1. Introduction
Biodiesel is a promising alternative to diesel, which is gained from fossil resources. Biodiesel from vegetable oils provides lower carbon dioxide and greenhouse gas emission compared to fossil fuels and, therefore, has a smaller environmental impact [
1]. Nowadays, biodiesel is industrially produced through transesterification of high fatty acids (triacylglycerols), which are mostly gained from soybean, rapeseed and palm oil [
2], and methanol or ethanol as aliphatic alcohol in a base-catalyzed transesterification reaction. As catalyst NaOH or KOH can be used. Since methanol is obtained from mineral oil, ethanol can be produced in the fermentation process from biomass. The food industry is deprived if using soybean oil or other cooking oils for biodiesel production. Applying waste cooking oil or non-edible oils like algae oil for the ethanolysis provides environmentally friendlier biodiesel [
3]. The suitability of the oil for the reaction depends on the number of triacylglycerols [
2]. The reaction mechanism includes three stages. In the first stage (1), triacylglycerol (T) is converted into diacylglycerol (D) while one equivalent of fatty acid ester (E) with ethanol is formed. In the second stage (2) another equivalent of alcohol (R-OH) reacts with D and forms monoacylglycerol (M) and glycerol (G) in the last step (3). With each of the reversible steps, one equivalent of R-OH is needed and one equivalent of fatty acid ethyl (FAEE) ester is formed.
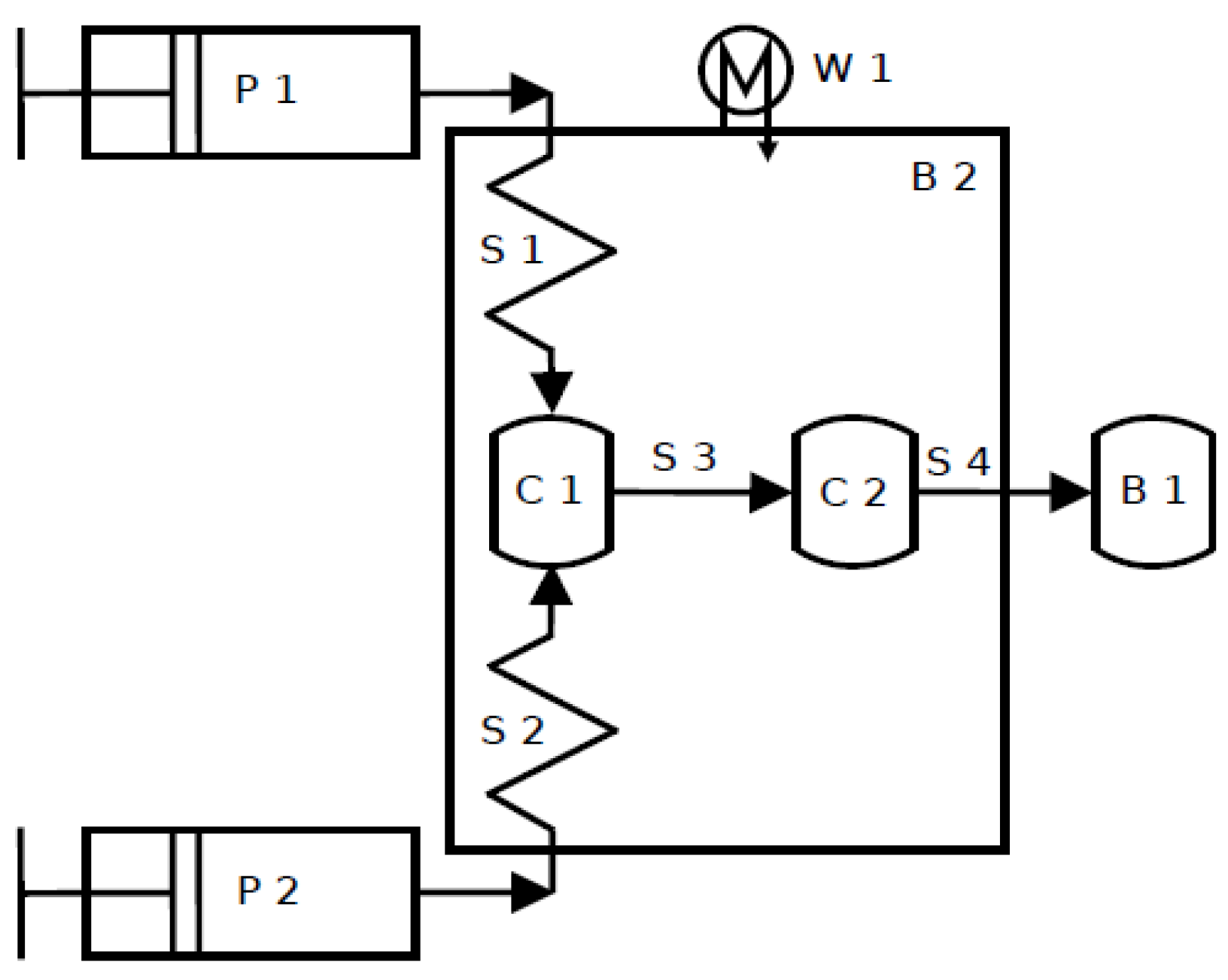
Previous studies have shown that it is possible to conduct the transesterification reaction in continuously operated microreactors [
4]. Microreactors provide advantages like higher controllability, safer dealing with toxic chemicals and easy scale-up by numbering-up. Moreover, microreactors make available micromixing of the reactants, which can lead to higher yields [
5]. Oil and alcohol phase (with the catalyst) are involved in the ethanolysis reaction leading to mass transfer limitations [
2]. Due to micromixing, a higher phase boundary interface can be obtained because of a higher surface-to-volume-ratio of the reactants and short diffusion distances [
6]. Methanolysis or ethanolysis reaction was investigated in several microreactors [
5,
7,
8,
9]. Wen et al. [
7] used zig-zag microreactors and observed a reaction yield of 97.3% at 56 °C, methanol–oil-ratio of 6:1, 1.6 wt.-% KOH and 18 s residence time. Schwarz et al. [
8] worked with a T-mixer and different microreactors, and obtained a maximum yield of 93.8% in a split-and-recombine micromixer at 50 °C, molar ethanol/oil ratio of 6:1, 1 wt.-% KOH and 11.3 min residence time. Sun et al. [
9] showed that at 1 wt.-% KOH catalyst, molar methanol/oil ratio of 6:1 and 60 °C reaction temperature the alcoholysis of rapeseed and cottonseed oil in a capillary microreactor with an inner diameter of 0.25 mm led to maximum yields of 99.4% FAEE after a reaction time of 5.89 min. The mixing of the reactants was performed in a separate container before the mixture was pumped through the capillary reactor. The authors found out that further increasing the catalyst concentration, the molar ethanol/oil ratio, the temperature or the reaction time was counterproductive for the investigated reactor system.
The aim of this paper is to determine optimal reaction conditions for base-catalyzed transesterification of different oil types and in different reactor systems using design of experiments.
3. Results and Discussion
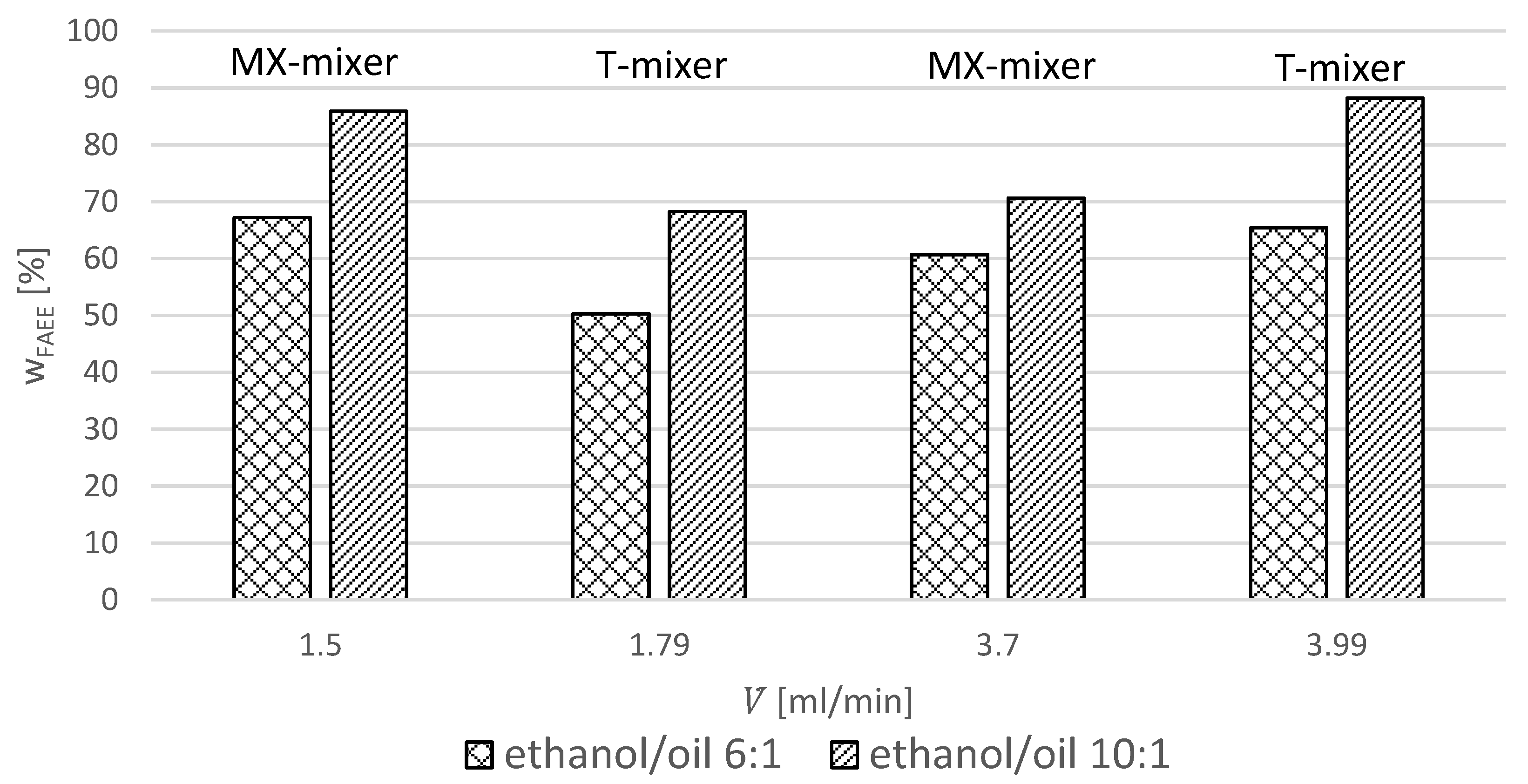
The FAEE yields in the continuous reaction system at different flow rates and molar ratios are listed in
Table 2 and displayed in
Figure 3.
Figure 3 shows that the MX-mixer provides higher yields at lower flow rates and otherwise similar parameters compared to the T-mixer. An opposite effect can be observed at higher flow rates. This could be explained by diverging flow dynamics [
8]. Because of the MX-mixer geometry, the efficient mixing of components at lower flow rates can be observed. In the T-mixer, the higher flow rate is required for good mixing.
In order to consider the significance of the parameters, the dimensionless coefficients (ethanol/oil ratio, flow rate and the mass percentage of catalyst) have to exceed the value for the Student’s t-distribution (
Table 3) [
11].
The regression analysis of the reaction in the MX-mixer shows that only the ethanol/oil ratio (a
1) has a significant impact on the yield of FAEE. The flow rate (a
2) has a negative sign and is not significant. In the T-mixer only flow rate (a
2) and ethanol/oil ratio (a
1) are significant. The variation of the flow rate in this system provides a higher impact on the yield than the molar ethanol/oil ratio. This result confirms that the flow rate has a great influence on the blending of the components in the T-mixer. The amount of the catalyst (a
3) shows no significant impact on the experiment in both reactor systems. The calculated dimension specific coefficients for the MX- and the T-mixer are presented in
Table 4.
In Equations (6) and (7) the significant coefficients for both investigated reactor systems (with MX-mixer and T-mixer) are presented.
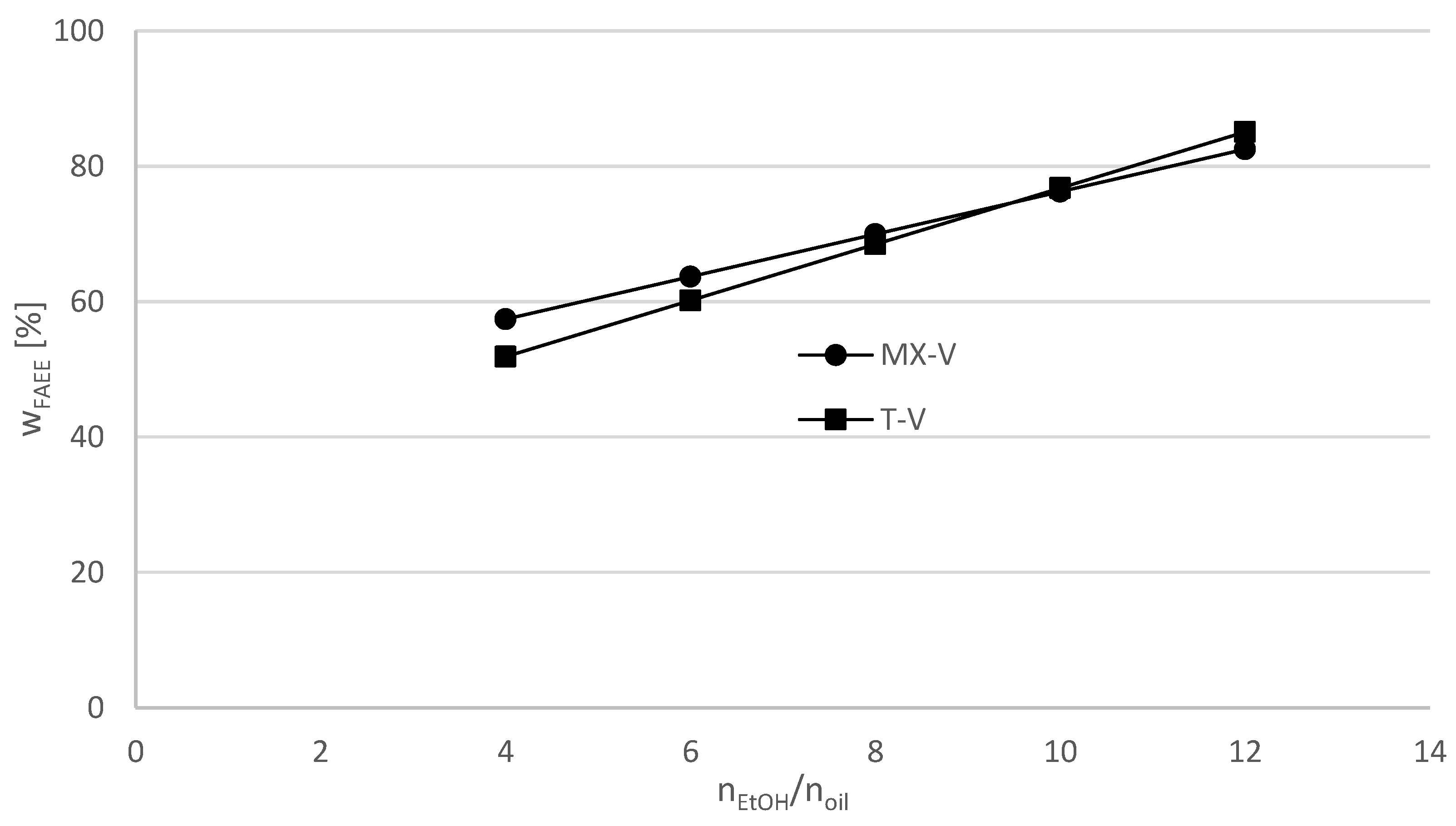
Regression equations depending on the ethanol/oil ratio at a constant flow rate x
2 = 2.89 mL/min in the MX-V and T-V reactor system can be formulated (
Figure 4). It shows that raising the ethanol/oil ratio in the reactor system with T-mixer would lead to higher yields of FAEE (w
FAEE [%]) than in the reactor system with MX-mixer. At ethanol/oil ratios under 9.5:1 the MX-V reactor system is more efficient than the T-V reactor system, while higher ethanol/oil ratio provides a slightly higher efficiency in the case of T-mixer.
The influence of the flow rate on the FAEE yield in the T-reactor system at a constant molar ethanol/oil ratio = 10:1 is shown in
Figure 5. A high positive impact of the flow rate in the T-mixer, and a reverse phenomenon in the MX-mixer can be observed. Due to a better blending in the T-mixer, the T-reactor system is more suitable for flow rates higher than 2.2 mL/min compared to the MX-V reactor system.
The optimization was conducted in both continuous systems according to the Box–Wilson method [
12]. New values for the parameters were calculated based on the regression coefficient with the greatest impact on the respective reactor system. The optimized parameters are presented in
Table 5 with the middle points (line 1 in
Table 1) of the plan matrix.
In both reactor systems, the optimized parameters provided an increase of the FAEE yield. Using the MX-mixer, the mass fraction of FAEE could be raised from 68.1% to 84.9%. With the T-mixer the yield of FAEE could be increased from 61.8% to 90.8%. Increased flow rate had a great positive impact on reaction yields in the system with the T-mixer.
The optimized parameters were applied to the experiments in the batch reactor and compared for soybean oil, original cooking and waste cooking oil (
Table 6).
All experimental results are shown in
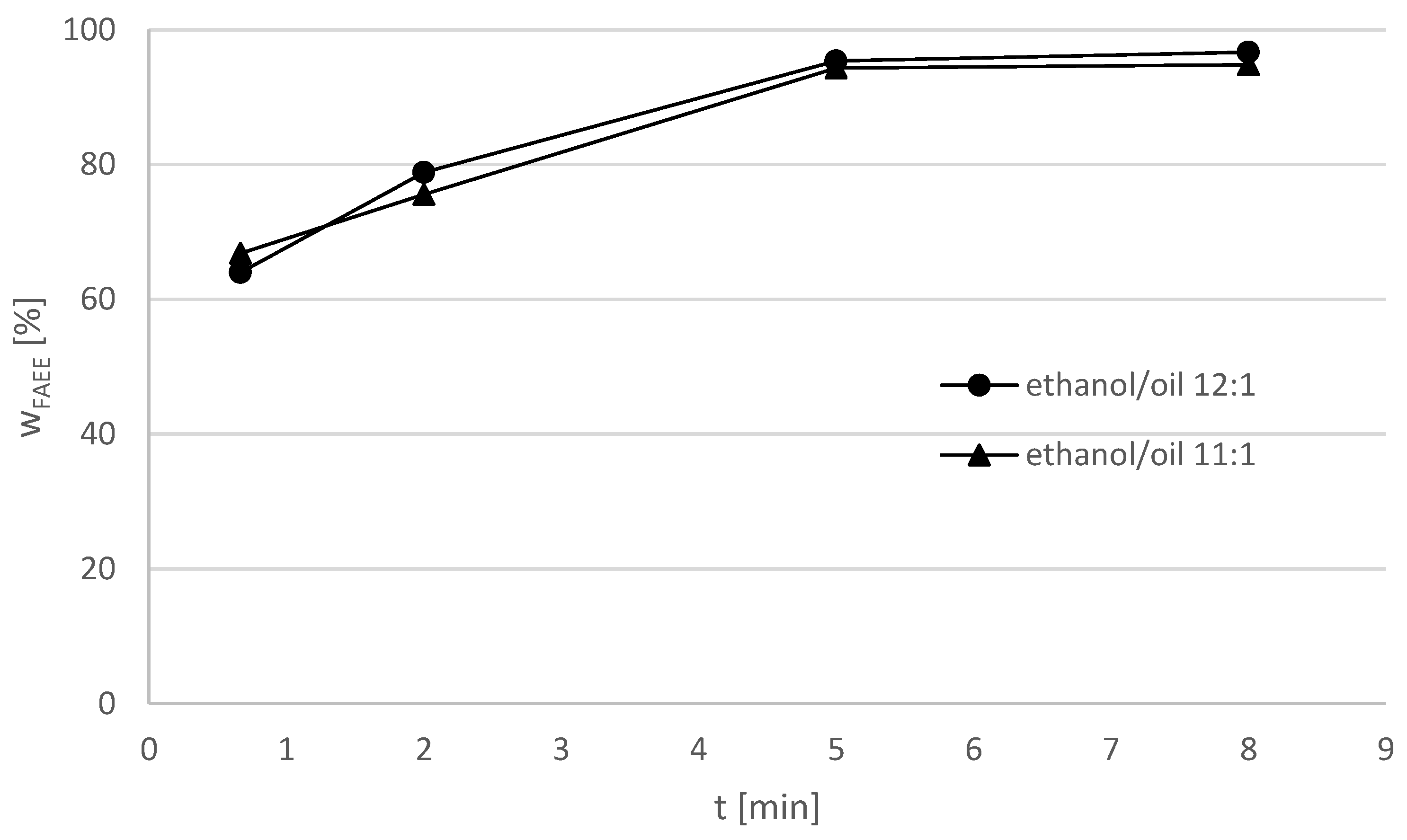
Table 7. The FAEE yield with soybean oil at ethanol–oil-ratio = 11:1 and 12:1 in the batch reactor is illustrated in
Figure 6. For a molar ethanol/oil ratio = 11:1, a maximum yield = 94.8% FAEE was detected after a reaction time of 8 min. The product yield remained nearly constant after a reaction time of 5 min, indicating that the reaction was practically finished. At the ethanol/oil ratio = 12:1 the maximum FAEE yield of 96.7% was detected after 8 min. The product yield of the experiment with the molar ethanol/oil ratio = 11:1 is exceeded, showing that a higher excess of ethanol has a positive effect on the reaction.
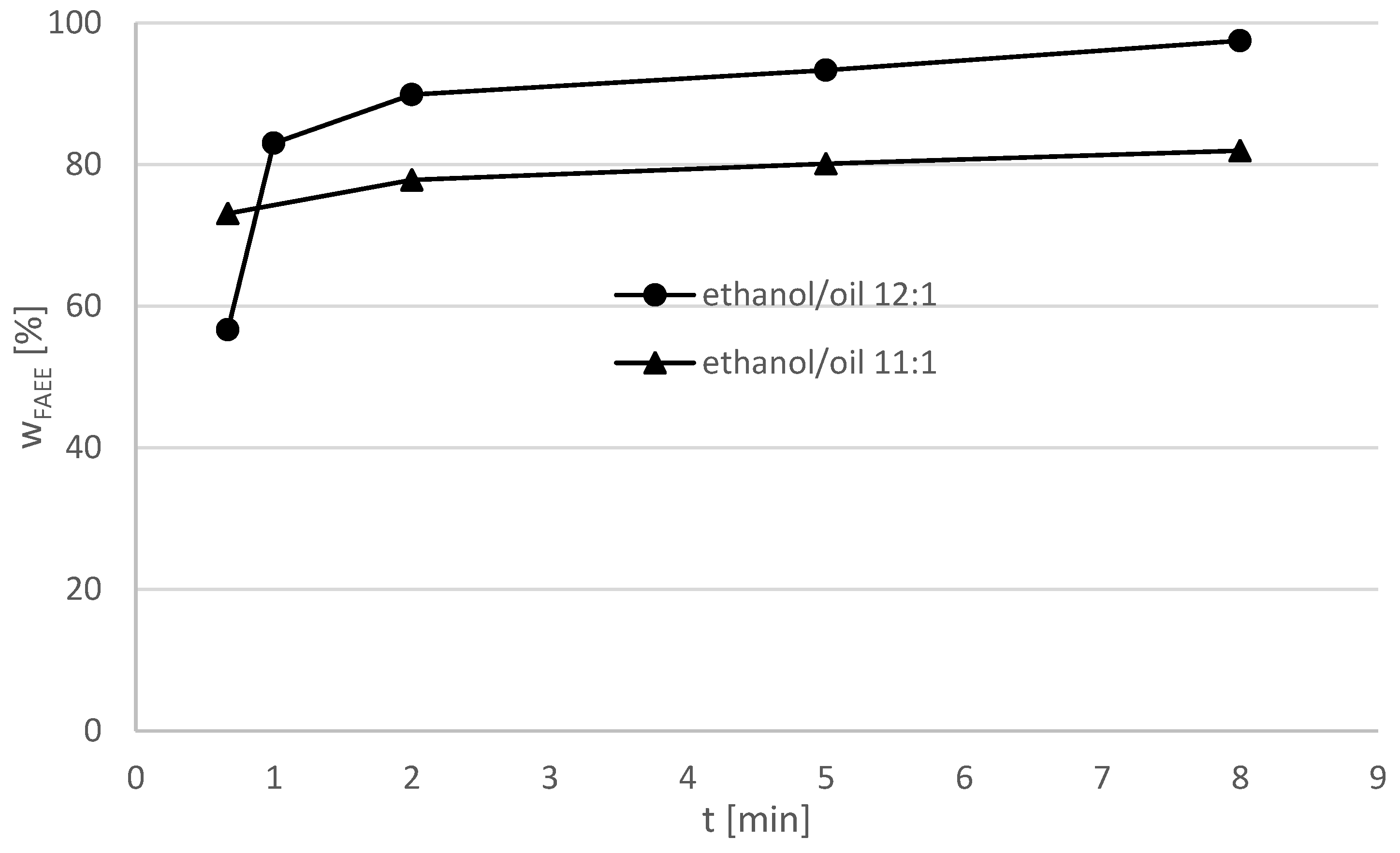
Figure 7 shows the reaction results of the experiment with the original cooking oil at molar ethanol/oil ratios = 11:1 and 12:1. The maximum yield = 96.6% for the ethanol–oil-ratio = 11:1 was detected after 8 min. It can be assumed that this value could be further increased by a longer reaction time. The highest yield in the reaction with the molar ethanol/oil ratio = 12:1 could be detected at 8.5 min. A higher amount of ethanol leads to higher yields indicating a need for high alcohol excess.
The same conclusion can be provided from the experimental results obtained with waste cooking oil (
Figure 8). The effect of ethanol excess on the FAEE yield is very significant. The product analysis for the molar ethanol/oil ratio = 11:1 showed that the amount of byproduct remained relatively high throughout the reaction. This could be partially provided due to contamination of the waste cooking oil with food debris, since only coarse particles could be filtered before usage. It is also known that used cooking or frying oil has a higher acid value which means it contains a higher amount of free fatty acids (FFA) than cooking oil [
13]. It decreases the biodiesel yield of the reaction because FFAs can be saponified by the catalyst [
14]. An excess amount of alcohol would counter this phenomenon by shifting the equilibrium of the saponification to the reactant’s side and thereby favoring the alcoholysis of the FFA.
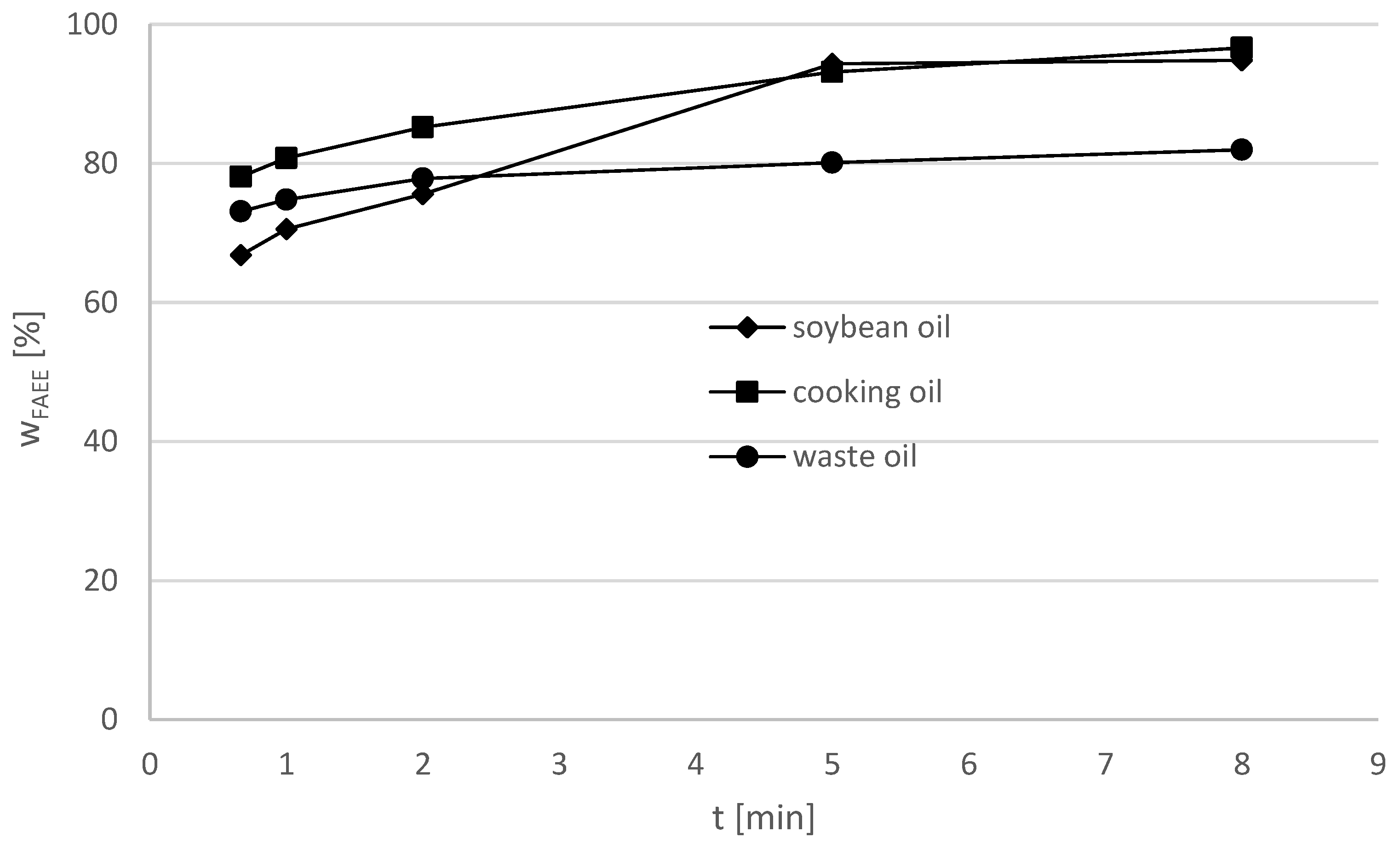
Figure 9 and
Figure 10 show the direct comparison of FAEE yields for different types of oils.
At a molar ethanol/oil ratio = 11:1 the original cooking oil provided a higher yield (96.6%) than the soybean oil (94.8%) at 8 min reaction time. Since the exact composition of the cooking oil is unknown it can only be assumed that the vegetable oils contained hold properties more appropriate for the transesterification. Using the waste cooking oil, the product yield was distinctively lower and a FAEE yield of 82.0% was obtained.
Figure 10 shows the experimental results at a molar ethanol/oil ratio of 12:1. The soybean oil provides slightly lower maximum yields (96.7%) of FAEE than the original cooking oil (97.9%) and the waste cooking oil (97.5%), indicating that the mix of vegetable oils in the cooking oil is more suitable for alcoholysis than soybean oil.
The increase of yields achieved can be described in a logarithmical function for the batch reactor experiments with soybean oil. By determining equations for this increase, the yields of the reactions after 4 min can be interpolated and then compared to the results in MX- and T-mixer reactor systems with soybean oil at the respectively optimal conditions from
Table 5 (
Table 8).
Applying the calculated optimal reaction parameters for the MX-V reactor system to the experiment in the batch reactor leads to a higher product yield of 86.4%. The product yield of 90.8% at optimal reaction conditions in the T-V reactor system could not be exceeded in the batch mode under otherwise equal reaction conditions.
In previous works, the maximum product yield of 93.8% FAEE using soybean oil in the MX-V reactor system was obtained at a reaction time of 11.3 min and molar ethanol/oil ratio = 6:1, the catalyst concentration 1wt.-%, the flow rate 0.565 mL/min and the temperature 50 °C [
14]. Interpolating the product yield of the reaction from a logarithmic equation depending on reaction time suggests the FAEE yield = 84.9% after 4 min. This result was reproduced using the same reactor system and reaction temperature but at a lower catalyst concentration of 0.86 wt.-%, higher molar ethanol/oil ratio of 12:1 and the higher flow rate = 2.6 mL/min. Using the T-V reactor system, the interpolated product yield of 43.9% obtained in [
15] with a flow rate of 0.535 mL/min and otherwise equal experimental conditions in the MX-V reactor system could be exceeded using a higher flow rate = 5.09 mL/min, a molar ethanol/oil ratio = 11:1 and catalyst concentration = 0.86 wt.-%, leading to 90.8% FAEE. These results show very clearly that an excess of alcohol has a positive influence on the reaction yield.
A good mixing of the reactants in the T-mixer is generated at a higher flow rate, which is crucial for obtaining high FAEE yields. This principle doesn’t apply to the MX-mixer. Leung and Guo [
13] found out that increasing the stirring speed in methanolysis experiments (producing fatty acid methyl ethers, FAME) conducted in batch mode had a positive effect on the product yield. Authors conducted experiments with used frying oil (UFO) and neat canola oil and obtained a maximum FAME yield = 88.8 wt.-% at optimal reaction conditions (1.1 wt.-% catalyst (NaOH), 60 °C reaction temperature, 15 min reaction time and a molar methanol/oil ratio of 7:1), while the optimal conditions for the neat canola oil (1 wt.-% NaOH, 45 °C, 15 min, methanol/oil ratio 6:1) provided a maximum FAME yield = 93.5%. Other researchers [
16] performed ethanolysis experiments with waste cottonseed oil in batch mode and found that at a molar ethanol/oil ratio of 12:1, 65 °C and 2.5 h reaction time, 98% FAEE were obtained, using 5 wt.-% of a 3 wt.-% Li/CaO catalyst.
Not only the reaction parameters but also the type and oil composition have a high impact on the FAEE yield. Previous works [
15,
17,
18], as well as the experiments carried out in this work, showed that the product yield for the alcoholysis strongly depends on the acid value (amount of FFA) of the oil, due to occurring saponification reaction.
Since the suitability of different oils for the alcoholysis could only be accurately determined by performing the reactions under otherwise completely equal conditions, a statement on this cannot be made. However, in the past, vegetable oils like sunflower, soybean, palm or rapeseed oil proved to be reasonable feedstocks [
13]. But the economic aspect of the production is a very important one, which implies that the oil yield of the crop should be as high as possible. Ghazali et al. [
19] mentioned in their review on the effects of different biodiesel feedstocks on the engine that about 70–80% of the total production cost account for the cost of the raw materials. In view of this, they stated that palm oil is the most suitable edible vegetable oil for biodiesel production, while Jatropha oil is the best non-edible one for this purpose. By optimizing the reaction conditions, the production costs could be further decreased due to possible energy-saving methods.
The efficiency of base-catalyzed transesterification, primarily conducted in batch reactors, can be substantially increased by a continuous process in microstructured reactors. Due to the much higher area/volume ratios, faster reaction rates and a faster mass and heat transfer in the base-catalyzed ethanolysis of waste cooking oils can be achieved in microreactors. Other enhancements are shorter diffusion distances and reduced residence times [
15].
The techno-economic implication of the atmospheric and subcritical heterogeneous transesterification [
20] and results from present work were compared. The authors report the time of reaction about 1–3 h at 70 °C. The reaction in microreactors needs several minutes at 50 °C providing lower costs and higher capacities for the industrial application.
4. Conclusions
Base catalyzed transesterification of oils with alcohols is an established batch process for biodiesel production. The application of a continuous flow process in microreactors leads to process intensification. The ethanolysis of soybean oil was studied in microreactors using Design of Experiments (DoE).
Experimental results show that the enhancement of the flow rate has a very-high positive impact on the FAEE yield in the system with T-mixer, while in the MX-V reactor system a longer residence time is important. In the system MX-mixer, the wFAEE could be raised from 68.1% to 84.9% after optimization procedure. High flow rates have a negative impact on the yield of the desired product in the MX-V reactor system. While the increased flow rate was counterproductive in the MX-mixer, it had a great positive impact on the reaction yields in the system with the T-mixer. The wFAEE in the system with T-mixer could be increased from 61.8% to 90.8%. Due to better blending in the T-mixer, the T-V reactor system is more suitable than the MX-V reactor system for flow rates higher than 2.2 mL/min. The batch reactor experiments lead to the highest product yields, though at an extended reaction time.
Usage of waste cooking oil provides reasonable product yields in the discontinuously conducted experiments at a molar ethanol/oil ratio = 12:1. The soybean oil provides slightly lower maximum FAEE yields = 96.7% than the original cooking oil 97.9% and the waste cooking oil (97.5%). This indicates that the mix of vegetable oils in the cooking oil is more suitable for alcoholysis reaction than the soybean oil.
It is important to apply these reaction parameters to a continuous mode process, particularly with waste oil and ethanol, because it is important to desist from edible oils as feedstock. As mentioned above, the continuous experiments were characterized by higher controllability, safer dealing with toxic chemicals and easy up-scaling.