Determination of a Complete Conversion Model for Gasification of Lignite Char
Abstract
1. Introduction
2. Theory
2.1. Kinetic Models
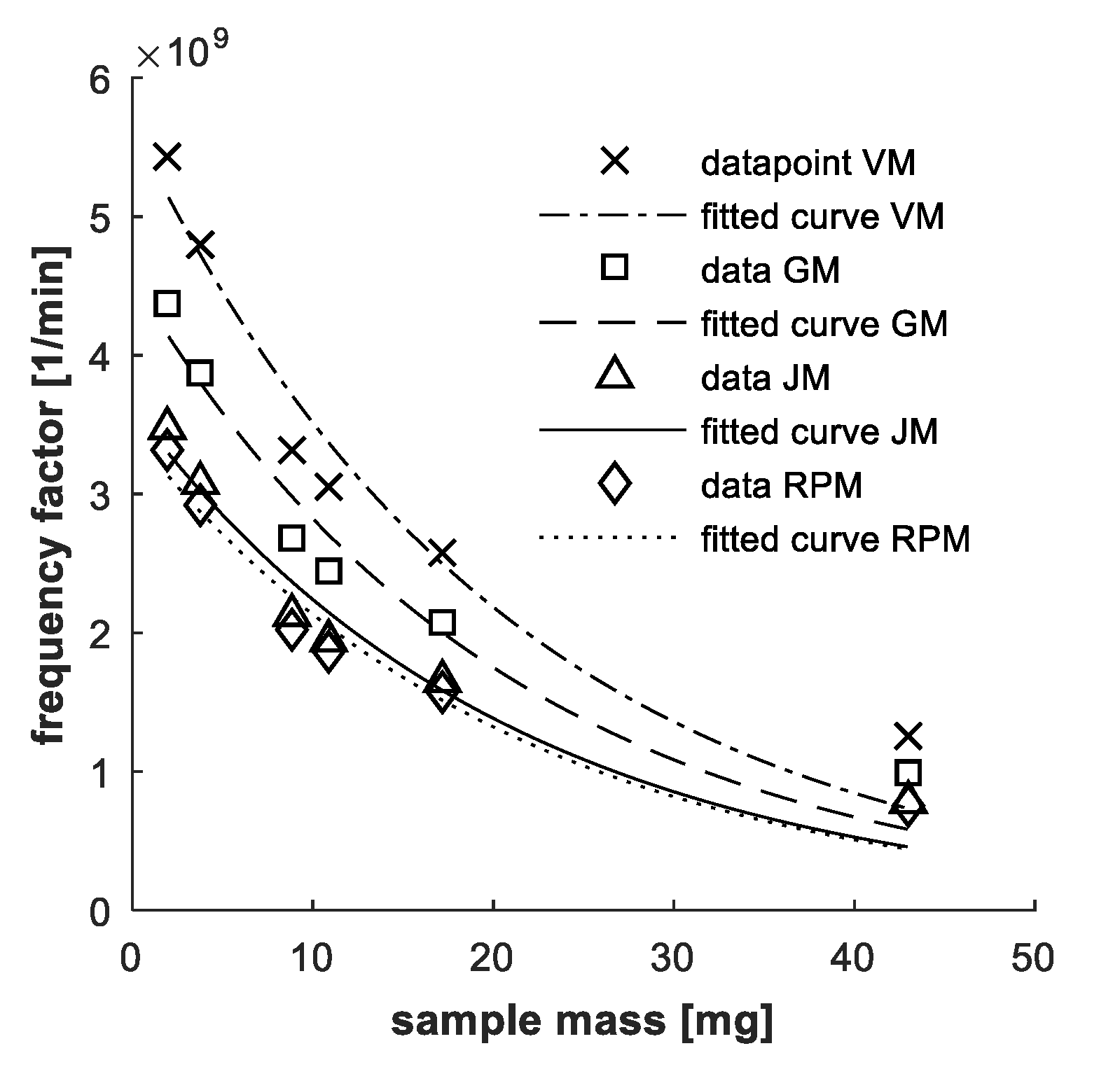
2.2. Mass Influence
3. Experimental
3.1. Fuel Sample and Char Preparation
3.2. Experimental Setup
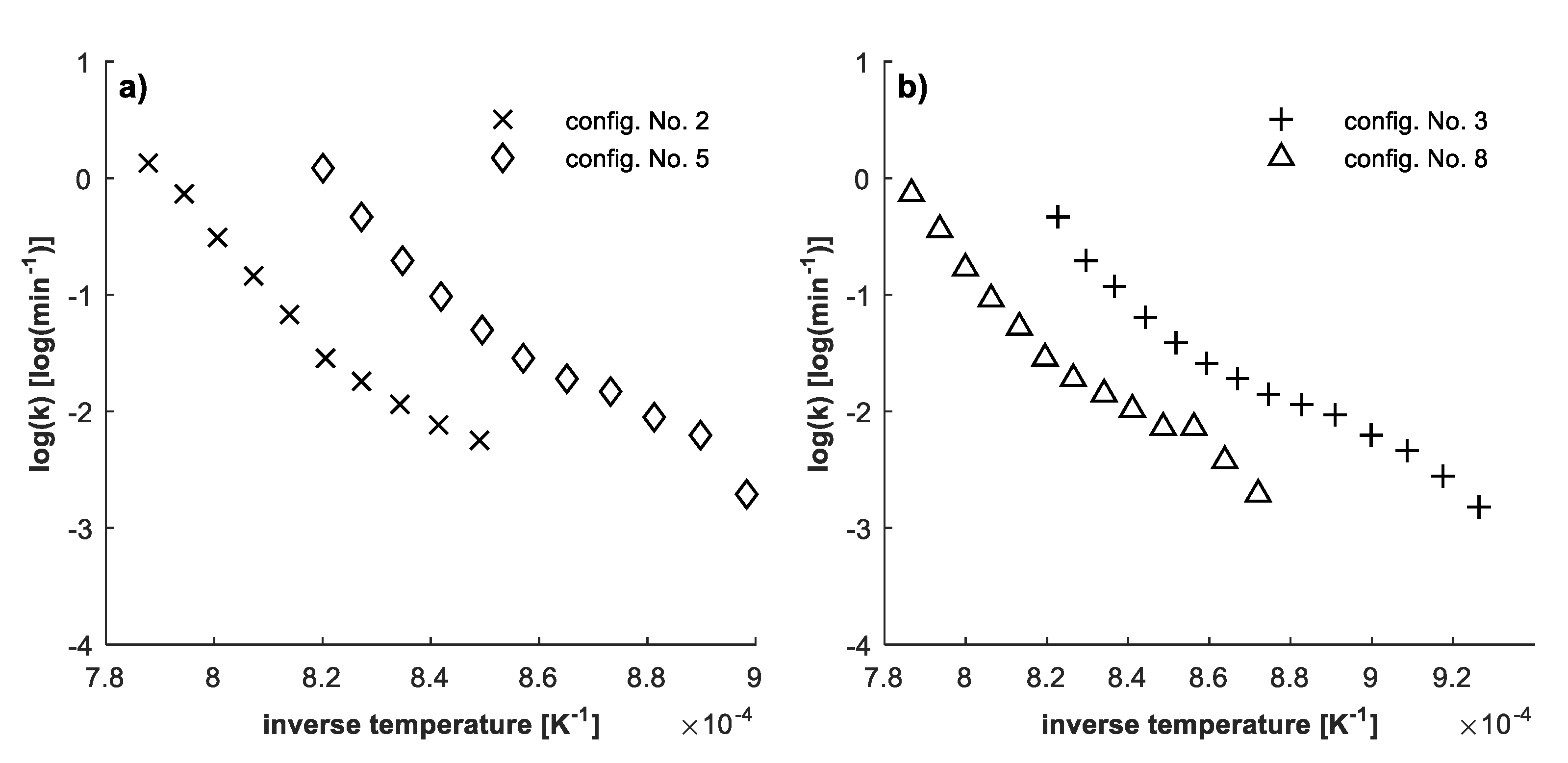
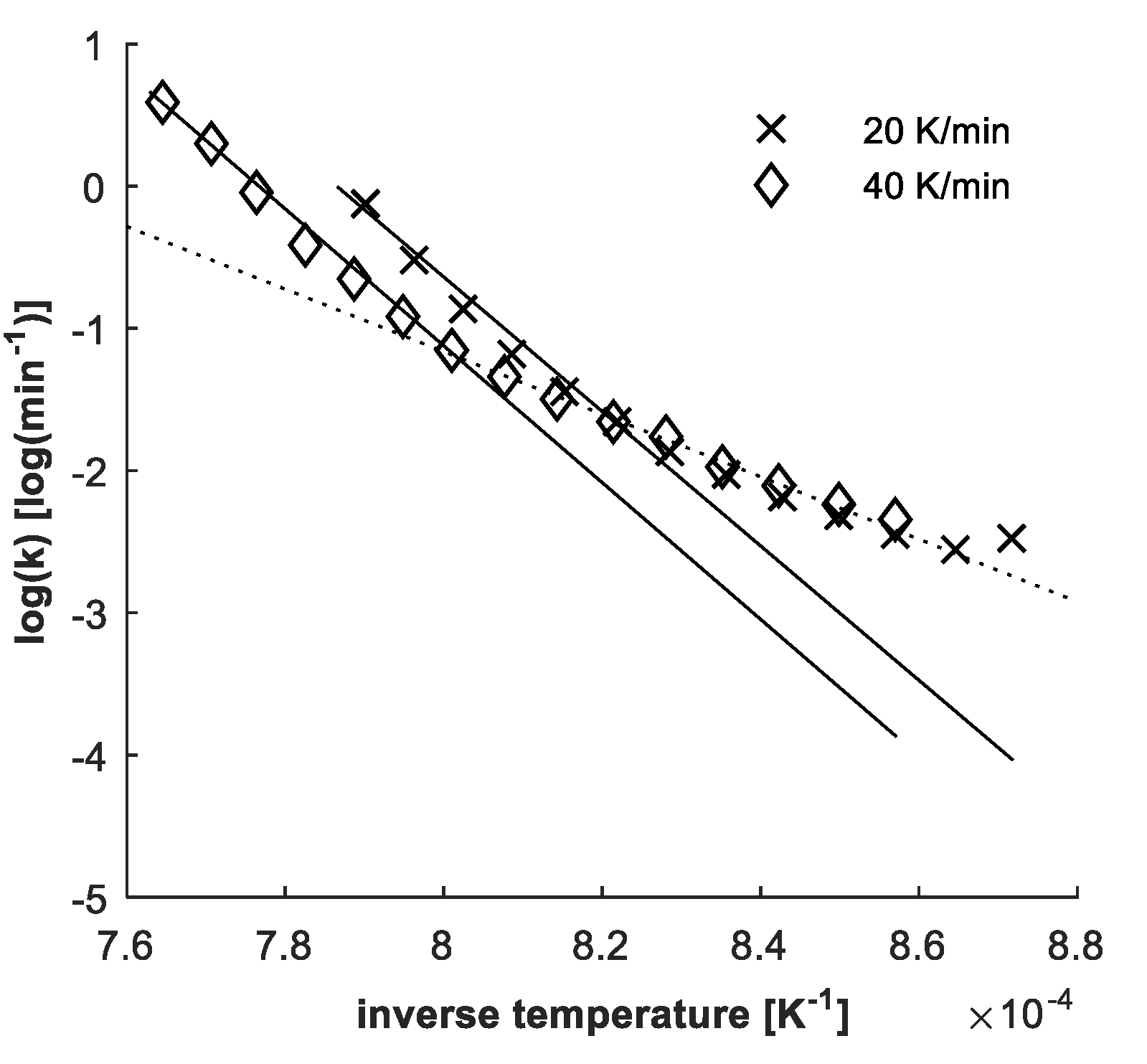
3.3. Data Preparation and Evaluation
4. Results and Discussion
4.1. Mass Influence
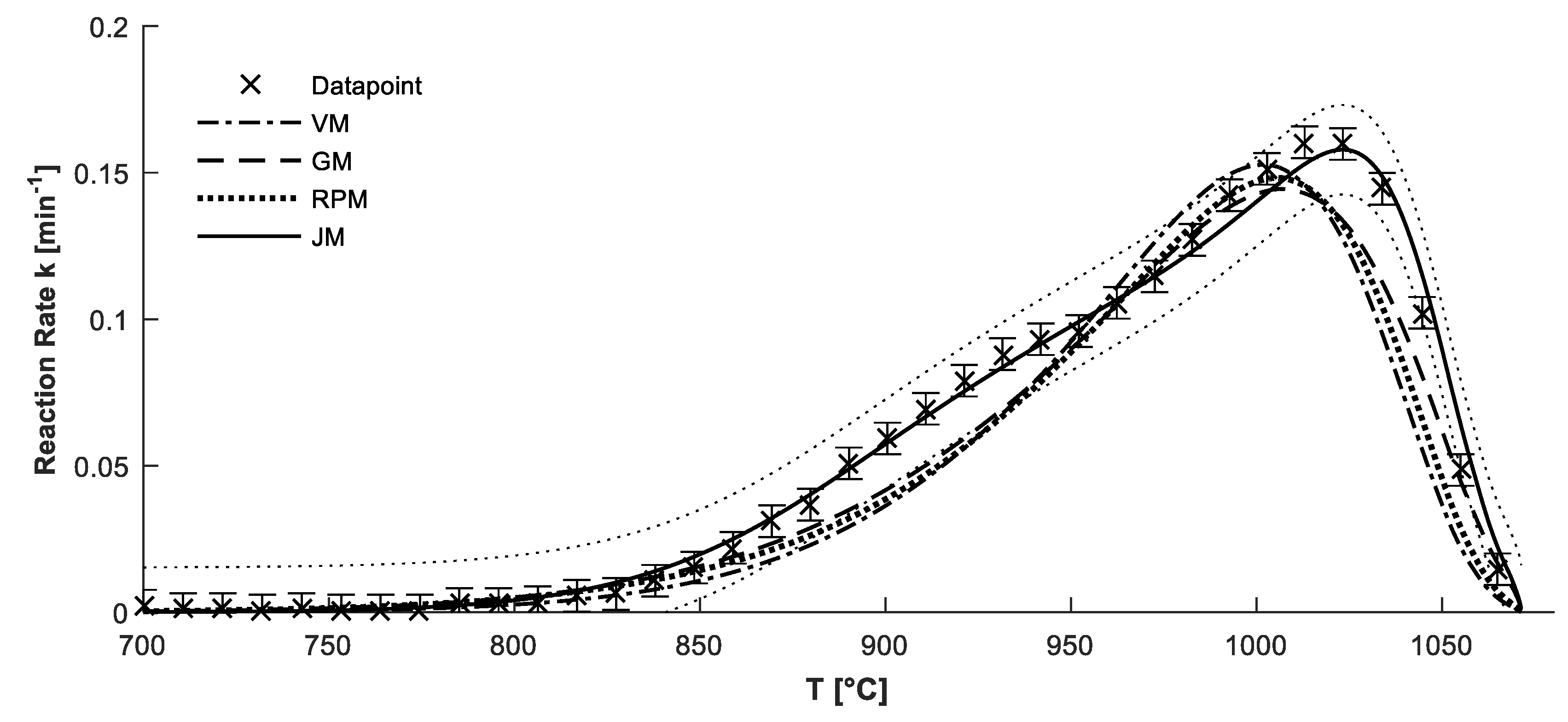
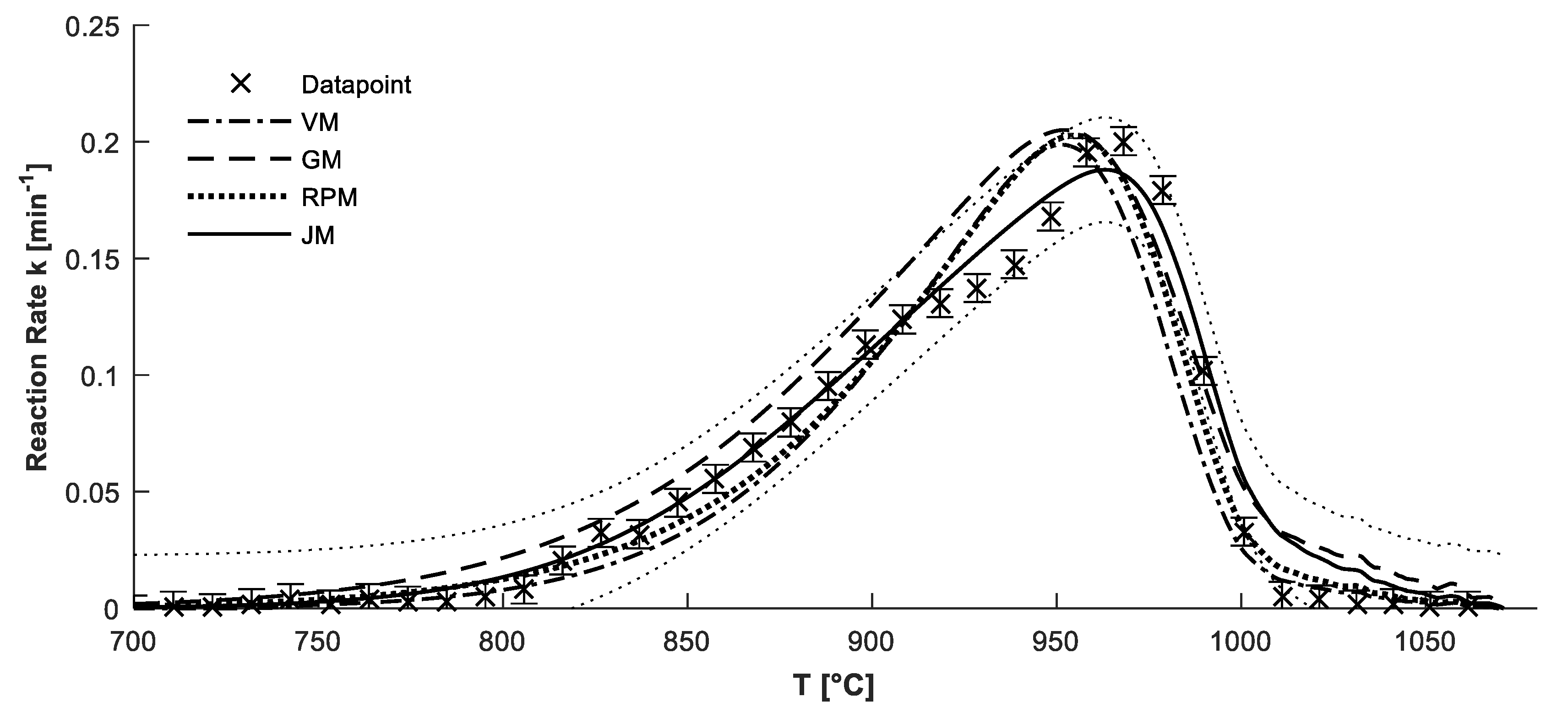
4.2. Confirmation of Reaction Model
4.3. Determination of Kinetic Parameters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IGCC | Integrated Gasification Combined Cycle |
| CFD | Computational Fluid Dynamic |
| L–H Model | Langmuir–Hinshelwood Reaction Model |
| VM | Volumetric Model |
| GM | Grain Model |
| RPM | Random Pore Model |
| JM | Johnson Model |
| TGA | Thermo-Gravimetric Analyzer |
References
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Farahani, E.; Kadner, S.; Seyboth, K.; Adler, A.; Baum, I.; Brunner, S.; Eickemeier, P. IPCC, 2014: Summary for policymakers—Sectoral and cross-sectoral mitigation pathways and measures. Clim. Chang. 2014, 17–26. [Google Scholar]
- Heinze, C.; Peters, J.; Ströhle, J.; Epple, B.; Hannes, J.; Ullrich, N.; Wulcko, I. Polygeneration von Strom und Kraftstoffen basierend auf der Wirbelschichtvergasung von Braunkohle. DGMK Tag. 2016, 2, 205–213. [Google Scholar]
- Irfan, M.F.; Usman, M.R.; Kusakabe, K. Coal gasification in CO2 atmosphere and its kinetics since 1948: Abrief review. Energy 2011, 36, 12–40. [Google Scholar] [CrossRef]
- Takarada, T.; Tamai, Y.; Tomita, A. Reactivities of 34 coals under steam gasification. Fuel 1985, 64, 1438–1442. [Google Scholar] [CrossRef]
- Ye, D.; Agnew, J.; Zhang, D. Gasification of a South Australian low-rank coal with carbon dioxide and steam: kinetics and reactivity studies. Fuel 1998, 77, 1209–1219. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Zhao, Y.; Zhang, H.; Yue, G.; Suda, T.; Narukawa, M. Kinetic studies of char gasification by steam and CO2 in the presence of H2 and CO. Fuel Process. Technol. 2010, 91, 843–847. [Google Scholar] [CrossRef]
- Fermoso, J.; Gil, M.V.; Pevida, C.; Pis, J.; Rubiera, F. Kinetic models comparison for non-isothermal steam gasification of coal–biomass blend chars. Chem. Eng. J. 2010, 161, 276–284. [Google Scholar] [CrossRef]
- Everson, R.C.; Neomagus, H.W.; Kasaini, H.; Njapha, D. Reaction kinetics of pulverized coal-chars derived from inertinite-rich coal discards: gasification with carbon dioxide and steam. Fuel 2006, 85, 1076–1082. [Google Scholar] [CrossRef]
- Laurendeau, N.M. Heterogeneous kinetics of coal char gasification and combustion. Prog. Energy Combust. Sci. 1978, 4, 221–270. [Google Scholar] [CrossRef]
- Lu, G.; Do, D. Comparison of structural models for high-ash char gasification. Carbon 1994, 32, 247–263. [Google Scholar] [CrossRef]
- Barrio, M.; Hustad, J. CO2 gasification of birch char and the effect of CO inhibition on the calculation of chemical kinetics. Prog. Thermochem. Biomass Convers. 2008, 47–60. [Google Scholar]
- Lussier, M.; Zhang, Z.; Miller, D.J. Characterizing rate inhibition in steam/hydrogen gasification via analysis of adsorbed hydrogen. Carbon 1998, 36, 1361–1369. [Google Scholar] [CrossRef]
- Ergun, S. Kinetics of the Reactions of Carbon Dioxide and Steam with Coke; US Government Printing Office: Washington, DC USA, 1962.
- Molina, A.; Mondragon, F. Reactivity of coal gasification with steam and CO2. Fuel 1998, 77, 1831–1839. [Google Scholar] [CrossRef]
- Szekely, J.; Evans, J. A structural model for gas—Solid reactions with a moving boundary. Chem. Eng. Sci. 1970, 25, 1091–1107. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980, 26, 379–386. [Google Scholar] [CrossRef]
- Johnson, J. Kinetics of bituminous coal char gasification with gases containing steam and hydrogen. Am. Chem. Soc. Coal Gasification (USA) 1974, 10, 145–178. [Google Scholar]
- Ollero, P.; Serrera, A.; Arjona, R.; Alcantarilla, S. Diffusional effects in TGA gasification experiments for kinetic determination. Fuel 2002, 81, 1989–2000. [Google Scholar] [CrossRef]
- Klutz, H.-J.; Moser, C.; Block, D. Development status of WTA fluidized-bed drying for lignite at RWE Power AG. Power Plant Technol. Secure Sustain. Energy Supply 2010, 2, 427–444. [Google Scholar]
- Liu, H.; Kaneko, M.; Luo, C.; Kato, S.; Kojima, T. Effect of pyrolysis time on the gasification reactivity of char with CO2 at elevated temperatures. Fuel 2004, 83, 1055–1061. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Werle, P.; Mücke, R.; Slemr, F. The limits of signal averaging in atmospheric trace-gas monitoring by tunable diode-laser absorption spectroscopy (TDLAS). Appl. Phys. B Lasers Opt. 1993, 57, 131–139. [Google Scholar] [CrossRef]
- Miura, K.; Silveston, P.L. Analysis of gas-solid reactions by use of a temperature-programmed reaction technique. Energy Fuels 1989, 3, 243–249. [Google Scholar] [CrossRef]
- Hüttinger, K.J.; Merdes, W.F. The carbon-steam reaction at elevated pressure: formations of product gases and hydrogen inhibitions. Carbon 1992, 30, 883–894. [Google Scholar] [CrossRef]
| Proximate Analysis (wt%) | Ultimate Analysis (wt%, daf) | |||||||
|---|---|---|---|---|---|---|---|---|
| Water | Ash | Volatile Matter | Fixed Carbon | C | H | O (Calculated) | N | S |
| 15.3 | 17.05 | 37.1 | 30.55 | 70.1 | 4.84 | 23.12 | 0.75 | 1.19 |
| Config. No. | Flow Rate CO2 (mL/min) | Flow Rate CO (mL/min) |
|---|---|---|
| 1 | 20 | 0 |
| 2 | 20 | 24 |
| 3 | 25 | 0 |
| 4 | 25 | 22.5 |
| 5 | 33 | 0 |
| 6 | 33 | 20 |
| 7 | 50 | 0 |
| 8 | 50 | 15 |
| Config. No. | Flow Rate H2O (mL/min) | Flow Rate H2 (mL/min) |
|---|---|---|
| 1 | 20 | 0 |
| 2 | 20 | 35 |
| 3 | 25 | 0 |
| 4 | 25 | 35 |
| 5 | 33 | 0 |
| 6 | 33 | 35 |
| 7 | 50 | 0 |
| 8 | 50 | 35 |
| Config. No. | Sample Mass (mg) | Flow Rate CO2 (mL/min) |
|---|---|---|
| 1 | 2.5 | 20 |
| 2 | 5 | 20 |
| 3 | 10 | 20 |
| 4 | 13 | 20 |
| 5 | 20 | 20 |
| 6 | 45 | 20 |
| Gasifying Agent | Carbon Dioxide | Steam | ||||||
|---|---|---|---|---|---|---|---|---|
| Conversion Model | VM | GM | RPM | JM | VM | GM | RPM | JM |
| R2 for Arrhenius Model [%] | 57.5 | 72.1 | 79.3 | 78.4 | 32.7 | 41.8 | 53.6 | 57.3 |
| R2 for L–H Model [%] | 85.1 | 71.8 | 90.6 | 94.8 | 86.1 | 92.4 | 89.4 | 98.4 |
| [kPa−1 min−1] | [kPa−1] | [kJ/mol] | [-] | [µg−1] | |||
| CO2 Gasification | |||||||
| 3.70 × 108 | 4.04 × 10−6 | 8.73 × 109 | 236.1 | −87.8 | 256.7 | 1.41 | 7.7 |
| Steam Gasification | |||||||
| 1.39 × 1012 | 5.14 × 10−3 | 3.25 × 1012 | 298.5 | −39.8 | 287.5 | 1.89 | 10.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinze, C.; Langner, E.; May, J.; Epple, B. Determination of a Complete Conversion Model for Gasification of Lignite Char. Appl. Sci. 2020, 10, 1916. https://doi.org/10.3390/app10061916
Heinze C, Langner E, May J, Epple B. Determination of a Complete Conversion Model for Gasification of Lignite Char. Applied Sciences. 2020; 10(6):1916. https://doi.org/10.3390/app10061916
Chicago/Turabian StyleHeinze, Christian, Eric Langner, Jan May, and Bernd Epple. 2020. "Determination of a Complete Conversion Model for Gasification of Lignite Char" Applied Sciences 10, no. 6: 1916. https://doi.org/10.3390/app10061916
APA StyleHeinze, C., Langner, E., May, J., & Epple, B. (2020). Determination of a Complete Conversion Model for Gasification of Lignite Char. Applied Sciences, 10(6), 1916. https://doi.org/10.3390/app10061916




