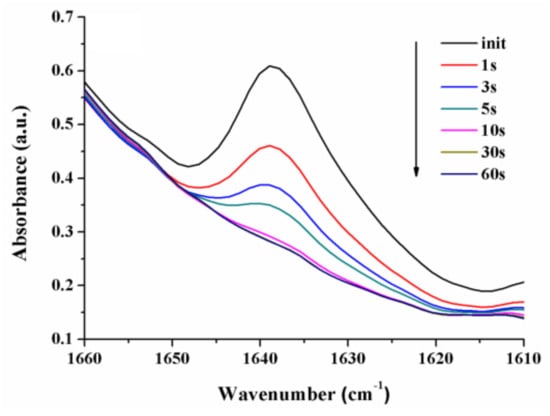
Figure 5.
Modification of the double bond FTIR absorption band for L1 formulation during the irradiation with UV light.
The results indicated that the kinetics of the photopolymerization processes was scarcely affected by the incorporation of the inorganic particles. However, the low difference obtained for the conversion degree could be explained in terms of the restriction effect manifested by ZnO or ZnO-SnO2 particles (Ps) on the mobility of the reactive centers, which led to the decrease of the methacrylic double-bond conversion degree []. In addition, the lower conversion values for both samples incorporating Ps could be due to the high ability of ZnO to capture the UV light [,].
Figure 6 illustrates the UV-Vis transmittance spectra of L1, L2, and L3 thin films. Accordingly, all films exhibited good transparency in the region stretching on the visible range. While the transmittance of L1 film was almost 85% for wavelengths beyond 350 nm, the same parameter for the films with 1wt.% ZnO and 1 wt.% ZnO-SnO2 was beyond 80% and 70%, respectively. The slightly lower value of UV-Vis transparency for L3 film could be explained by the electron transitions between the valence and conduction bands. Also, the lower transparency of the film containing doped particles could be due to the UV and visible photons captured by the particles incorporated into the film [].
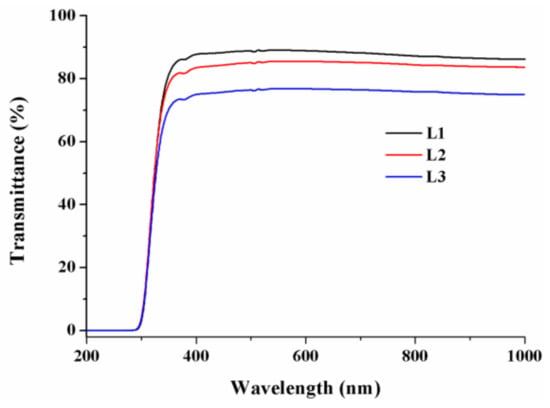
Figure 6.
Transmittance spectra of L1–L3 thin films.
To thoroughly examine the morphology of the obtained polymeric films, they were broken in liquid nitrogen and then analyzed in the fracture section. The film homogeneity and particle distribution in the hybrid composites were assessed by scanning electron microscopy, as shown in Figure 7. The SEM image taken in fracture for the L1 sample, which had no particles in the composition (Figure 7a), indicated a homogenous structure without pores, the noticed irregularities being attributed to the way the film was broken. On the other hand, as observed in Figure 7b, the L2 formulation contained ZnO in the form of stars, homogeneously distributed in the polymer matrix, while the L3 fracture (Figure 7c) evidenced cube-like forms of ZnO doped with SnO2 incorporated into the polymer matrix.
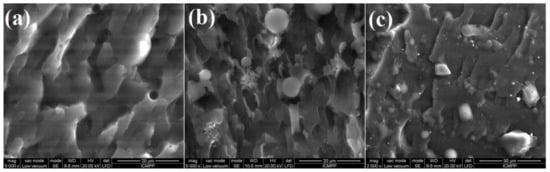
Figure 7.
SEM images of polymeric films (in fracture) for L1 without particles (a), L2 with ZnO (b) and L3 with ZnO-SnO2 (c).
The composition and spatial atoms distribution into the composite films based on L1, L2, and L3 formulations were surveyed by EDX pectroscopy and elemental dot mapping of the fractured films, which allowed us to asses the main atoms scattered in the samples (Figure 8).
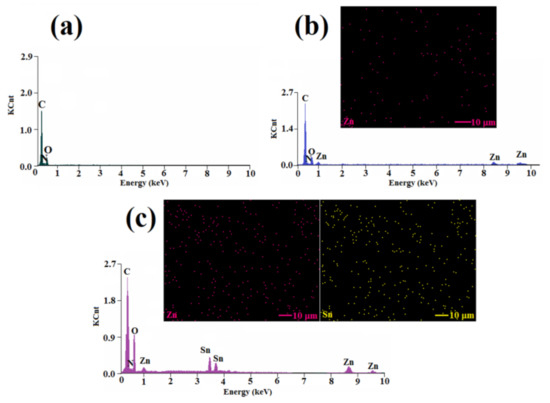
Figure 8.
EDX analysis results for L1 (a) and the mapping images registered for L2 (b) and L3 (c) formulations.
Thus, the EDX spectrum of the L1 formulation was characterized by the presence of three peaks that corresponded to C, O, and N atoms (Figure 8a), in good agreement with the composition of the photopolymerized film without nanoparticles. For the L2 sample, besides the peaks for C, N, and O, some peaks characteristic for Zn atoms were clearly evidenced (Figure 8b), demonstrating that ZnO particles were successfully incorporated into the polymeric film. Moreover, the bright spots in the mapping image of Zn atoms indicated a uniform distribution of the atoms in the film. The EDX analysis of the L3 fractured film, illustrated in Figure 8c, denoted the presence of clear peaks characteristic for C, O, N, Zn, and Sn, a clear proof for the incorporation of ZnO-SnO2 particles into the film during photopolymerization. Also, the uniform distribution of zinc and tin atoms inside the L3 photocrosslinked film could be remarked. The EDX analysis allowed the estimation of the content of Zn atoms to be as 0.88 wt.% in the L2 film, while in the L3 film, the content of the Zn and Sn atoms was found to be 0.96 wt.% and 0.92 wt.%, respectively.
2.4. Photocatalytic Activity
As mentioned in the literature, ZnO, in combination with other metal particles, can degrade various organic dyes in wastewater, including methylene blue (MB) [] or rhodamine B [], among others []. Consequently, we considered challenging to investigate the photocatalytic degradation of malachite green (MG) dye under irradiation with simulated solar light, by using L2 and L3 composite films as photocatalysts. To highlight the efficiency of photocatalysts L2 and L3, we firstly studied the photo-optical behavior of MG dye during the irradiation in the absence of any photocatalyst and when L1 film was used (Figure S3 SI). Although malachite green (MG) has a high photostability, it was observed that the absorption band at 616 nm was reduced with only 9% after 180 min of simulated solar light irradiation in the absence of any photocatalyst (Figure S3a). In the presence of L1 film (Figure S3b), there was a slight influence of about 3% (12% degradation after 60 min of irradiation) due to an absorption process, which produced a color change in this film from translucent white to a bluish color. This was more likely due to the absorption of the dye on the surface of the film, rather than a degradation process.
Figure 9 illustrates the evolution of the UV-Vis absorption spectra of MG dye in aqueous solutions at a concentration of 10−5 M, as was induced by composite films L2 and L3 under simulated solar light exposure, recorded after different irradiation times.
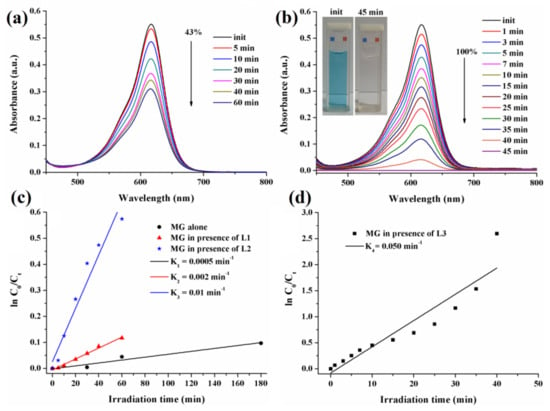
Figure 9.
Irradiation-induced evolution of UV-Vis absorption spectra of malachite green (MG) solutions when L2 (a) and L3 (b) were used as photocatalysts and the corresponding kinetic curves for MG dye degradation (c,d).
The characteristic absorption band of the MG dye at 616 nm registered an absorbance decrease of 43% after 60 min of irradiation when assisted by L2 film (Figure 9a), while it totally disappeared when L3 hybrid film was used as photocatalyst after only 45 min exposure to simulated solar light (Figure 9b), proving 100% photodegradation activity of L3 film with no signature for intermediary products (Figure S4, SI). This change could be viewed with the naked eye (Figure 9b, inset). These experimental features highlighted that the L3 film, containing doped ZnO-SnO2 particles, exhibited higher photocatalytic performance than the L2 film, which contained only ZnO particles. The superior photocatalytic response of ZnO-SnO2 could be associated with the cubic shape of the particles incorporated in the polymer matrix, increased light absorption, and reduced electron-hole pair recombination.
To further asses the performance of L2 and L3 photopolymerized hybrid films as photocatalysts, the kinetic rate constant K was determined by using the formula Kt = ln C0/Ct, where t is the irradiation time (min), C0 is the initial concentration of MG dye, and Ct represents the concentration after t min of irradiation, by assuming that the process follows pseudo-first-order kinetics []. Figure 9c,d show the ln C0/Ct plots as a function of the irradiation time. The kinetic rate constant for MG alone was K1 = 0.0005 min−1, and it increased to K2 = 0.002 min−1 for L1 film, K3 = 0.01 min−1 for L2 film, and K4 = 0.050 min−1 for L3 film. These results brought additional proof that the ZnO-SnO2 cubes embedded in the polymer matrix exhibited a superior photocatalytic response compared to the equivalent samples containing ZnO star shapes.
The mechanism followed by different dyes during the photocatalytic decomposition is described in detail in the literature [,], as well as the reactions that occurred on the surface of the film [].
To evaluate the reusability of the L3 composite film, repeated photocatalytic tests were performed using this sample. As illustrated in Figure 10, the photodegradation activity of the ZnO-SnO2 composite was retained even after five consecutive cycles of reuse with an insignificant reduction of the photocatalytic performance. Such behavior suggested an excellent photostability of the photocatalyst. Photocrosslinked films were used since they maintain their integrity, and the particle leaching does not occur. Also, materials could be reused more easily than by using only simple particles, as previously demonstrated in the literature [,,].
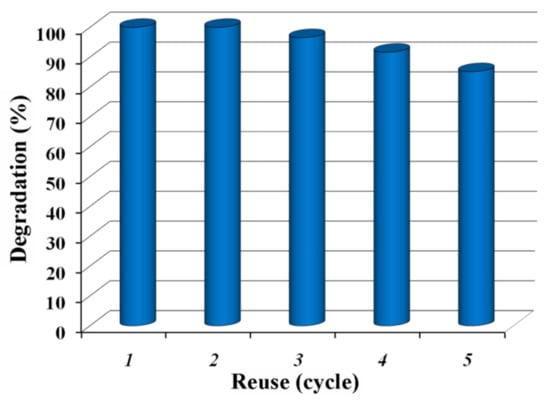
Figure 10.
Reusability test for the L3 composite film (the estimated degradationin % corresponds to 45 min irradiation time).
Additionally, to demonstrate that dye degradation took place as previously described, the 1H NMR spectra and total organic carbon (TOC) analysis of MG water solution before and after irradiation were registered. 1H NMR spectroscopy was used to identify the products that might appear during the degradation of MG. In Figure S5a, the appearance of the methyl group protons of MG at 1.28 ppm and 3.24 ppm were observed, while the signals corresponding to the aromatic protons were found between 5.82 ppm and 7.74 ppm. After visible irradiation in the presence of an L3 composite film containing ZnO-SnO2, the disappearance of the peaks corresponding to the MG dye along with solution discoloration occurred (Figure S5b), demonstrating that the degradation process took place. This also demonstrated that the film was not subjected to photodegradation and was stable under irradiation with simulated solar light.
The mineralization of MG dye and its decomposition to CO2 was investigated by measuring the concentration of total organic carbon (TOC) in the solution irradiated with simulated solar light in the presence of the L3 film. The TOC analysis indicated mineralization of about 81%, the result suggesting that the present photocatalyst showed effective activity in the discoloration process and removal of dye organic carbon under simulated solar radiation.
3. Materials and Methods
3.1. Materials
Poly(propylene glycol) (Mw =1000 g/mol), 2-isocyanatoethyl methacrylate, dibutyltin dilaurate, N,N-diisopropylcarbamoyl chloride, 2-hydroxyethyl methacrylate (HEMA), triethylamine, zinc nitrate hexahydrate, phenyl-bis(2,4,6-trimethylbenzoyl) phosphine oxide (Irg819), ammonia solution (25%), tin(IV) chloride pentahydrate, malachite green (MG), tetrahydrofuran (THF), methylene chloride, and deionized water were provided from Sigma-Aldrich, (Merck, Germany) and used without further purifications.
3.2. Synthesis
3.2.1. Poly(propylene glycol) Dimethacrylate (PO-UDMA)
Poly(propylene glycol) dimethacrylate (PO-UDMA) was prepared following a published procedure []. Briefly, in a three-necked round bottom flask equipped with a magnetic stirrer, poly(propylene glycol) (4 mmol) and 2-isocyanatoethyl methacrylate (8 mmol) were mixed in 10 mL anhydrous THF, by using dibutyltin dilaurate as a catalyst. The product evolution was monitored by FTIR, following the disappearance of the absorption band characteristic for the isocyanate unit at ~2260 cm−1. The reaction was continuously stirred for 24 h at 38 °C, and after that, the solvent was removed under reduced pressure. Finally, the monomer was obtained as a viscous liquid.
3.2.2. N,N-(diisopropylcarbamoyloxy)Ethyl Methacrylate (N-MA)
In a 50 mL three-necked round-bottomed flask equipped with a magnetic stirrer, a dropping funnel, and a thermometer, 5 g (31.17 mmol) of N,N-diisopropylcarbamoyl chloride and 4.33 mL (31.17 mmol) of triethylamine were placed in 10 mL methylene chloride. Then, the reaction was allowed to cool to 0 °C, and 3.82 mL (31.17 mmol) of HEMA in 5 mL methylene chloride was dropped with stirring for 1 h. After 24 h of the mixture at room temperature, the liquid monomer was obtained after solvent removal under reduced pressure.
FTIR (KBr, cm−1): 2971-2877 (aliphatic C–H stretching); 1723 (C=O stretching), 1638 and 815 (C=C stretching); 1291 (C-O-C stretching).
13C NMR in CDCl3 (δ, ppm): 166.16 and 154.38 (>C=O), 135.08 and 124.88 (>C=CH2), 61.96 and 59.68 (-CH2-), 46.41-44.87 (-CH-), 19.57 and 17.35 (CH3).
3.2.3. ZnO Particles
This synthesis was carried out in a 500 mL three-necked flask, by using 13.9 mmol of zinc nitrate hexahydrate and 350 mL deionized water. The pH was allowed to reach 10-11 by using ammonia (25%) when the solution evolved from turbid to clear. Then, the resulting mixture was refluxed under vigorous stirring for 3 h to allow the evolution of ZnO particles. The solid product was separated by centrifugation, followed by washing with deionized water and drying for 1 day at room temperature. Then, the as-resulted solid was calcinated in the air at 500 °C for 3 h, when ZnO particles were achieved in the form of white powder (bandgap for ZnO particles is 3.3 eV, values were calculated according to the literature [,]).
3.2.4. ZnO-Doped SnO2 Particles
Doped ZnO particles were prepared using the identical procedure described above. A molar ratio of 1:1 zinc nitrate hexahydrate (Zn) to tin (IV) chloride pentahydrate (Sn) was used. After isolation by centrifugation and washing with deionized water, the product was dried for 1 day at room temperature and calcinated at 500 °C for 3 h. This sample was abbreviated as ZnO-SnO2 (bandgap for ZnO-SnO2 particles is 2.9 eV, values were calculated according to the literature [,]).
3.3. Films Preparation
The polymer films used in this study were synthesized through the photopolymerization of monomer mixtures, using Irg819 as photoinitiator, the experimental composition for films being presented in Table 1. In order to achieve homogeneity, several drops of acetone were introduced, and then the mixtures were cast on glass or Teflon plates and photopolymerized. The irradiation was carried out with a Hg–Xe lamp (Hamamatsu Lightningcure Type LC8, Model L9588) at 365 nm, having a light intensity of 40 mW/cm2.
3.4. Photocatalytic Activity Investigation
The photopolymerized films were studied with regard to their photocatalytic efficiency by investigating the degradation process of malachite green (MG) in aqueous solutions (pH = 5), under continuous stirring, in ambient conditions and under irradiation with light from visible range. The same device, Hamamatsu Lightningcure Type LC8, Model L9588 was used for the degradation experiments, only that the lamp was changed to a Xe type visible light source (λ = 400–800 nm, maximum at 437 nm, UV contribution of ~ 5 mW/cm2), which mimics sunlight. Apart from that, each photopolymerized film (~1 g) was introduced into 50 mL of MG solutions. This mixture was irradiated by a programmed time regime with the visible light source coming from the Xe lamp. At a given time interval, 1.5 mL of the solution was taken and further analyzed by UV-vis spectrometry. The experimental set-up was added to the Supporting Information (SI) section, Figure S6.
3.5. Measurements
The structure of the synthesized monomers was identified by FTIR, 1H, and 13C NMR spectroscopies. 1H and 13C NMR spectra were registered on a Bruker 400MHz spectrometer at room temperature (BRUKER, Karlsruhe, Germany). The NMR experiments were recorded with a 5 mm four nuclei direct detection z-gradient probe. Chemical shifts were reported in δ units (ppm) and were referenced to the residual solvent signal (D2O at 4.78 ppm for 1H). NMR spectra were recorded using standard pulse sequence as delivered by Bruker with TopSpin 4.0.5 spectrometer control and processing software. All the spectra were registered at room temperature (24 °C) in D2O water at pH = 5, with acquisition parameters ns = 64. The Fourier transform infrared (FTIR) spectra were recorded on a Bruker Vertex 70 FTIR spectrometer (BRUKER, Karlsruhe, Germany). To evaluate the conversion degree by FTIR analysis, homogeneous mixtures of photopolymerizable monomers were synthesized by following the data listed in Table 1 and then manually coated on KBr pellets and irradiated with UV light. The FTIR absorption spectra were registered at different irradiation times, and the modification, which appeared in the C=C stretching vibration at 1638 cm−1, was used to estimate the degree of conversion. The irradiation of the monomer mixtures containing ZnO or ZnO-SnO2 was carried out by using the UV light with an intensity of 40 mW/cm2 generated by a Hg-Xe lamp (Hamamatsu Lightningcure Type LC58, Model L9588, HAMAMATSU PHOTONICS K.K., Iwata City, Shizuoka Pref., Japan). The UV-Vis absorption spectra were obtained on a Perkin Elmer Lambda 2 spectrophotometer (PerkinElmer Inc., Wellesley, MA, United States). XRD spectra were measured on a Bruker D8 ADVANCE (BRUKER, Karlsruhe, Germany) diffractometer by using Cu Kα radiation (λ = 0.1541 nm). The spectral patterns were registered from 20° to 100°, at a scan rate of 0.02° min−1. Raman spectroscopy experiments were performed by using a Renishaw in Via confocal microscope equipped with a He-Ne laser at 632.8 nm (17 mW) and a CCD detector coupled to a Leica DM 2500 M microscope. All experiments were performed in backscattering geometry, using a 100× objective with a numerical aperture of 0.75, at room temperature and atmospheric pressure. All spectra were read with the WiRE 3.2 software (Renishaw, New Mills, GL, UK). TEM and SEM micrographs were obtained at 120 kV, by using a Hitachi High-Tech HT7700 (Hitachi High-Tech GLOBAL, Tokyo, Japan) instrument and an environmental scanning electron microscope QUANTA200 coupled with an energy-dispersive X-ray spectroscope (ESEM/EDX), respectively (FEI Company, Netherlands). The total organic carbon (TOC) of the MG solution before and after visible irradiation in the presence of L3 film was measured by using a Multi N/C 2100 Analyticjena analyzer (Analytik Jena AG, Jena, Germany).
4. Conclusions
A new monomer, namely, N,N-(diisopropylcarbamoyloxy)ethyl methacrylate (N-MA), was synthesized and used together with PO-UDMA and metal nanoparticles in the development of hybrid composites by photopolymerization. To this scope, flower-shaped ZnO and unique cube-shaped ZnO-SnO2 particles were prepared and subsequently incorporated into photopolymerizable formulations. According to FTIR spectra, the monomers displayed a good photoreactivity during the photopolymerization process, regardless of its occurrence in the absence or in the presence of metal particles. The particles were investigated with respect to their size, shape, and morphology by using several methods, such as ESEM/EDX, TEM, XRD, and Raman analyses, all proving their shape and purity. The incorporation and homogeneous distribution of ZnO and ZnO-SnO2 particles in the organic phase were fully confirmed by ESEM/EDX measurements. The photocatalytic activity tests highlighted that the prepared cube-shaped ZnO-SnO2-based photocatalyst displayed an excellent photocatalytic activity. After only 45 min of irradiation, the discoloration efficiency of MG dye in aqueous solutions achieved 100% with this photocatalyst, while for the flower-shaped ZnO composite film, degradation of 43% was obtained after 60 min of irradiation. Overall, the above results indicated that the newly prepared film based on cubic ZnO-SnO2 was an efficient photocatalyst and could be considered for future treatment of wastewater containing dyes.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/6/1954/s1.
Author Contributions
Writing—original draft preparation, V.-E.P., writing—review and editing, V.-E.P. and M.-D.D., supervision, M.-D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research and Innovation, CNCS - UEFISCDI, project number PN-III-P1-1.1-PD-2016-1718 (36/2018), within PNCDI III.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Khraisheh, M.A.M.; Al-Ghouti, M.A.; Allen, S.J.; Ahmad, M.N. Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res. 2005, 39, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, L. The adsorption of basic dyes from aqueous solution on modified peat–resin particle. Water Res. 2003, 37, 1535–1544. [Google Scholar] [CrossRef]
- Wan Ngaha, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Sun, J.H.; Sun, S.P.; Wang, G.L.; Qiao, L.P. Degradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes Pigments 2007, 74, 647–652. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Zhang, M.; Sheng, G.; Fu, J.; An, T.; Wang, X.; Hu, X. Novel preparation of nanosized ZnO–SnO2 with high photocatalytic activity by homogeneous co-precipitation method. Mater. Lett. 2005, 59, 3641–3644. [Google Scholar] [CrossRef]
- Ismail, A.A. Single-step synthesis of a highly active photocatalyst for oxidation of trichloroethylene. Appl. Catal. B Environ. 2008, 85, 33–39. [Google Scholar] [CrossRef]
- Hwangbo, M.; Claycomb, E.C.; Liu, Y.; Alivio, T.E.G.; Banerjee, S.; Chu, K.H. Effectiveness of zinc oxide-assisted photocatalysis for concerned constituents in reclaimed wastewater: 1,4-Dioxane, trihalomethanes, antibiotics, antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs). Sci. Total Environ. 2019, 649, 1189–1197. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Qin, R.; Meng, F.; Khan, M.W.; Yu, B.; Li, H.; Fan, Z.; Gong, J. Fabrication and enhanced photocatalytic property of TiO2-ZnO composite photocatalysts. Mater. Lett. 2019, 240, 84–87. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Fu, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl. Catal. B Environ. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, J.; Liu, N. Characterization of the structure and catalytic activity of copper modified palygorskite/TiO2 (Cu2+-PG/TiO2) catalysts. Mater. Sci. Eng. A 2006, 431, 256–262. [Google Scholar] [CrossRef]
- Magnone, E.; Kim, M.K.; Lee, H.J.; Park, J.H. Testing and substantial improvement of TiO2/UV photocatalysts in the degradation of methylene blue. Ceram. Int. 2019, 45, 3359–3367. [Google Scholar] [CrossRef]
- Martinez-Ortiz, M.J.; Fetter, G.; Dominguez, J.M.; Melo-Banda, J.A.; Ramos-Gomez, R. Catalytic hydrotreating of heavy vacuum gas oil on Al- and Ti-pillared clays prepared by conventional and microwave irradiation methods. Micropor. Mesopor. Mater. 2003, 58, 73–80. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Christoforidis, K.C.; Fernandez-Garcia, M.; Li, H.; Shu, Y.; Sato, T.; Katsuki, H. Three-phase nanocomposites of two nanoclays and TiO2: Synthesis, characterization and photacatalytic activities. Appl. Catal. B Environ. 2014, 147, 526–533. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Nikolopoulou, A.; Papoulis, D.; Komarneni, S.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, G.H.; Zhang, P.; Yin, S.; Sato, T. Solvothermal preparation of TiO2/saponite nanocomposites and photocatalytic activity. Appl. Clay Sci. 2009, 46, 363–368. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Nikolopoulou, A.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, H.G.; Zhang, P.; Yin, S.; Sato, T.; Katsuki, H. Palygorskite- and Halloysite-TiO2 nanocomposites: Synthesis and photocatalytic activity. Appl. Clay Sci. 2010, 50, 118–124. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Li, D.; Pan, W.; Zhang, Q.; Xu, B.; Gu, L.; Nan, C.; Wu, H. Arc-melting to narrow the bandgap of oxide semiconductors. Adv. Mater. 2015, 27, 2589–2594. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Hu, S.; Li, J. Preparation and enhanced photoelectrochemical performance of coupled bicomponent ZnO-TiO2 nanocomposites. J. Phys. Chem. C 2008, 112, 117–122. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D. Photocatalytic removal of tartrazine dye from aqueous samples on LaFeO3/ZnO photocatalysts. Chem. Eng. Trans. 2016, 52, 847–852. [Google Scholar]
- Etacheri, V.; Roshan, R.; Kumar, V. Mg-doped ZnO nanoparticles for efficient sunlight-driven photocatalysis. ACS Appl. Mater. Interfaces 2012, 4, 2717–2725. [Google Scholar] [CrossRef]
- Ali, M.B.; Barka-Bouaifel, F.; Sieber, B.; Elhouichet, H.; Addad, A.; Boussekey, L.; Ferid, M.; Boukherroub, R. Preparation and characterization of Ni-doped ZnO-SnO2 nanocomposites: Application in photocatalysis. Superlattices Microst. 2016, 91, 225–237. [Google Scholar]
- Jin, C.; Ge, C.; Jian, Z.; Wei, Y. Facile synthesis and high photocatalytic degradation performance of ZnO-SnO2 hollow spheres. Nanoscale Res. Lett. 2016, 11, 526. [Google Scholar] [CrossRef]
- Khanchandani, S.; Kundu, S.; Patra, A.; Ganguli, A.K. Shell thickness dependent photocatalytic properties of ZnO/CdS core−shell nanorods. J. Phys. Chem. C 2012, 116, 23653–23662. [Google Scholar] [CrossRef]
- Yi, J.B.; Pan, H.; Lin, J.Y.; Ding, J.; Feng, Y.P.; Thongmee, S.; Liu, T.; Gong, H.; Wang, L. Ferromagnetism in ZnO nanowires derived from electro-deposition on AAO template and subsequent oxidation. Adv. Mater. 2008, 20, 1170–1174. [Google Scholar] [CrossRef]
- Wu, H.; Lin, D.; Zhang, R.; Pan, W. ZnO Nanofiber field-effect transistor assembled by electrospinning. J. Am. Ceram. Soc. 2008, 91, 656–659. [Google Scholar] [CrossRef]
- Wang, X.; Summers, C.J.; Wang, Z.L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004, 4, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhu, J. ZnO based advanced functional nanostructures: Synthesis, properties and applications. J. Mater. Chem. 2011, 21, 599–614. [Google Scholar] [CrossRef]
- Jitaru, F.; Buruiana, T.; Podasca, V.; Buruiana, E.C. Preparation and properties of new carbazole methacrylates and their polymer composites with ZnO for photocatalysis and sensing applications. Soft Mater. 2015, 13, 67–76. [Google Scholar] [CrossRef]
- Podasca, V.E.; Buruiana, T.; Buruiana, E.C. UV-cured polymeric films containing ZnO and silver nanoparticles with UV–vis light-assisted photocatalytic activity. Appl. Surf. Sci. 2016, 377, 262–273. [Google Scholar] [CrossRef]
- Podasca, V.E.; Buruiana, T.; Buruiana, E.C. Photocatalytic degradation of rhodamine B dye by polymeric films containing ZnO, Ag nanoparticles and polypyrrole. J. Photochem. Photobiol. A Chem. 2019, 371, 188–195. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A Highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A high performance cobalt-doped ZnO visible light photocatalyst and its photogenerated charge transfer properties. Nano Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Achouri, F.; Corbel, S.; Balan, L.; Mozet, K.; Girot, E.; Medjahdi, G.; Said, M.B.; Ghrabi, A.; Schneider, R. Porous Mn-doped ZnO nanoparticles for enhanced solar and visible light photocatalysis. Mater. Des. 2016, 101, 309–316. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, C.; Hu, X.; Huang, W.; Aref, A.A.; Wang, B.; Liu, Z.; Sun, Y.; Zhou, W.; Tang, Y. Dye-sensitized solar cells based on nanoparticle-decorated ZnO/SnO2 core/shell nanoneedle arrays. Appl. Surf. Sci. 2014, 292, 111–116. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. ZnO doped SnO2 nanoparticles heterojunction photo-catalyst for environmental remediation. J. Alloy. Compd. 2015, 653, 327–333. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Ma, J.; Zhan, W.; Wang, L.; Guo, Y.; Guo, Y.; Lu, G. Catalytic combustion of methane over Pd/SnO2 catalysts. Chin. J. Catal. 2017, 38, 1322–1329. [Google Scholar] [CrossRef]
- Ozgur, M.; Pat, S.; Mohammadigharehbagh, R.; Musaoglu, C.; Demirkol, U.; Elmas, S.; Ozen, S.; Korkmaz, S. Sn doped ZnO thin film deposition using thermionic vacuum arc technique. J. Alloy. Comp. 2019, 774, 1017–1023. [Google Scholar] [CrossRef]
- Ganesh, V.; Yahia, I.S.; AlFaify, S.; Shkir, M. Sn-doped ZnO nanocrystalline thin films with enhanced linear and nonlinear optical properties for optoelectronic applications. J. Phys. Chem. Solids 2017, 100, 115–125. [Google Scholar] [CrossRef]
- Adhyapak, P.V.; Meshrama, S.P.; Mulla, I.S.; Pardeshi, S.K.; Amalnerkar, D.P. Controlled synthesis of zinc oxide nanoflowers by succinate-assisted hydrothermal route and their morphology-dependent photocatalytic performance. Mat. Sci. Semicon. Proc. 2014, 27, 197–206. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, S.; Wu, W.; Sun, L.; Lei, M.; Pan, J.; Xiong, X. Self-assemble SnO2@TiO2 porous nanowire-nanosheet heterostructures for enhanced photocatalytic property. CrystEngComm 2014, 16, 10863–10869. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Yang, S.; Wu, H.; Wu, Y.; Wu, L.; Pan, J.; Xiong, X. In situ construction of an SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- Lee, S.J.; Hwang, C.S.; Pi, J.E.; Yang, J.H.; Byun, C.W.; Chu, H.Y.; Cho, K.I.; Cho, S.H. High-performance amorphous multilayered ZnO-SnO2 heterostructure thin-film transistors: Fabrication and characteristics. ETRI J. 2015, 37, 1135–1142. [Google Scholar] [CrossRef]
- Russo, V.; Ghidelli, M.; Gondoni, P.; Casari, C.S.; Li Bassi, A. Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J. Appl. Phys. 2014, 115, 073508. [Google Scholar] [CrossRef]
- Huang, X.; Shao, L.; She, G.W.; Wang, M.; Chenac, S.; Meng, X.M. Catalyst-free synthesis of single crystalline ZnO nanonails with ultrathin caps. CrystEngComm 2012, 14, 8330–8334. [Google Scholar] [CrossRef]
- Liu, L.Z.; Li, T.H.; Wu, X.L.; Shen, J.C.; Chu, P.K. Identification of oxygen vacancy types from Raman spectra of SnO2 nanocrystals. J. Raman Spectrosc. 2012, 43, 1423–1426. [Google Scholar] [CrossRef]
- Kar, A.; Sain, S.; Rossouw, D.; Knappett, B.R.; Pradhan, S.K.; Wheatley, A.E.H. Facile synthesis of SnO2–PbS nanocomposites with controlled structure for applications in photocatalysis. Nanoscale 2016, 8, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Pal, U.; Serrano, J.G.; Ucer, K.B.; Williams, R.T. Photoluminescence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Phys. Stat. Sol. C 2006, 3, 3577–3581. [Google Scholar]
- Song, C.; Dong, X. Preparation and characterization of tetracomponent ZnO/SiO2/SnO2/TiO2 composite nanofibers by electrospinning. Adv. Chem. Eng. Sci. 2012, 2, 108–112. [Google Scholar] [CrossRef]
- Melinte, V.; Chibac, A.; Buruiana, T.; Buruiana, E.C. Hybrid nanocomposites prepared by in situ photopolymerization using photoinitiator-modified montmorillonite. Prog. Org. Coat. 2017, 104, 125–134. [Google Scholar] [CrossRef]
- Chibac, A.; Melinte, V.; Buruiana, T.; Balan, L.; Buruiana, E.C. One-pot synthesis of photocrosslinked sol–gel hybrid composites containing silver nanoparticles in urethane-acrylic matrixes. Chem. Eng. J. 2012, 200–202, 577–588. [Google Scholar] [CrossRef]
- Xue, X.; Zang, W.; Deng, P.; Wang, Q.; Xing, L.; Zhang, Y.; Wang, Z.L. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. [Google Scholar] [CrossRef]
- Zhan, Z.; Zheng, L.; Pan, Y.; Sun, G.; Li, L. Self-powered, visible-light photodetector based on thermally reduced grapheme oxide–ZnO (rGO–ZnO) hybrid nanostructure. J. Mater. Chem. 2012, 22, 2589–2595. [Google Scholar] [CrossRef]
- Sonker, R.K.; Sharma, A.; Shahabuddin, M.; Tomar, M.; Gupta, V. Low temperature sensing of NO2 gas using SnO2-ZnO nanocomposite sensor. Adv. Mat. Lett. 2013, 4, 196–201. [Google Scholar] [CrossRef]
- Chang, T.; Li, Z.; Yun, G.; Jia, Y.; Yang, H. Enhanced photocatalytic activity of ZnO/CuO nanocomposites synthesized by hydrothermal method. Nano Micro Lett. 2013, 5, 163–168. [Google Scholar] [CrossRef]
- Uddin, T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Muller, M.M.; Kleebe, H.J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2−ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Well-crystalline porous ZnO–SnO2 nanosheets: An effective visible-light driven photocatalyst and highly sensitive smart sensor material. Talanta 2015, 131, 490–498. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Y.; Xiao, A.; Liu, H. Preparation and photocatalytic property of mesoporous ZnO/SnO2 composite nanofibers. J. Alloy. Compd. 2010, 503, 103–110. [Google Scholar] [CrossRef]
- Chibac, A.L.; Buruiana, T.; Melinte, V.; Buruiana, E.C. Photocatalysis applications of some hybrid polymeric composites incorporating TiO2 nanoparticles and their combinations with SiO2/Fe2O3. Beilstein J. Nanotechnol. 2017, 8, 272–286. [Google Scholar] [CrossRef]
- Guy, N.; Ozacar, M. The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int. J. Hydrog. Energ. 2016, 41, 20100–20112. [Google Scholar] [CrossRef]
- Liu, T.; Chen, W.; Hua, Y.; Liu, X. Au/ZnO nanoarchitectures with Au as both supporter and antenna of visible-light. Appl. Surf. Sci. 2017, 392, 616–623. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).