Strong Effect of Process Parameters on the Properties of Boron-Containing Phenolic Resins with High Char Yield
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of B-Containing PR
2.3. Sample Characterization
3. Results and Discussion
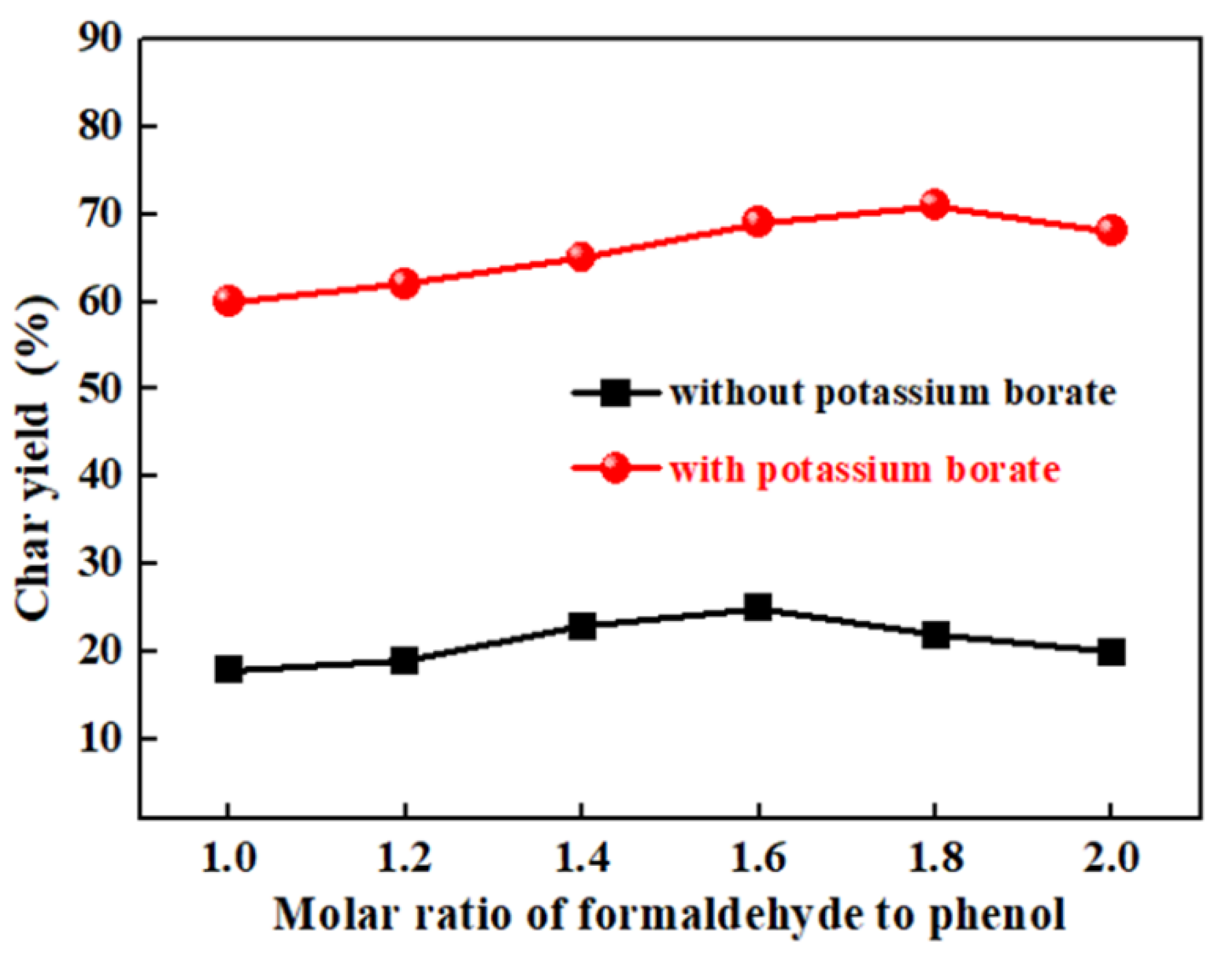
3.1. The Effect of Formaldehyde/Phenol Molar Ratio on the Char Yield of B-Containing PR
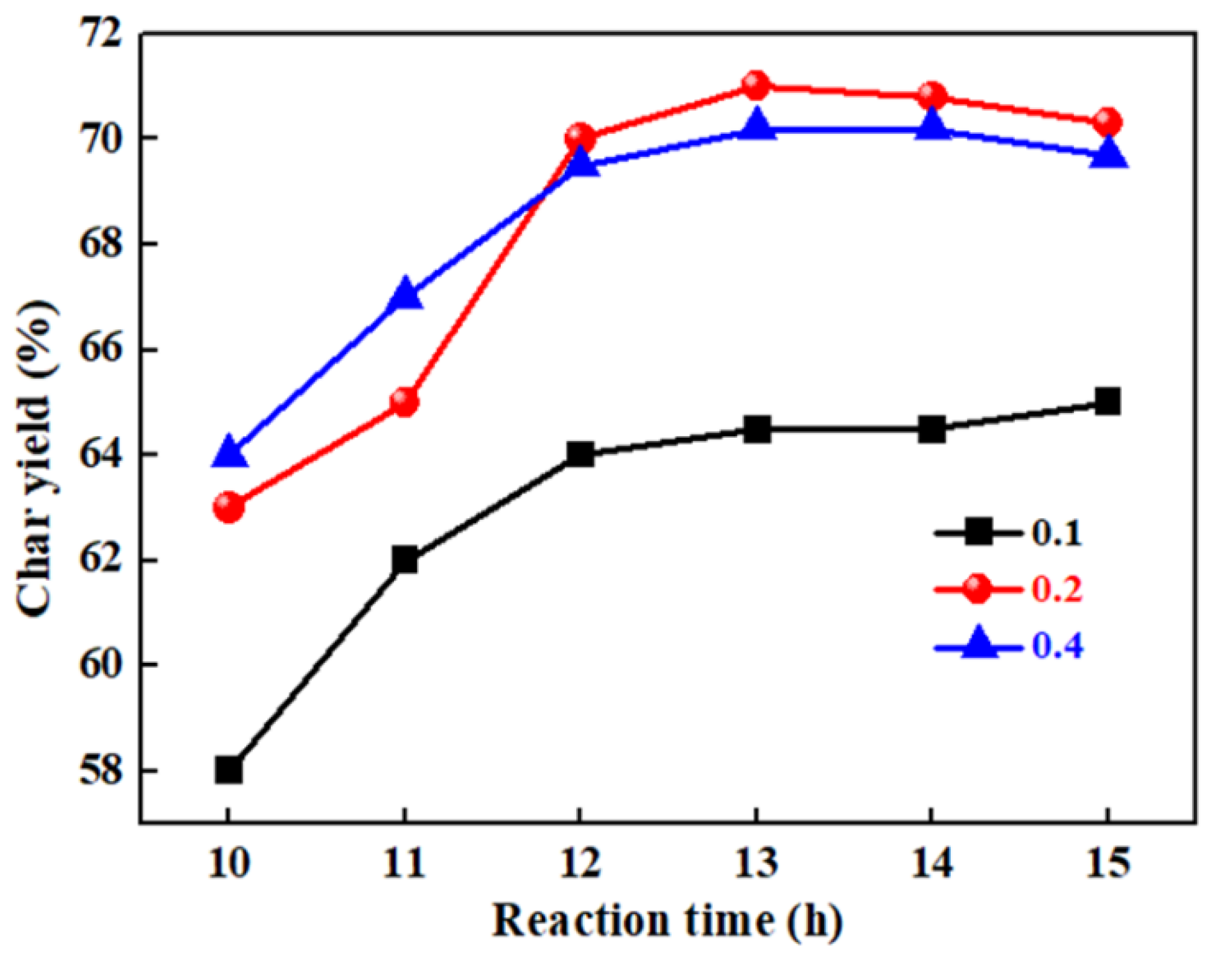
3.2. The Effects of Potassium Borate/Phenol Molar Ratio and Reaction Time on the Char Yield of B-Containing PR
3.3. Comparison of Thermal Stabilities of Common PR and B-Containing PR
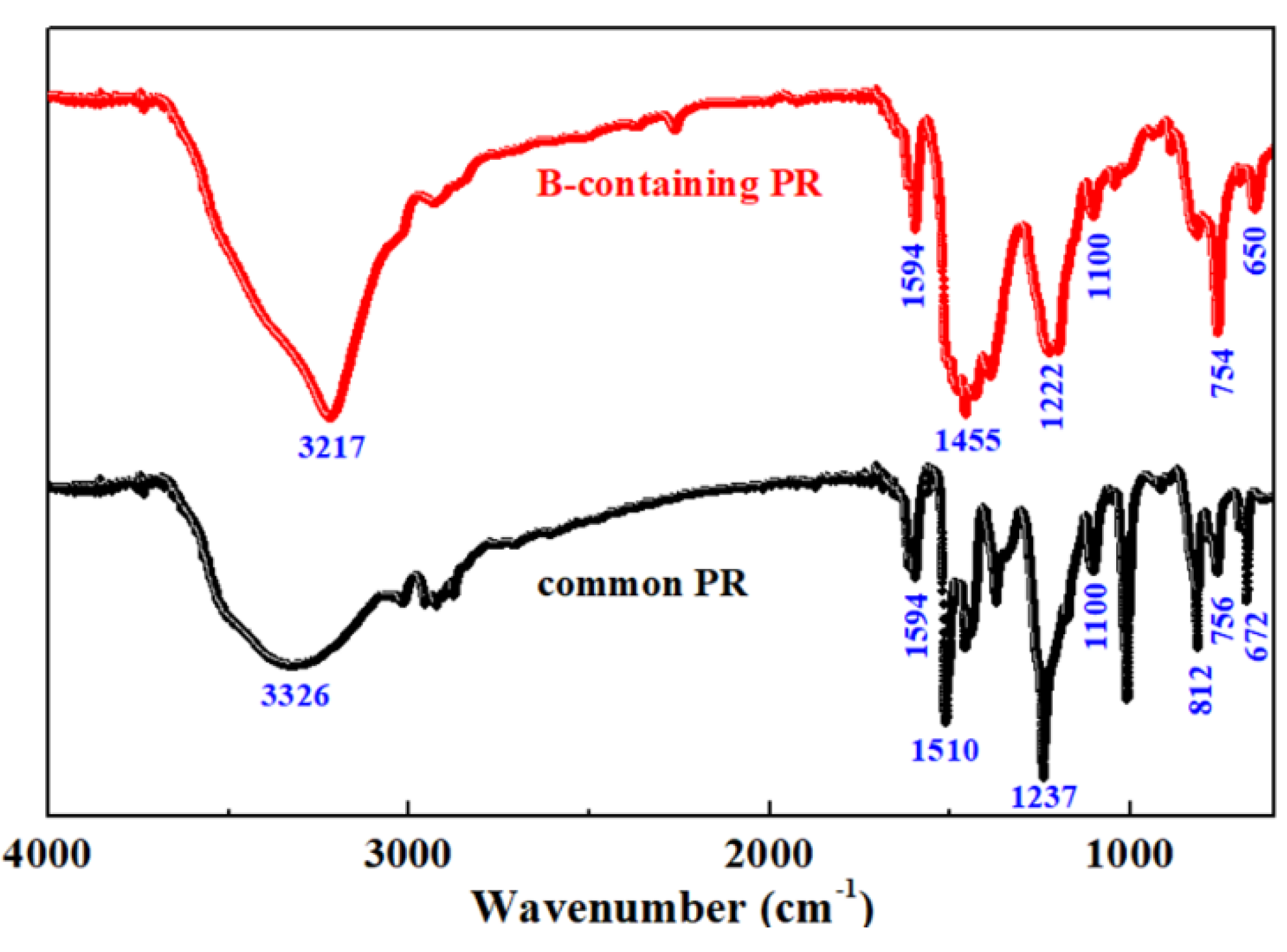
3.4. Structure of Common PR and B-Containing PR
3.5. Surface Tensions of Common PR and B-Containing PR Solutions
3.6. Wettability Between B-Containing PR Solution and Carbon Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natali, M.; Kenny, J.M.; Torre, L. Science and technology of polymeric ablative materials for thermal protection systems and propulsion devices: A review. Prog. Mater. Sci. 2016, 84, 192–275. [Google Scholar] [CrossRef]
- Harpale, A.; Sawant, S.; Kumar, R.; Levin, D.; Chew, H.B. Ablative thermal protection systems: Pyrolysis modeling by scale-bridging molecular dynamics. Carbon 2018, 130, 315–324. [Google Scholar] [CrossRef]
- Cheon, J.H.; Shin, E.S. Assessment of the ablation characteristics of carbon/phenolic composites using X-ray microtomography. Compos. Sci. Technol. 2019, 182, 107740. [Google Scholar] [CrossRef]
- Gong, C.L.; Gou, J.J.; Hu, J.X.; Gao, F. A novel TE-material based thermal protection structure and its performance evaluation for hypersonic flight vehicles. Aerosp. Sci. Technol. 2018, 77, 458–470. [Google Scholar] [CrossRef]
- Le, V.T.; Goo, N.S.; Kim, J.Y. Thermomechanical behavior of superalloy thermal protection system under aerodynamic heating. J. Spacecr. Rocket. 2019, 56, 1432–1448. [Google Scholar] [CrossRef]
- De Cesare, M.; Savino, L.; Ceglia, G.; Alfano, D.; Di Carolo, F.; French, A.D.; D’Onofrio, A. Applied radiation physics techniques for diagnostic evaluation of the plasma wind and thermal protection system critical parameters in aerospace re-entry. Prog. Aerosp. Sci. 2019, 112, 100550. [Google Scholar] [CrossRef]
- Bessire, B.K.; Lahankar, S.A.; Minton, T.K. Pyrolysis of phenolic impregnated carbon ablator (PICA). ACS Appl. Mater. Int. 2014, 7, 1383–1395. [Google Scholar] [CrossRef]
- Riccio, A.; Raimondo, F.; Sellitto, A.; Carandente, V.; Scigliano, R.; Tescione, D. Optimum design of ablative thermal protection systems for atmospheric entry vehicles. Appl. Therm. Eng. 2017, 119, 541–552. [Google Scholar] [CrossRef]
- Torres-Herrador, F.; Meurisse, J.B.; Panerai, F.; Blondeau, J.; Lachaud, J.; Bessire, B.K.; Mansour, N.N. A high heating rate pyrolysis model for the Phenolic Impregnated Carbon Ablator (PICA) based on mass spectroscopy experiments. J. Anal. Appl. Pyrol. 2019, 141, 104625. [Google Scholar] [CrossRef]
- Rivier, M.; Lachaud, J.; Congedo, P.M. Ablative thermal protection system under uncertainties including pyrolysis gas composition. Aerosp. Sci. Technol. 2019, 84, 1059–1069. [Google Scholar] [CrossRef]
- Bessire, B.K.; Minton, T.K. Decomposition of phenolic impregnated carbon ablator (PICA) as a function of temperature and heating rate. ACS Appl. Mater. Inter. 2017, 9, 21422–21437. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Huang, H.; Xu, X. Optimization of the curing process of phenolic impregnated carbon ablator. J. Appl. Polym. Sci. 2018, 135, 46230. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Chen, L.; Qin, Y.; Zhang, X.; Gu, J.; Guo, Z. Enhanced thermal stabilities and char yields of carbon fibers reinforced boron containing novolac phenolic resins composites. J. Polym. Res. 2017, 24, 176. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Yang, L. Thermal stability of boron-containing phenol formaldehyde resin. Polym. Degrad. Stabil. 1999, 63, 19–22. [Google Scholar] [CrossRef]
- Kawamoto, A.M.; Pardini, L.C.; Diniz, M.F.; Lourenço, V.L.; Takahashi, M.F.K. Synthesis of a boron modified phenolic resin. J. Aerosp. Technol. Manag. 2010, 2, 169–182. [Google Scholar] [CrossRef]
- Wang, S.; Jing, X.; Wang, Y.; Si, J. Synthesis and characterization of novel phenolic resins containing aryl-boron backbone and their utilization in polymeric composites with improved thermal and mechanical properties. Polym. Advan. Technol. 2014, 25, 152–159. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Ferrari, F.; Striani, R.; Visconti, P.; Greco, A. An innovative green process for the stabilization and valorization of organic fraction of municipal solid waste (OFMSW): Optimization of the curing process II part. Appl. Sci. 2019, 9, 3702. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Ferrari, F.; Striani, R.; Minosi, S.; Pollini, M.; Paladini, F.; Greco, A. An Innovative Green Process for the Stabilization and Valorization of the Organic Fraction of Municipal Solid Waste. Appl. Sci. 2019, 9, 4516. [Google Scholar] [CrossRef]
- Zhang, W.F.; Liu, C.L.; Ying, Y.G.; Dong, W.S. The preparation and characterization of boron-containing phenolic fibers. Mater. Chem. Phys. 2010, 121, 89–94. [Google Scholar] [CrossRef]
- Gu, A.; Liang, G.; Lan, L. Modification of polyaralkyl–phenolic resin and its copolymer with bismaleimide. J. Appl. Polym. Sci. 1996, 59, 975–979. [Google Scholar] [CrossRef]
- Espinosa, M.A.; Cádiz, V.; Galia, M. Synthesis and characterization of benzoxazine-based phenolic resins: Crosslinking study. J. Appl. Polym. Sci. 2003, 90, 470–481. [Google Scholar] [CrossRef]
- Wang, Y.G.; Shi, T.J.; Li, Z. Synthesis and characterization of phenolic resin modified with zirconium. Chem. Propellants Polym. Mater. 2009, 4, 37–39. [Google Scholar]
- Ding, J.; Huang, Z.; Luo, H.; Qin, Y.; Shi, M. The role of microcrystalline muscovite to enhance thermal stability of boron-modified phenolic resin, structural and elemental studies in boron-modified phenolic resin/microcrystalline muscovite composite. Mater. Res. Innov. 2015, 19, S8–S605. [Google Scholar] [CrossRef]
- Gao, G.Y. Study on boron phenol-formaldehyde resin synthesized by one-step method and sodium borate or potassium borate as catalyst. China Adhes. 2016, 25, 6–9. [Google Scholar]
- Wang, D.C.; Chang, G.W.; Chen, Y. Preparation and thermal stability of boron-containing phenolic resin/clay nanocomposites. Polym. Degrad. Stabil. 2008, 93, 125–133. [Google Scholar] [CrossRef]
- Strelko, V.V.; Kuts, V.S.; Thrower, P.A. On the mechanism of possible influence of heteroatoms of nitrogen, boron and phosphorus in a carbon matrix on the catalytic activity of carbons in electron transfer reactions. Carbon 2000, 38, 1499–1503. [Google Scholar] [CrossRef]
- Wang, S.; Jing, X.; Wang, Y.; Si, J. High char yield of aryl boron-containing phenolic resins: The effect of phenylboronic acid on the thermal stability and carbonization of phenolic resins. Polym. Degrad. Stabil. 2014, 99, 1–11. [Google Scholar] [CrossRef]
- Yun, J.; Chen, L.; Zhao, H.; Zhang, X.; Ye, W.; Zhu, D. Boric Acid as a coupling agent for preparation of phenolic resin containing boron and silicon with enhanced char yield. Macromol. Rapid Commun. 2019, 40, 1800702. [Google Scholar] [CrossRef]
- Martin, C.; Ronda, J.C.; Cádiz, V. Novel flame-retardant thermosets: Diglycidyl ether of bisphenol A as a curing agent of boron-containing phenolic resins. J. Polym. Sci. Pol. Chem. 2016, 44, 1701–1710. [Google Scholar] [CrossRef]
- Lin, C.T.; Lee, H.T.; Chen, J.K. Preparation and properties of bisphenol-F based boron-phenolic resin/modified silicon nitride composites and their usage as binders for grinding wheels. Appl. Surf. Sci. 2015, 330, 1–9. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Gu, J.; He, Z.; Yun, J.; Wang, X. Synthesis and characterization of aryl boron-containing thermoplastic phenolic resin with high thermal decomposition temperature and char yield. J. Polym. Res. 2016, 23, 97. [Google Scholar] [CrossRef]
- Park, J.M.; Wang, Z.J.; Kwon, D.J.; Gu, G.Y.; Lee, W.I.; Park, J.K.; DeVries, K.L. Interfacial properties and self-sensing of single carbon fiber reinforced CNT-phenolic nanocomposites using electro-micromechanical and wettability tests. Compos. Part B Eng. 2012, 43, 1171–1177. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, D.O.; Lee, J.H.; Lee, P.C.; Lee, M.H.; Lee, Y.; Nam, J.D. Solvent-assisted graphite loading for highly conductive phenolic resin bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2010, 195, 3794–3801. [Google Scholar] [CrossRef]
- Wang, B.C.; Huang, Y.D.; Liu, L. Effects of fibre surface silanisation on silica fibre/phenolics composites produced by resin transfer moulding process. Mater. Sci. Technol. 2006, 22, 206–212. [Google Scholar] [CrossRef]
- Durkić, T.; Perić, A.; Laušević, M.; Dekanski, A.; Nešković, O.; Veljković, M.; Laušević, Z. Boron and phosphorus doped glassy carbon: I. Surface properties. Carbon 1997, 35, 1567–1572. [Google Scholar] [CrossRef]
- Tan, X.M.; Huang, N.Y.; Shang, Y.H.; Li, Y. Complexation reaction between boric acid and resole phenol-formaldehyde resin and the structure of the complexes. Chem. Res. Appl. 2002, 14, 145–148. [Google Scholar]


| Sample | Temperature (°C) | Concentration (g/ML) | Surface Tension (mN/m) |
|---|---|---|---|
| Common PR | 25 | 0.40 | 32.10 |
| 25 | 0.80 | 29.45 | |
| 35 | 0.40 | 29.23 | |
| 35 | 0.80 | 26.23 | |
| 45 | 0.40 | 25.06 | |
| 45 | 0.80 | 23.12 | |
| B-containing PR | 25 | 0.40 | 28.45 |
| 25 | 0.80 | 25.62 | |
| 35 | 0.40 | 26.02 | |
| 35 | 0.80 | 23.17 | |
| 45 | 0.40 | 22.71 | |
| 45 | 0.80 | 20.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Sun, Y. Strong Effect of Process Parameters on the Properties of Boron-Containing Phenolic Resins with High Char Yield. Appl. Sci. 2020, 10, 1408. https://doi.org/10.3390/app10041408
Sun Y, Sun Y. Strong Effect of Process Parameters on the Properties of Boron-Containing Phenolic Resins with High Char Yield. Applied Sciences. 2020; 10(4):1408. https://doi.org/10.3390/app10041408
Chicago/Turabian StyleSun, Yu, and Yuguo Sun. 2020. "Strong Effect of Process Parameters on the Properties of Boron-Containing Phenolic Resins with High Char Yield" Applied Sciences 10, no. 4: 1408. https://doi.org/10.3390/app10041408
APA StyleSun, Y., & Sun, Y. (2020). Strong Effect of Process Parameters on the Properties of Boron-Containing Phenolic Resins with High Char Yield. Applied Sciences, 10(4), 1408. https://doi.org/10.3390/app10041408




