Adsorption between Quercetin Derivatives and β-Glucan Studied with a Novel Approach to Modeling Adsorption Isotherms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Spectrophotometric Method for Total Polyphenols (Folin-Ciocalteu Method)
2.3. Adsorption of Quercetin Derivatives onto β-Glucan
2.4. The Conservation of Mass
2.5. Adsorption Isotherms and Data Modeling
2.5.1. Non-Linear Regression
2.5.2. Improved Non-Linear Regression
2.5.3. Linear Regression
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camouse, M.M.; Hanneman, K.K.; Conrad, E.P.; Baron, E.D. Protective effects of tea polyphenols and caffeine. Expert Rev. Anticanc. 2005, 5, 1061–1068. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer growth. Rev. Physiol. Biochem. Pharm. 2007, 159, 79–113. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and diseases. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantifications methods and mechanism. Crit. Rev. Food Sci. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Dhital, S.; López-Rubio, A.; Gidley, M.J. Dietary polyphenols bind to potato cells and cellular components. J. Funct. Food 2017, 37, 283–292. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; de la Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- dos Santos Costa, T.; Rogez, H.; da Silva Pena, R. Adsorption capacity of phenolic compounds onto cellulose and xylan. Food Sci. Technol. 2015, 35, 314–320. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Phan, A.D.T.; D’Arcy, B.R.; Gidley, M.J. Polyphenol-cellulose interactions: Effects of pH, temperature and salt. Int. J. Food Sci. Technol. 2016, 51, 203–211. [Google Scholar] [CrossRef]
- Laroche, C.; Michaud, P. New developments and prospective applications for β (1,3) glucans. Recent Pat. Biotechnol. 2007, 1, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. Beta-glucans in the treatment of diabetes and associated cardiovascular risks. Vasc. Health Risk Manag. 2008, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant activity of β-glucan. ISRN Pharm. 2012, 2012, 125864. [Google Scholar] [PubMed]
- Queenan, K.M.; Stewart, M.L.; Smith, K.N.; Thomas, W.; Fulchner, R.G.; Slavin, J.L. Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr. J. 2007, 6, 1–8. [Google Scholar] [CrossRef]
- Gao, R.; Liu, H.; Peng, Z.; Wu, Z.; Wang, Y.; Zhao, G. Adsorption of (-)-epigallocatechin-3-gallate (EGCG) onto oat β-glucan. Food Chem. 2012, 132, 1936–1943. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Ming, J.; Zhao, G. Optimization of adsorption of tea polyphenols onto oat β-glucan using response surface methodology. J. Agric. Food Chem. 2011, 59, 378–385. [Google Scholar] [CrossRef]
- Marsal, A.; Maldonado, F.; Cuadros, S.; Bautista, M.E.; Manich, A.M. Adsorption isotherm, thermodynamics and kinetics studies of polyphenols onto tannery shavings. Chem. Eng. J. 2012, 183, 21–29. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, F.; Zhao, G. Effects of molecular structure of polyphenols on their noncovalent interactions with oat β-glucan. J. Agric. Food Chem. 2013, 61, 4533–4538. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Wu, Z.; Ming, J.; Zhao, G. Interaction of barley β-glucan and tea polyphenols on glucose metabolism in streptozotocin-induced diabetic rats. J. Food Sci. 2012, 77, H128–H134. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Nielsen, M.S.; Christensen, N.J.; Christensen, U.; La Cour, T.V.; Motawia, M.S.; Jespersen, B.P.M.; Engelsen, S.B.; Møller, B.L. Molecular interactions between barley and oat β-glucans and phenolic derivatives. J. Agric. Food Chem. 2009, 57, 2056–2064. [Google Scholar] [CrossRef]
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorík, V.; Veverková, E.; Mičušik, M.; Šimon, P. Beta-glucan complexes with selected nutraceuticals: Synthesis, characterization, and stability. J. Funct. Food 2014, 8, 309–318. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Šic Žlabur, J.; Skendrović, B.M. Traditional, Indigenous apple varieties, a fruit with potential for beneficial effects: Their quality traits and bioactive polyphenol contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Arias-Santé, M.F.; Atala, E.; Pastene, E.; Kogan, M.J.; Speisky, H. Low nanomolecular concentrations of a quercetin oxidation product, which naturally occurs in onion peel, protects cells against oxidative damage. Food Chem. 2020, 126166. [Google Scholar] [CrossRef] [PubMed]
- Babaeivelni, K.; Khodadoust, A.P. Adsorption of fluoride onto crystalline titanium dioxide: Effect of pH, ionic strength, and co-existing ions. J. Colloid Interf. Sci. 2013, 394, 419–427. [Google Scholar] [CrossRef]
- Dalal, R.C. Application of Dubinin-Radushkevich adsorption isotherm for phosphorus sorption by soils. Soil Sci. 1979, 128, 65–69. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insight into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Samatya, S.; Kabay, N.; Yüksel, Ü.; Arda, M.; Yüksel, M. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Functpolym. 2006, 66, 1206–1214. [Google Scholar] [CrossRef]
- Barron, A.; Benšić, M.; Buljeta, I.; Jakobek, L. A statistically accurate algorithm for fitting adsorption isotherms with application to the interaction between polyphenols and β-glucan. In Book of abstract of 13th World Congress on Polyphenols Applications, Valletta, Malta, 30 September to 1 October 2019; Shieber, A., Ed.; International Society f Antioxidants in Nutrition and Health: Paris, France, 2019; p. 69. [Google Scholar]
- Jakobek, L.; Matić, P.; Barron, R.A. The application of adsorption isotherms with proper fitting to interpret polyphenol bioaccessibility in vitro. In Book of abstract of Euro-Global Conference on Food Science, Agronomy and Technology, Rome, Italy, 20–22 September 2018; Magnus Group: London, UK, 2018; p. 61. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagens. Method Enzym. 1999, 299, 152–178. [Google Scholar]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of spectrophotometric methods for the determination of total polyphenol and total flavonoid content. J. Aoac. Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material part I. Effect of some environmental parameters. Biochim. Biophys. Acta 2004, 1672, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part II: Quantification and impact of cell wall drying. Biochim. Biophys. Acta 2005, 1725, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Bouchet, B.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material. Part III; Study on model polysaccharides. Biochim. Biophys. Acta 2005, 1725, 10–18. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Saleh, Z.S.; Carle, R.; Stanley, R.A. Adsorptive recovery of phenolic compounds from apple juice. Eur. Food Res. Technol. 2007, 224, 605–613. [Google Scholar] [CrossRef]
- Liu, D.; Lopez-Sanchez, P.; Martinez-Sanz, M.; Gilbert, E.P.; Gidley, M.J. Adsorption isotherm studies on the interaction between polyphenols and apple cell walls: Effects of variety, heating and drying. Food Chem. 2019, 282, 58–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Jia, Z.; Scarlett, C.J.; Sheng, Z. Adsorption/desorption characteristics and enrichment of quercetin, luteolin and apigenin from Flos populi using macroporous resin. Rev. Bras. Farm. 2019, 29, 69–76. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Ursueguía, D.; Díaz, E.; Ordóñez, S. Adsorption of methane and nitrogen on basolite mofs: Equilibrium and kinetic studies. Micropor. Mesopor. Mat. 2020, 298, 110048. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Niu, Q.; Pan, J.; Wang, H.; Wang, Z. CO2 adsorption and swelling of coal under constrained conditions and their stagechange relationship. J. Nat. Gas. Sci. Eng. 2020, 76, 103205. [Google Scholar] [CrossRef]
- Li, P.; Gao, B.; Li, A.; Yang, H. Evaluation of the selective adsorption of silica-sand/anionizedstarch composite for removal of dyes and Cupper(II) from their aqueous mixtures. Int. J. Biol. Macromol. 2020, 149, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zong, Y.; Jassby, D.; Wang, J.; Tian, J. The interactions and adsorption mechanisms of ternary heavy metals on boron nitride. Environ. Res. 2020, 109240. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, J.; Li, M.; Guo, C.; Long, H.; Zhang, Y.; Xin, L. Mechanistic study of selective adsorption and reduction of Au (III) to gold nanoparticles by ion-imprinted porous alginate microspheres. Chem. Eng. J. 2020, 385, 123897. [Google Scholar] [CrossRef]
- Shabir, F.; Sultan, M.; Miyazaki, T.; Saha, B.B.; Askalany, A.; Ali, I.; Zhou, Y.; Ahmad, R.; Shamshiri, R.R. Recent updates on the adsorption capacities of adsorbent-adsorbate pairs for heat transformation applications. Renew. Sust. Energ. Rev. 2020, 119, 109630. [Google Scholar] [CrossRef]
- Gaona-Forero, A.; Agudelo-Rodríguez, G.; Herrera, A.O.; Castellanos, D.A. Modeling and simulation of an active packaging system with moisture adsorption for fresh produce. Application in ‘Hass’ avocado. Food Packag. Shelf. 2018, 17, 187–195. [Google Scholar] [CrossRef]
- Yang, A.; Liu, H.; Li, Z.; Li, L.; Li, W.; Liu, K. Green synthesis of β-cyclodextrin metal-organic frameworks and the adsorption of quercetin and emodin. Polyhedron 2019, 159, 116–126. [Google Scholar] [CrossRef]
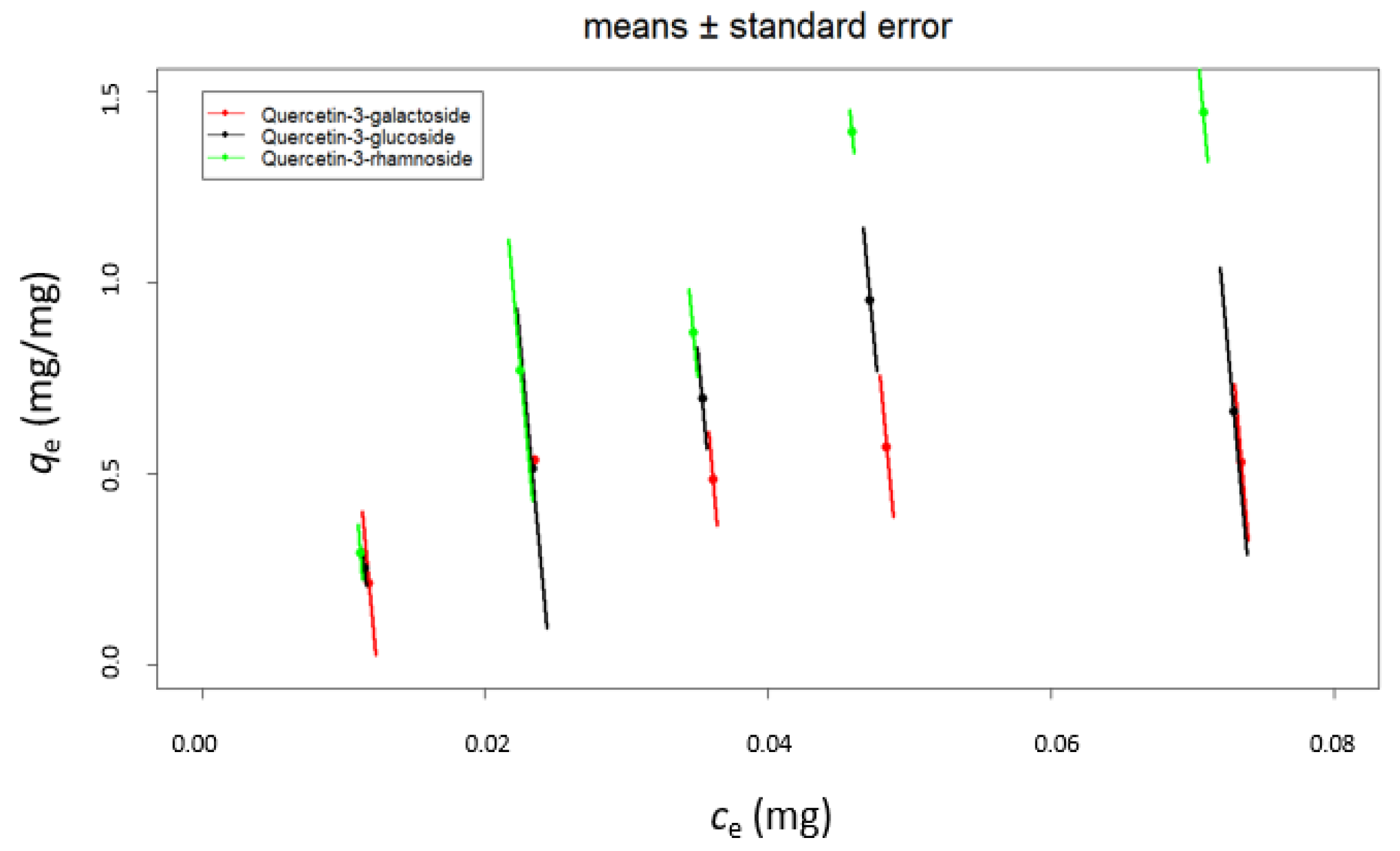

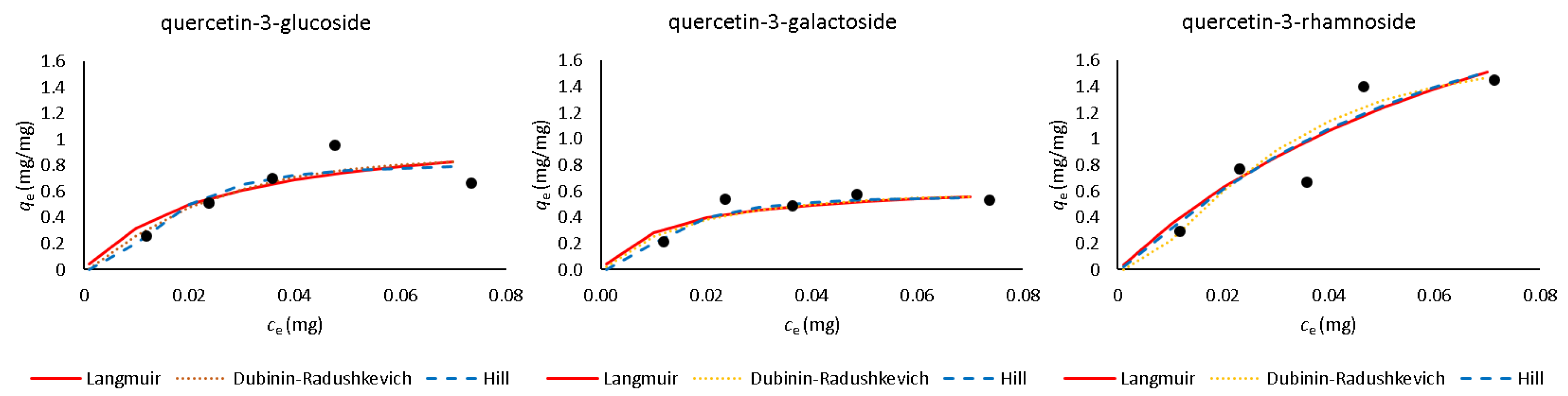
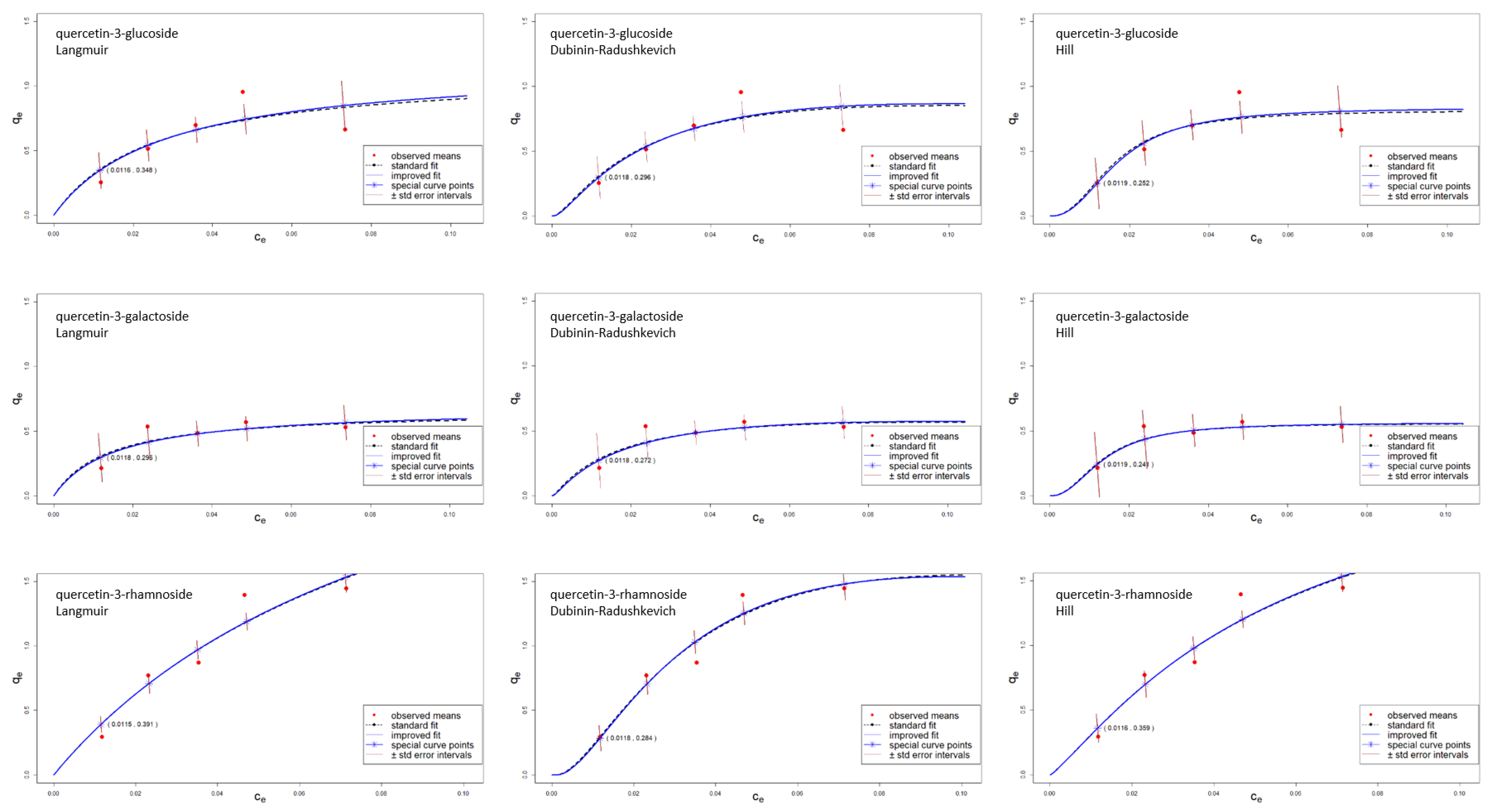
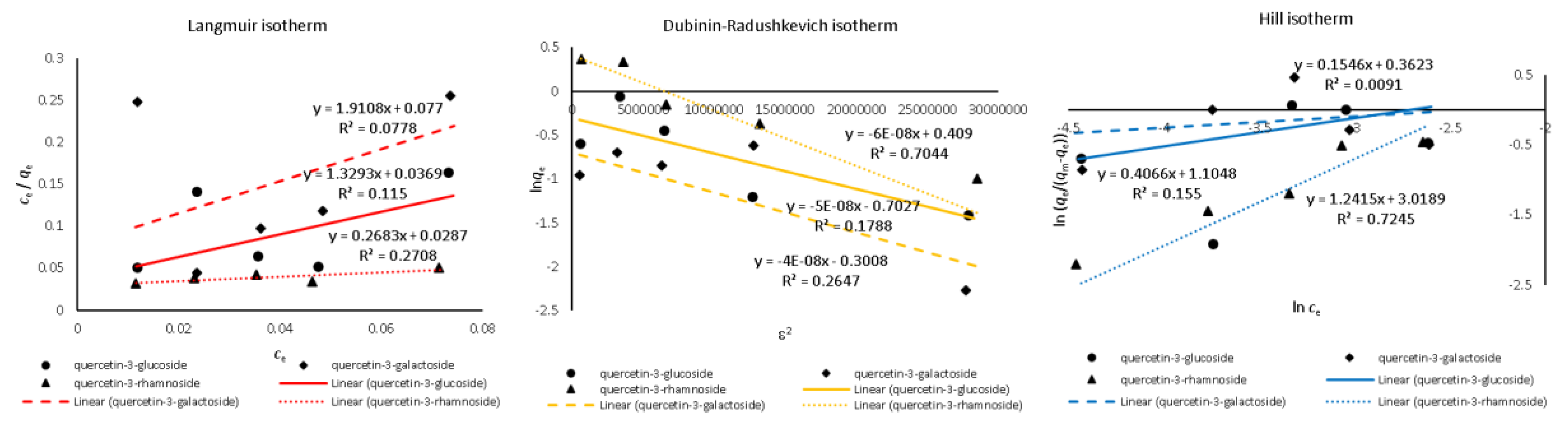

| Quercetin Derivative | Range mg L−1 | Equation | r2 | LOD mg L−1 | LOQ mg L−1 |
|---|---|---|---|---|---|
| quercetin-3-glucoside | 1–200 | Y = 0.001x + 0.0055 | 0.9972 | 1.17 | 3.54 |
| quercetin-3-galactoside | 1–100 | Y = 0.001x + 0.0031 | 0.9932 | 0.22 | 0.67 |
| quercetin-3-rhamnoside | 1–200 | Y = 0.0013x + 0.00003 | 0.9985 | 0.17 | 0.52 |
| Quercetin Derivative | Langmuir | Dubinin-Radushkevich | Hill | |
|---|---|---|---|---|
| qe | qe(calc) | qe(calc) | qe(calc) | |
| (mg/mg) | (mg/mg) | (mg/mg) | (mg/mg) | |
| quercetin-3-glucoside | 0.25 ± 0.05 | 0.35 | 0.30 | 0.25 |
| quercetin-3-galactoside | 0.21 ± 0.19 | 0.30 | 0.28 | 0.24 |
| quercetin-3-rhamnoside | 0.30 ± 0.07 | 0.40 | 0.27 | 0.35 |
| Quercetin-derivatives | Langmuir | Dubinin-Radushkevich | Hill | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/mg) | KL (1/mg) | se | qs (mg/mg) | cs (mg) | E (J/mol) | se | qm (mg/mg) | nH | KD (mg)nH | se | |
| Nonlinear modeling | |||||||||||
| quercetin-3-glucoside | 1.12 | 39.9 | 0.330 | 0.85 | 0.1 | 3721 | 0.339 | 0.82 | 2.23 | 0.000103 | 0.335 |
| quercetin-3-galactoside | 0.66 | 73.0 | 0.324 | 0.57 | 0.1 | 4483 | 0.335 | 0.56 | 2.15 | 0.000091 | 0.334 |
| quercetin-3-rhamnoside | 3.47 | 11.0 | 0.248 | 1.55 | 0.1 | 2909 | 0.246 | 2.67 | 1.18 | 0.0331 | 0.253 |
| Improved nonlinear modeling | |||||||||||
| quercetin-3-glucoside | 1.17 | 36.4 | 0.324 | 0.87 | 0.1 | 3623 | 0.318 | 0.84 | 2.26 | 0.000103 | 0.328 |
| quercetin-3-galactoside | 0.69 | 64.3 | 0.322 | 0.58 | 0.1 | 4327 | 0.321 | 0.57 | 2.17 | 0.000091 | 0.331 |
| quercetin-3-rhamnoside | 3.51 | 10.9 | 0.235 | 1.54 | 0.098 | 2854 | 0.232 | 2.69 | 1.19 | 0.0331 | 0.240 |
| Linear modeling | |||||||||||
| quercetin-3-glucoside | 0.75 | 36.0 | 0.410 | 0.74 | 0.1 | 3536 | 0.357 | 0.41 | 0.331 | 0.499 | |
| quercetin-3-galactoside | 0.52 | 24.8 | 0.404 | 0.50 | 0.1 | 3162 | 0.360 | 0.15 | 0.696 | 0.439 | |
| quercetin-3-rhamnoside | 3.72 | 9.3 | 0.156 | 1.51 | 0.1 | 2887 | 0.256 | 1.24 | 0.049 | 0.271 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobek, L.; Matić, P.; Kraljević, Š.; Ukić, Š.; Benšić, M.; Barron, A.R. Adsorption between Quercetin Derivatives and β-Glucan Studied with a Novel Approach to Modeling Adsorption Isotherms. Appl. Sci. 2020, 10, 1637. https://doi.org/10.3390/app10051637
Jakobek L, Matić P, Kraljević Š, Ukić Š, Benšić M, Barron AR. Adsorption between Quercetin Derivatives and β-Glucan Studied with a Novel Approach to Modeling Adsorption Isotherms. Applied Sciences. 2020; 10(5):1637. https://doi.org/10.3390/app10051637
Chicago/Turabian StyleJakobek, Lidija, Petra Matić, Šima Kraljević, Šime Ukić, Mirta Benšić, and Andrew R. Barron. 2020. "Adsorption between Quercetin Derivatives and β-Glucan Studied with a Novel Approach to Modeling Adsorption Isotherms" Applied Sciences 10, no. 5: 1637. https://doi.org/10.3390/app10051637
APA StyleJakobek, L., Matić, P., Kraljević, Š., Ukić, Š., Benšić, M., & Barron, A. R. (2020). Adsorption between Quercetin Derivatives and β-Glucan Studied with a Novel Approach to Modeling Adsorption Isotherms. Applied Sciences, 10(5), 1637. https://doi.org/10.3390/app10051637






