Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals
Abstract
Featured Application
Abstract
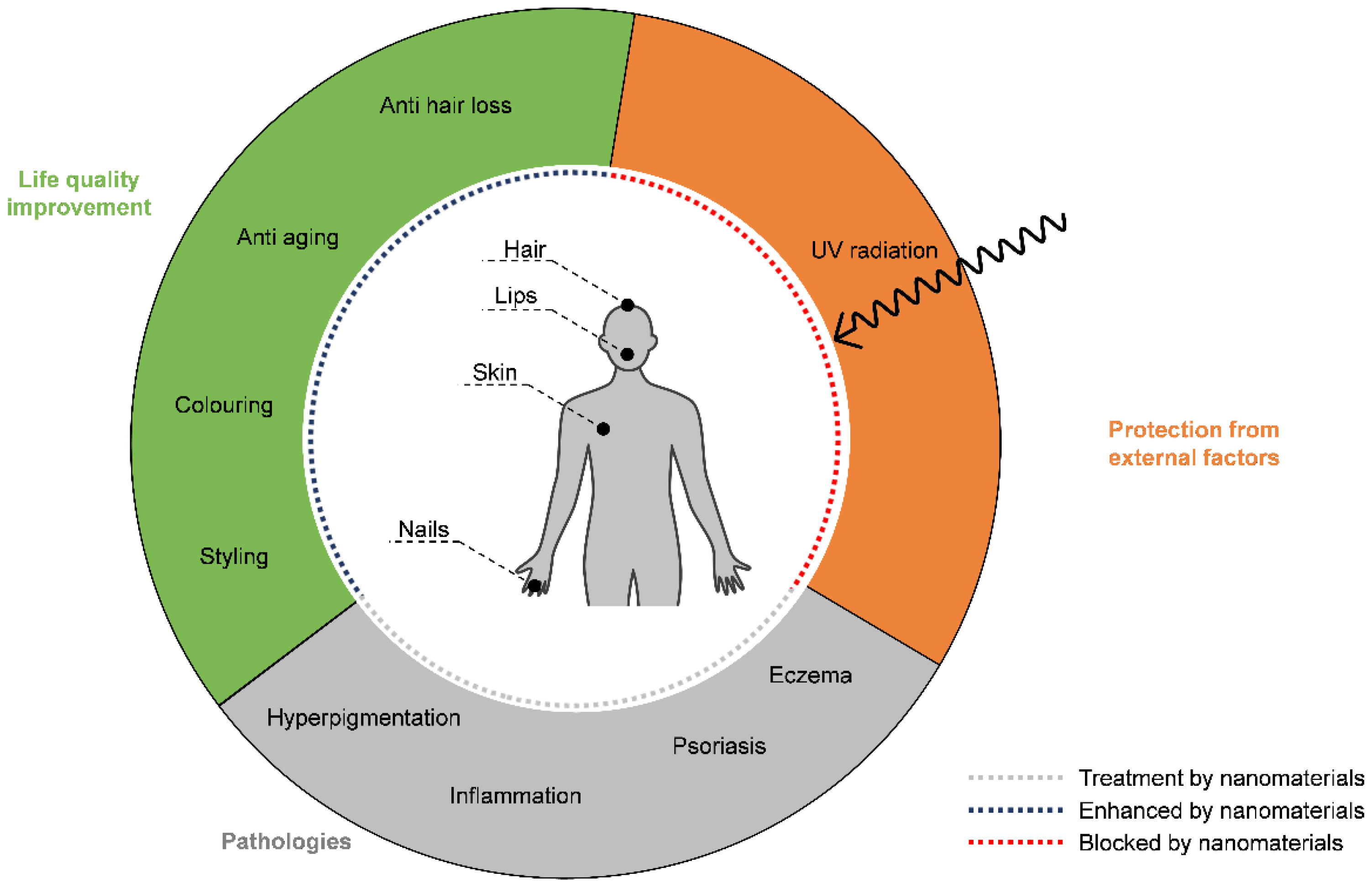
1. Introduction
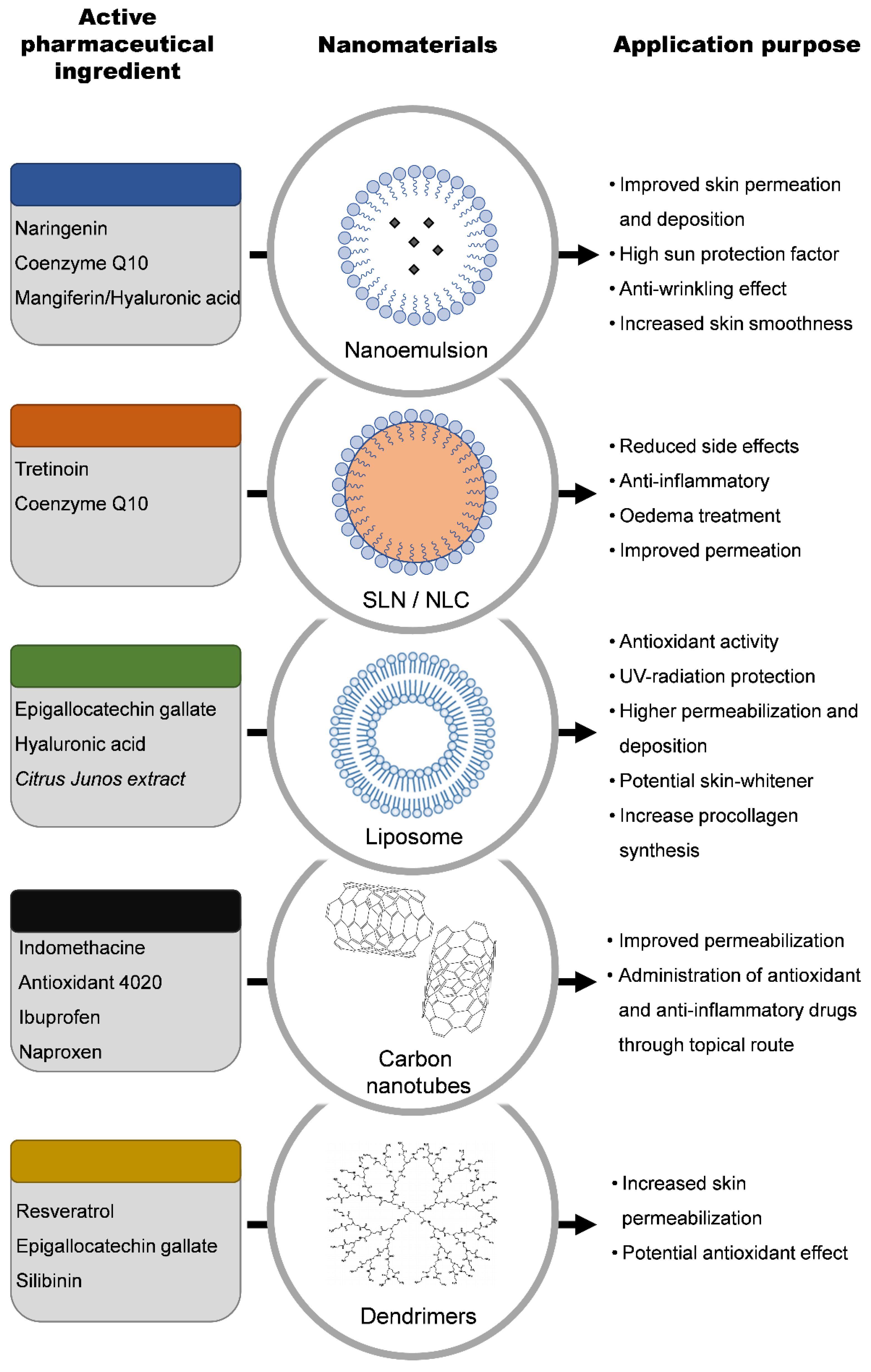
2. Types of Nanomaterials Used in Cosmetic Formulations
2.1. Nanoemulsions
2.2. Solid Lipid Nanoparticles (SLN) and the Nanostructured Lipid Carriers (NLC)
2.3. Nanocrystals
2.4. Dendrimers
2.5. Liposomes
2.6. Niosomes
2.7. Metal Nanoparticles
2.8. Polymeric Nanoparticles
2.9. Carbon Nanotubes
2.10. Polymersomes
2.11. Cubosomes
3. Nanomaterials in Anti-Aging Formulations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Dias-Ferreira, J.; Oliveira, J.; Sanchez-Lopez, E.; Lopez-Machado, A.; Espina, M.; Garcia, M.L.; Souto, S.B.; Martins-Gomes, C.; Silva, A.M. Trends in Atopic Dermatitis-From Standard Pharmacotherapy to Novel Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 5659. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Fangueiro, J.F.; Ferreira, S.V.; Basso, R.; Chaud, M.V.; Santana, M.H.; Rosmaninho, A.; Souto, E.B. Nanoemulsions and nanoparticles for non-melanoma skin cancer: Effects of lipid materials. Clin. Transl. Oncol. 2013, 15, 417–424. [Google Scholar] [CrossRef]
- Souto, E.B.; Ribeiro, A.F.; Ferreira, M.I.; Teixeira, M.C.; Shimojo, A.A.M.; Soriano, J.L.; Naveros, B.C.; Durazzo, A.; Lucarini, M.; Souto, S.B.; et al. New Nanotechnologies for the Treatment and Repair of Skin Burns Infections. Int. J. Mol. Sci. 2020, 21, 393. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sousa, I.M.O.; Goncalves, F.; Eberlin, S.; Davila, J.L.; Jozala, A.F.; Chaud, M.V.; Sanchez-Lopez, E.; et al. Flavonoid-Enriched Plant-Extract-Loaded Emulsion: A Novel Phytocosmetic Sunscreen Formulation with Antioxidant Properties. Antioxidants (Basel) 2019, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.C.; Nascimento, L.E.D.; Bani, C.; Almeida, T.; Nery, M.; Santos, R.S.; Menezes, L.R.O.; Zielinska, A.; Fernandes, A.R.; Cardoso, J.C.; et al. Development, Cytotoxicity and Eye Irritation Profile of a New Sunscreen Formulation Based on Benzophenone-3-poly(epsilon-caprolactone) Nanocapsules. Toxics 2019, 7, 51. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Eberlin, S.; da Silva Goncalves, F.C.; Fernandes, A.R.; Marto, J.; Ribeiro, H.M.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. In vitro SPF and Photostability Assays of Emulsion Containing Nanoparticles with Vegetable Extracts Rich in Flavonoids. AAPS Pharmscitech 2018, 20, 9. [Google Scholar] [CrossRef]
- Xia, Q.; Saupe, A.; Muller, R.H.; Souto, E.B. Nanostructured lipid carriers as novel carrier for sunscreen formulations. Int. J. Cosmet. Sci. 2007, 29, 473–482. [Google Scholar] [CrossRef]
- Souto, E.B.; Anselmi, C.; Centini, M.; Muller, R.H. Preparation and characterization of n-dodecyl-ferulate-loaded solid lipid nanoparticles (SLN). Int. J. Pharm. 2005, 295, 261–268. [Google Scholar] [CrossRef]
- Osmond-McLeod, M.J.; Oytam, Y.; Rowe, A.; Sobhanmanesh, F.; Greenoak, G.; Kirby, J.; McInnes, E.F.; McCall, M.J. Long-term exposure to commercially available sunscreens containing nanoparticles of TiO2 and ZnO revealed no biological impact in a hairless mouse model. Part Fibre Toxicol. 2016, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Moraes, L.F.; Zanchetta, B.; Souto, E.B.; Santana, M.H.A. Elastic liposomes containing benzophenone-3 for sun protection factor enhancement. Pharm. Dev. Technol. 2012, 17, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Khanna, S.; Nasa, A. Cosmeceuticals for the skin: An overview. Asian J. Pharm. Clin. Res. 2011, 4, 1–6. [Google Scholar]
- Ahmadi Ashtiani, H.R.; Bishe, P.; Lashgari, N.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in Cosmetics. J. Ski. Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Carbone, C.; Teixeira, M.D.C.; Sousa, M.D.C.; Martins-Gomes, C.; Silva, A.M.; Souto, E.M.B.; Musumeci, T. Clotrimazole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Doktorovova, S. Chapter 6-Solid lipid nanoparticle formulations pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzymol. 2009, 464, 105–129. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Saha, R. Cosmeceuticals and herbal drugs: Practical uses. Int. J. Pharm. Sci. Res. 2012, 3, 59. [Google Scholar]
- Dureja, H.; Kaushik, D.; Gupta, M.; Kumar, V.; Lather, V. Cosmeceuticals: An emerging concept. Indian J. Pharmacol. 2005, 37, 155. [Google Scholar] [CrossRef]
- Srinivas, K. The current role of nanomaterials in cosmetics. J. Chem. Pharm. Res. 2016, 8, 906–914. [Google Scholar]
- Brandt, F.S.; Cazzaniga, A.; Hann, M. Cosmeceuticals: Current trends and market analysis. Semin. Cutan. Med. Surg. 2011, 30, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Dias-Ferreira, J.; Shegokar, R.; Durazzo, A.; Santini, A. Ethical issues in research and development of nanoparticles. In Drug Delivery Aspects; Shegokar, R., Ed.; Drug Delivery Trends; Elsevier: AE Amsterdam, The Netherlands, 2020; Volume 3, Chapter 7. [Google Scholar]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Doktorovova, S.; Shegokar, R.; Rakovsky, E.; Gonzalez-Mira, E.; Lopes, C.M.; Silva, A.M.; Martins-Lopes, P.; Muller, R.H.; Souto, E.B. Cationic solid lipid nanoparticles (cSLN): Structure, stability and DNA binding capacity correlation studies. Int. J. Pharm. 2011, 420, 341–349. [Google Scholar] [CrossRef]
- Doktorovova, S.; Silva, A.M.; Gaivao, I.; Souto, E.B.; Teixeira, J.P.; Martins-Lopes, P. Comet assay reveals no genotoxicity risk of cationic solid lipid nanoparticles. J. Appl. Toxicol. 2014, 34, 395–403. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers-a systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arraez-Roman, D.; Martinez-Ferez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols-A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-based cosmetics for hair care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Sundari, P.T.; Anushree, H. Novel delivery systems: Current trend in cosmetic industry. Eur. J. Pharm. Med. Res. 2017, 4, 617–627. [Google Scholar]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against T richophyton rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Santos, D.L.; Costa, I.; Andreani, T.; Souto, E.B.; Silva, A.M. Cationic solid lipid nanoparticles interfere with the activity of antioxidant enzymes in hepatocellular carcinoma cells. Int. J. Pharm. 2014, 471, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm. Dev. Technol. 2019, 24, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharm. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, V.N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharm. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals-shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharm. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Bhadoriya, S.S.; Mangal, A.; Madoriya, N.; Dixit, P. Bioavailability and bioactivity enhancement of herbal drugs by “Nanotechnology”: A review. J. Curr. Pharm. Res. 2011, 8, 1–7. [Google Scholar]
- Dokhani, A.; Amini, J.; Gortzi, O.; Danaei, M.; Mozafari, M.R.; Maherani, B. Enhanced Efficacy and Bioavailability of Skin-Care Ingredients Using Liposome and Nano-liposome Technology. Mod. Appl. Bioequiv. Availab. 2017, 2, 555584. [Google Scholar]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduno-Ramirez, M.L.; Clares, B.; Garcia, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Calpena, A.C.; Parra, A.; Abrego, G.; Alvarado, H.; Fangueiro, J.F.; Souto, E.B. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: Effect on skin permeation. Int. J. Pharm. 2014, 473, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A. Nanotechnology and dermatology: Part II—risks of nanotechnology. Clin. Dermatol. 2010, 28, 581–588. [Google Scholar] [CrossRef]
- Soriano-Ruiz, J.L.; Calpena-Capmany, A.C.; Canadas-Enrich, C.; Febrer, N.B.; Suner-Carbo, J.; Souto, E.B.; Clares-Naveros, B. Biopharmaceutical profile of a clotrimazole nanoemulsion: Evaluation on skin and mucosae as anticandidal agent. Int. J. Pharm. 2019, 554, 105–115. [Google Scholar] [CrossRef]
- Macedo, A.S.; Quelhas, S.; Silva, A.M.; Souto, E.B. Nanoemulsions for delivery of flavonoids: Formulation and in vitro release of rutin as model drug. Pharm. Dev. Technol. 2014, 19, 677–680. [Google Scholar] [CrossRef]
- Souto, E.B.; Nayak, A.P.; Murthy, R.S. Lipid nanoemulsions for anti-cancer drug therapy. Pharmazie 2011, 66, 473–478. [Google Scholar]
- Teixeira, M.C.; Severino, P.; Andreani, T.; Boonme, P.; Santini, A.; Silva, A.M.; Souto, E.B. d-alpha-tocopherol nanoemulsions: Size properties, rheological behavior, surface tension, osmolarity and cytotoxicity. Saudi Pharm. J. 2017, 25, 231–235. [Google Scholar] [CrossRef]
- Nand, K. Proniosome Gel: Potential Carrier System in Topical/Transdermal Delivery for Drugs and Cosmetics. Cosmeceuticals Pharmainfo. Net 2010, 16, 35. [Google Scholar]
- Özgün, S. Nanoemulsions in cosmetics. Anadolu Univ. 2013, 1, 3–11. [Google Scholar]
- Souto, E.B.; Muller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. In Drug Delivery; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 115–141. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. Cosmetic features and applications of lipid nanoparticles (SLN, NLC). Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef]
- Arora, N.; Agarwal, S.; Murthy, R. Latest technology advances in cosmaceuticals. Int. J. Pharm. Sci. Drug Res. 2012, 4, 168–182. [Google Scholar]
- Patidar, A.; Thakur, D.S.; Kumar, P.; Verma, J. A review on novel lipid based nanocarriers. Int. J. Pharm. Pharm. Sci. 2010, 2, 30–35. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- AlZahabi, S.; Sakr, O.S.; Ramadan, A.A. Nanostructured lipid carriers incorporating prickly pear seed oil for the encapsulation of vitamin A. J. Cosmet. Derm. 2019, 18, 1875–1884. [Google Scholar] [CrossRef]
- Suter, F.; Schmid, D.; Wandrey, F.; Zulli, F. Heptapeptide-loaded solid lipid nanoparticles for cosmetic anti-aging applications. Eur. J. Pharm. Biopharm. 2016, 108, 304–309. [Google Scholar] [CrossRef]
- Severino, P.; Andreani, T.; Chaud, M.V.; Benites, C.I.; Pinho, S.C.; Souto, E.B. Essential oils as active ingredients of lipid nanocarriers for chemotherapeutic use. Curr. Pharm. Biotechnol. 2015, 16, 365–370. [Google Scholar] [CrossRef]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.M.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E.B. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. SLN and NLC for topical delivery of ketoconazole. J. Microencapsul. 2005, 22, 501–510. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J. Microencapsul. 2006, 23, 377–388. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. The use of SLN and NLC as topical particulate carriers for imidazole antifungal agents. Pharmazie 2006, 61, 431–437. [Google Scholar] [PubMed]
- Vieira, R.; Severino, P.; Nalone, L.A.; Souto, S.B.; Silva, A.M.; Lucarini, M.; Durazzo, A.; Santini, A.; Souto, E.B. Sucupira Oil-Loaded Nanostructured Lipid Carriers (NLC): Lipid Screening, Factorial Design, Release Profile, and Cytotoxicity. Molecules 2020, 25, 685. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Ferreira, N.R.; Durazzo, A.; Lucarini, M.; Cicero, N.; Mamouni, S.E.; Silva, A.M.; Nowak, I.; Santini, A.; Souto, E.B. Development and Optimization of Alpha-Pinene-Loaded Solid Lipid Nanoparticles (SLN) Using Experimental Factorial Design and Dispersion Analysis. Molecules 2019, 24, 2683. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Martins-Gomes, C.; Ferreira, N.R.; Silva, A.M.; Nowak, I.; Souto, E.B. Anti-inflammatory and anti-cancer activity of citral: Optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer(R). Int. J. Pharm. 2018, 553, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Borchard, G. Drug Nanocrystals. In Non-Biological Complex Drugs: The Science and the Regulatory Landscape; Crommelin, D.J.A., de Vlieger, J.S.B., Eds.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Ye, Y.; Mao, S.; He, S.; Xu, X.; Cao, X.; Wei, Z.; Gunasekaran, S. Ultrasensitive electrochemical genosensor for detection of CaMV35S gene with Fe3O4-Au@Ag nanoprobe. Talanta 2020, 206, 120205. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yao, G.; Liu, X.; Yin, H. Preparation Nanocrystals of Poorly Soluble Plant Compounds Using an Ultra-Small-Scale Approach. AAPS Pharmscitech 2017, 18, 2610–2617. [Google Scholar] [CrossRef]
- Chaudhri, N.; Soni, G.C.; Prajapati, S. Nanotechnology: An advance tool for nano-cosmetics preparation. Int. J. Pharma Res. Rev. 2015, 4, 28–40. [Google Scholar]
- Lai, F.; Schlich, M.; Pireddu, R.; Fadda, A.M.; Sinico, C. Nanocrystals as Effective Delivery Systems of Poorly Water-soluble Natural Molecules. Curr. Med. Chem. 2019, 26, 4657–4680. [Google Scholar] [CrossRef]
- Liu, T.; Muller, R.H.; Moschwitzer, J.P. Production of drug nanosuspensions: Effect of drug physical properties on nanosizing efficiency. Drug Dev. Ind. Pharm. 2018, 44, 233–242. [Google Scholar] [CrossRef]
- Nimesh, S. Dendrimers. In Gene Therapy-Potential Applications of Nanotechnology, 1st ed.; Nimesh, S., Ed.; Woodhead Publishing: Cambridge, UK, 2013; Chapter 13. [Google Scholar] [CrossRef]
- Arif, T.; Adil, M. Nanotechnology-Dermatological Perspective. Int. J. Nanomed. Nanosurg. 2016, 2. [Google Scholar] [CrossRef]
- Maignan, J.; Genard, S. Use of Hyperbranched Polymers and Dendrimers Comprising a Particular Group as Film-Forming Agent, Film-Forming Compositions Comprising Same and Use Particularly in Cosmetics and Pharmaceutics. U.S. Patent US6432423B1, 13 August 2002. [Google Scholar]
- Adams, G.; Ashton, M.R.; Khoshdel, E. Cosmetic or Dermatological Topical Compositions Containing Dendritic Polyesters. European Patent EP0987017, 13 June 2001. [Google Scholar]
- Bangham, A.D.; Horne, R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668, IN2-IN10. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: The acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids Surf. B Biointerfaces 2018, 171, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B. A special issue on Lipid-based delivery systems (liposomes, lipid nanoparticles, lipid matrices and medicines). J. Biomed. Nanotechnol. 2009, 5, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Pashirova, T.N.; Sapunova, A.S.; Lukashenko, S.S.; Burilova, E.A.; Lubina, A.P.; Shaihutdinova, Z.M.; Gerasimova, T.P.; Kovalenko, V.I.; Voloshina, A.D.; Souto, E.B.; et al. Synthesis, structure-activity relationship and biological evaluation of tetracationic gemini Dabco-surfactants for transdermal liposomal formulations. Int. J. Pharm. 2020, 575, 118953. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Schmid, M.H.; Korting, H.C. Liposomes for atopic dry skin: The rationale for a promising approach. Clin. Investig. 1993, 71, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J. Toxicol. 2011, 2011, 152474. [Google Scholar] [CrossRef]
- Arif, T.; Nisa, N.; Amin, S.S.; Shoib, S.; Mushtaq, R.; Shawl, M.R. Therapeutic and diagnostic applications of nanotechnology in dermatology and cosmetics. J. Nanomed. Biother. Discov. 2015, 5, 1. [Google Scholar]
- Ghyczy, M.; Nissen, H.; Biltz, H. The treatment of acne vulgaris by phosphatidylcholine from soybeans, with a high content of linoleic acid. J. Appl. Cosmetol. 1996, 14, 137–146. [Google Scholar]
- Mota, A.d.C.V.; de Freitas, Z.M.F.; Ricci Júnior, E.; Dellamora-Ortiz, G.M.; Santos-Oliveira, R.; Ozzetti, R.A.; Vergnanini, A.L.; Ribeiro, V.L.; Silva, R.S.; dos Santos, E.P. In vivo and in vitro evaluation of octyl methoxycinnamate liposomes. Int. J. Nanomed. 2013, 8, 4689–4701. [Google Scholar] [CrossRef]
- Zhao, X.; Lai, E.P.C. Titania and Zinc Oxide Nanoparticles: Coating with Polydopamine and Encapsulation within Lecithin Liposomes—Water Treatment Analysis by Gel Filtration Chromatography with Fluorescence Detection. Separations 2018, 5, 13. [Google Scholar] [CrossRef]
- Blume, G. Flexible liposomes for topical applications in cosmetics. Sci. Appl. Skin Deliv. Syst. 2008, 269–282. [Google Scholar]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374. [Google Scholar]
- Chandu, V.P.; Arunachalam, A.; Jeganath, S.; Yamini, K.; Tharangini, K.; Chaitanya, G. Niosomes: A novel drug delivery system. Int. J. Novel Trends Pharm. Sci. 2012, 2, 25–31. [Google Scholar]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Gandhi, M.; Sanket, P.; Mahendra, S. Niosomes: Novel drug delivery system. Int. J. Pure Appl. Biosci. 2014, 2, 267–274. [Google Scholar]
- Verma, S.; Singh, S.; Syan, N.; Mathur, P.; Valecha, V. Nanoparticle vesicular systems: A versatile tool for drug delivery. J. Chem. Pharm. Res. 2010, 2, 496–509. [Google Scholar]
- Varun, T.; Sonia, A.; Bharat, P.; Patil, V. Niosomes and liposomes-vesicular approach towards transdermal drug delivery. Int. J. Pharm. Chem. Sci. 2012, 1, 632–644. [Google Scholar]
- Nasir, A.; Harikumar, S.; Amanpreet, K. Niosomes: An excellent tool for drug delivery. Int. J. Res. Pharm. Chem. 2012, 2, 479–487. [Google Scholar]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Kokura, S.; Handa, O.; Takagi, T.; Ishikawa, T.; Naito, Y.; Yoshikawa, T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine 2010, 6, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Grabowska, A.; Chwastowski, J.; Majka, T.M.; Banach, M. Safety of the application of nanosilver and nanogold in topical cosmetic preparations. Colloids Surf. B Biointerfaces 2019, 183, 110416. [Google Scholar] [CrossRef]
- Kodoth, A.K.; Ghate, V.M.; Lewis, S.A.; Prakash, B.; Badalamoole, V. Pectin-based silver nanocomposite film for transdermal delivery of Donepezil. Int. J. Biol. Macromol. 2019, 134, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Alaqad, K.; Saleh, T.A. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 2016, 6, 525–2161. [Google Scholar] [CrossRef]
- Thakor, A.; Jokerst, J.; Zavaleta, C.; Massoud, T.; Gambhir, S. Gold nanoparticles: A revival in precious metal administration to patients. Nano Lett. 2011, 11, 4029–4036. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef]
- Tahir, M.A.; Ali, M.E.; Lamprecht, A. Nanoparticle formulations as recrystallization inhibitors in transdermal patches. Int. J. Pharm. 2020, 575, 118886. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Garduno, M.L.; Garcia, M.L.; Calpena, A.C. Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization. Int. J. Pharm. 2016, 501, 350–361. [Google Scholar] [CrossRef]
- Gilbert, E.; Roussel, L.; Serre, C.; Sandouk, R.; Salmon, D.; Kirilov, P.; Haftek, M.; Falson, F.; Pirot, F. Percutaneous absorption of benzophenone-3 loaded lipid nanoparticles and polymeric nanocapsules: A comparative study. Int. J. Pharm. 2016, 504, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019, 6, 191184. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Takahashi, T.; Kanamura, K.; Kawakami, H. Possibility for the development of cosmetics with PLGA nanospheres. Drug Dev. Ind. Pharm. 2013, 39, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduno-Ramirez, M.L.; Garcia, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan-PVP-titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef]
- Ibrahim, K.S. Carbon nanotubes? properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar]
- Hirlekar, R.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V. Carbon nanotubes and its applications: A review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Zhang, M.; Li, J. Carbon nanotube in different shapes. Mater. Today 2009, 12, 12–18. [Google Scholar] [CrossRef]
- Kim, S.-H.; Shum, H.C.; Kim, J.W.; Cho, J.-C.; Weitz, D.A. Multiple polymersomes for programmed release of multiple components. J. Am. Chem. Soc. 2011, 133, 15165–15171. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, P.-Y. Polymersomes in nanomedicine-A review. Curr. Nanosci. 2017, 13, 124–129. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Osmani, R.A.; Harkare, B.R.; Ghodake, P.P. Cubosomes: The inimitable nanoparticulate drug carriers. Sch. Acad. J. Pharm. 2013, 2, 481–486. [Google Scholar]
- Thadanki, M.; Kumari, P.S.; Prabha, K.S. Overview of cubosomes: A nano particle. Int. J. Res. Pharm. Chem. 2011, 1, 535–541. [Google Scholar]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef]
- Hu, Z.; Liao, M.; Chen, Y.; Cai, Y.; Meng, L.; Liu, Y.; Lv, N.; Liu, Z.; Yuan, W. A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone. Int. J. Nanomed. 2012, 7, 5719. [Google Scholar]
- Ünlü, E.; Erdem, C. Deri yaşlanmasında korunma ve tedavi yöntemleri. Dermatoz 2010, 1, 23–31. [Google Scholar]
- Otlatıcı, G.; Yeğen, G.; Güngör, S.; Aksu, B. Overview on nanotechnology based cosmeceuticals to prevent skin aging. Istanb. J. Pharm. 2018, 48, 55–62. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- Türsen, Ü. Deri Yaşlanmasının Topikal Ajanlarla Önlenmesi. Dermatose 2006, 4, 267–283. [Google Scholar]
- de Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.R.; Severino, P.; Ferreira, C.S.; Zielinska, A.; Santini, A.; Souto, S.B.; Souto, E.B. Linseed Essential Oil-Source of Lipids as Active Ingredients for Pharmaceuticals and Nutraceuticals. Curr. Med. Chem 2019, 26, 4537–4558. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Zielinska, A.; Ferreira, N.R.; Silva, A.M.; Souto, E.B. Optimization of linalool-loaded solid lipid nanoparticles using experimental factorial design and long-term stability studies with a new centrifugal sedimentation method. Int. J. Pharm. 2018, 549, 261–270. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus (L.): Bioactive Components’ Beneficial Properties-Focused on Antidiabetic Role-For Sustainable Health Applications. Molecules 2018, 24, 38. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Ferrari Nicoli, S.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Taghouti, M.; Schäfer, J.; Bunzel, M.; Silva, A.M.; Nunes, F.M. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Allemann, I.B.; Baumann, L. Botanicals in skin care products. Int. J. Dermatol. 2009, 48, 923–934. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Souto, E.B.; Cosme, F.; Nunes, F.M.; Silva, A.M. Thymus carnosus extracts induce anti-proliferative activity in Caco-2 cells through mechanisms that involve cell cycle arrest and apoptosis. J. Funct. Foods 2019, 54, 128–135. [Google Scholar] [CrossRef]
- Sorg, O.; Kuenzli, S.; Kaya, G.; Saurat, J.H. Proposed mechanisms of action for retinoid derivatives in the treatment of skin aging. J. Cosmet. Dermatol. 2005, 4, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, A. Future prospect of nanotechnology in development of anti-ageing formulations. Int. J. Pharm. Pharm. Sci. 2012, 4, 57–66. [Google Scholar]
- Lazarus, M.C.; Baumann, L.S. The use of cosmeceutical moisturizers. Dermatol. Ther. 2001, 14, 200–207. [Google Scholar] [CrossRef]
- Graf, J. Anti-aging skin care ingredient technologies. In Cosmetic Dermatology; Springer: Berlin, Germany, 2005; pp. 17–28. [Google Scholar]
- Gondikas, A.P.; Kammer, F.v.d.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old Danube recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Colao, C. Biofunctional Textiles for Aging Skin. Biomedicines 2019, 7, 51. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Ni, Y.; Khan, A.; Wang, B. Chitosan-Nanocellulose Composites for Regenerative Medicine Applications. Curr. Med. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Schutz, C.; Sort, J.; Bacsik, Z.; Oliynyk, V.; Pellicer, E.; Fall, A.; Wagberg, L.; Berglund, L.; Bergstrom, L.; Salazar-Alvarez, G. Hard and transparent films formed by nanocellulose-TiO2 nanoparticle hybrids. PLoS ONE 2012, 7, e45828. [Google Scholar] [CrossRef]
| Brand-Product | Characteristics and Uses |
|---|---|
| Agera®-Nano Eye Lift | Anti-aging skin care. Make the skin around the eyes softer |
| Bionova-Nano Skin Tech Tennis Player Sun and Wind Protection | Confers UV protection, using nanocomplex of naturally existing UV Chromophores and UV Protectant. Increase the protection against the sun radiation. Widely used in dry skin (nanocomplex of multiple antioxidants, oil, water-soluble vitamins with their specific coenzymes) |
| Chantecaille-Nano Gold Energizing Cream | Anti-aging power due to the incorporation of 24-karat gold and silk bound, together with pullalan/algae and plantago extracts, and other natural antioxidants (vitamin E) and natural oils. Decrease significantly the lines, wrinkles, dullness and the dehydration. Antioxidant and anti-inflammatory activity. |
| StriVectinTM-Specialised Hand Care System | Offers specialized hand care, for the treatment of age and/or sun spots, ageing skin, fine Lines and wrinkles. NanoExfoliate action (with thermo-active formula, exfoliate without causing redness or damage on the skin). Hand Cream ultra-concentrated to nourish, protect and hydrate the hand’s skin (contains hyaluronic acid, UV blockers, etc). |
| Rosactive® Phytoceutical Skin care-Biomixyl | Anti-aging line with several products designed to reduce wrinkles and lines by stimulating collagen production, using a bio-peptide complex and natural oils and extracts from several plants. |
| Salcura® Natural Skin Therapy—e.g., Bioskin Zeoderm Skin Repair System | The products consist of natural colloidal solution delivery systems based on biotechnology and nanotechnology. Aimed to treat dry skin and irritation symptoms, uses natural extracts, oils and other ingredients. Used for the treatment of diseases, such as, eczema, psoriasis, dermatitis and other skin allergies. |
| Shakti® Face and Body Resculpting Cream™ | With encapsulated black currant seed oil (also contains amino-acids, vitamin C, oil from fragrant Bulgarian roses, etc.), to promote natural antibacterial and anti-inflammatory action, while conferring lifting and moisturizing to the skin from head to toe. |
| Nanoceuticals™ Citrus Mint Shampoo | With nanoscale ingredients (in suspended nanoparticles), allow the scavenging of free radicals, the stimulation of the source energy, increased hydration, the balance of pH, and others. Nanoparticles in the composition provide to the hair a healthy shine. |
| Serge Lutens Blusher (Barneys New York®) Nano Dispersion technology | Make up products produced with resource to nanotechnology. Due to the dispersion technology, this powder has excellent elasticity, extreme softness and light diffusion. |
| Apagard® Royal-Sangi | It is a re-mineralizing toothpaste (contains nano medical hydroxyapatite for protection against caries). Promotes oral health by preventing caries, remineralization and whitening using natural healing. |
| Nanomaterial | Active Ingredients and Applications | Brand/Producer |
|---|---|---|
| Nanosomes™ | Plant extracts (e.g., Gingko biloba), oils (e.g., almond and lavender) and natural compounds (caffeine, amino acids and polyphenols). With high cationic charge density, enhances the deposition of functional active ingredients and fragrances at target site and reduces wash-off during rinse. e.g., Viterol. A (Cream for Wrinkles and Expression Lines), Revita® shampoo (Anti-hair loss, hair follicle stimulation) | DS Laboratories |
| Liposomes | Coenzyme Q10, vitamins (A, C and E) and green tea extract. Encapsulation using Rovisome™, with enhanced sun protection and antioxidant effect of the active ingredients. e.g., Rovisome ACE Plus, Rovisome Defence, Rovisome Q10 | Rovi Cosmetics International GmbH |
| Coenzyme Q10. Enhances antioxidant, anti-wrinkle and penetration properties, as well as collagen and elastin production of connective tissue. e.g., Ageless Face Firming Serum, Ageless Anti-Wrinkle Serum | I-Wen Naturals | |
| Natural extracts and oils (Chondrus crispus, Prunus armeniaca, Aloe barbadensis, Symphytum officinale, Rosa damascena). Liposome encapsulation improves natural products delivery to target site, with higher antioxidant activity and skin hydration. e.g., Liposome Concentrate | Russell Organics | |
| Vitamin E, hyaluronic acid, super oxide dismutase (SOD) and sunflower oil. The use of encapsulated vitamin E, SOD and hyaluronic acid increases skin protection to sun damage and oxidative stress. Skin rejuvenation in enhanced. e.g., Liposome Face and Neck Lotion | Clinicians Complex | |
| Liposomes formulation containing ginseng to mitigate skin aging signs. E.g., Anti-Age Response Cream Simply Man Match | Nouvelle, HSA® cosmetics | |
| SLN and NLC | Includes plant extracts and oils, that promote skin regeneration and provide anti-inflammatory and anti-scaling activities. E.g., Phyto NLC Active Cell Repair | Sirehemas™ |
| Q10, hemp and macadamia nut oils, oil, Edelweiss extract. The encapsulation improved skin’s elasticity and regeneration. E.g., Xcelent Uplift Q10 | Dr. Rimpler | |
| Nanoemulsion | Vitamin E and B3, provitamin B5. Enhanced penetration of the active ingredients, that provide anti-pollution and enhance skin’s hydration and regeneration. E.g., Bepanthol-Protect Facial Cream Ultra | Bayer HealthCare |
| UVA and UVB filters, loaded in transparent cationic Nanoemulsions, for hair protection. E.g., Nano-lipobelle™ | Mibelle Biochemistry, Switzerland | |
| Nanocomplex with TiO2 and ZnO | Contain Zinc oxide particles that act as UV radiation blockers for sun protection. E.g., Sheer Zinc Dry-Touch Sunscreen Broad Spectrum SPF 50 | Neutrogena® |
| Contains iron oxide, titanium dioxide and plant extracts and vitamins. Bronze mineral pigments in its composition provide sun tan effect. E.g., Instant bronze body butter. | Mineral Fusion® | |
| Gold nanoparticles | Composed of 24 k gold and silk bounded together, botanical (extracts and oils) and plant stem cell extracts, promoting antioxidant, smoothing, clarity and luminosity properties. with anti-aging, anti-wrinkle and anti-redness properties. Skin care products (e.g., Nano Gold Energizing Cream and Nano Gold Energizing Eye Serum) | Chantecaille |
| The active ingredients include gold powder, plant extracts (coffee Seed (Coffee arabica), Aloe barbadensis, Cucurbita pepo (Pumpkin) seed), vitamins (A, C and E) and hyaluronic acid. Skin care product with anti-aging properties. E.g., Nano Gold Anti-Aging Lifting Serum | Nuvoderm | |
| Nanospheres | Plant extracts and oils (olive, Canadian Willowherb™, green tea), vitamin E and hyaluronic acid. Provide anti-wrinkle activity, anti-inflammatory activity and stimulate fibroblasts and collagen production. e.g., Eye Tender® | Kara Vita |
| Contain natural products (soy firming agent), vitamin E and UVA/UVB filters. Nanospheres provide a sustained release of the active ingredients. The formulation promotes moisturizing and anti-inflammatory activities as well as skin repair on scarred tissues and UV radiation protection. The photo-reflecting agent confers radiance to the complexion. E.g., DNA Filler Intense Cream | CellAct Switzerland |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. https://doi.org/10.3390/app10051594
Souto EB, Fernandes AR, Martins-Gomes C, Coutinho TE, Durazzo A, Lucarini M, Souto SB, Silva AM, Santini A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Applied Sciences. 2020; 10(5):1594. https://doi.org/10.3390/app10051594
Chicago/Turabian StyleSouto, Eliana B., Ana Rita Fernandes, Carlos Martins-Gomes, Tiago E. Coutinho, Alessandra Durazzo, Massimo Lucarini, Selma B. Souto, Amélia M. Silva, and Antonello Santini. 2020. "Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals" Applied Sciences 10, no. 5: 1594. https://doi.org/10.3390/app10051594
APA StyleSouto, E. B., Fernandes, A. R., Martins-Gomes, C., Coutinho, T. E., Durazzo, A., Lucarini, M., Souto, S. B., Silva, A. M., & Santini, A. (2020). Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Applied Sciences, 10(5), 1594. https://doi.org/10.3390/app10051594








