Prediction System for Prostate Cancer Recurrence Using Machine Learning
Abstract
1. Introduction
2. Materials and Methods
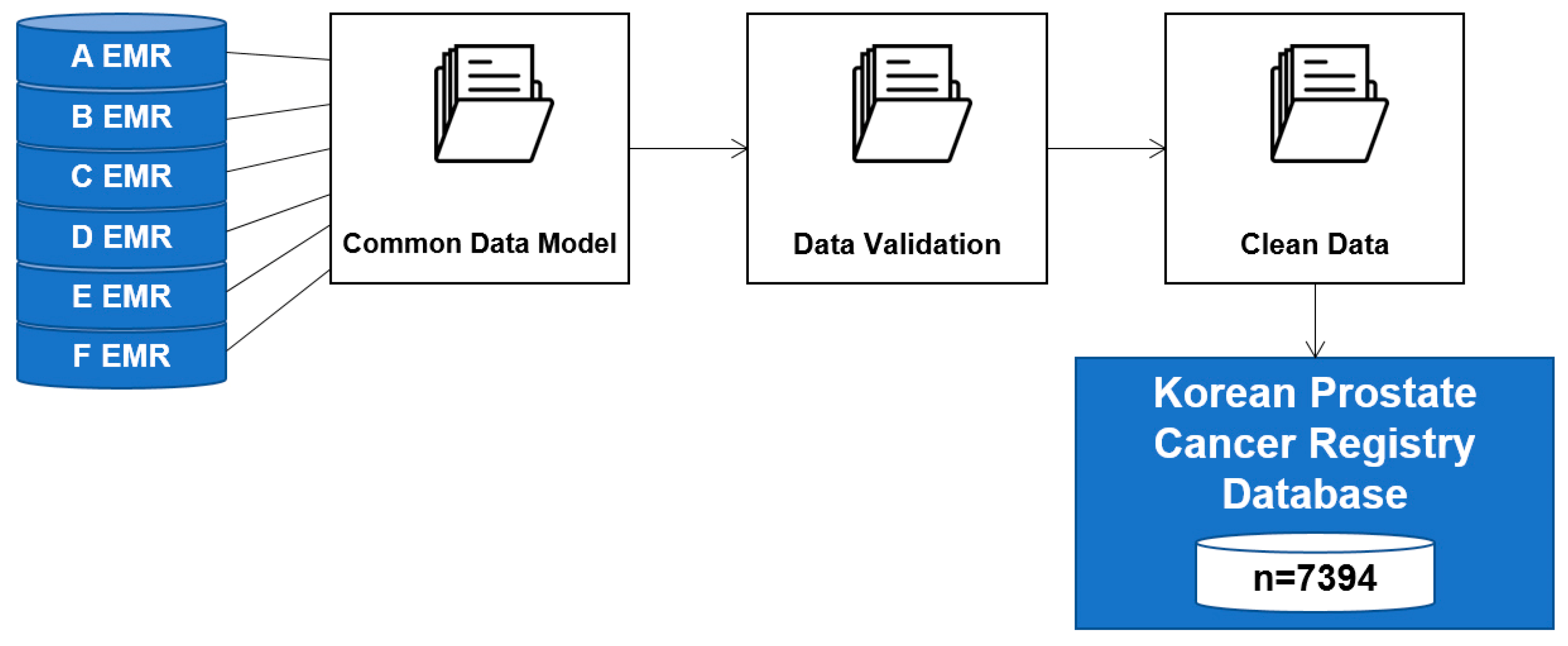
2.1. BCR Prediction Data
- There were 7394 potentially relevant records in the KPCR database
- 2280 records, including those of non-Koreans (51), those who did not have a full follow-up period of at least one year (633), those who did not have a neoclassical prostatectomy (80), those who performed pre-supplementary therapy (230), and those for whom critical data were missing (1286) were excluded
- We identified 5114 individual records as being statistically usable and relevant
- We classified the 5114 patient data items into BCR (1207) and Non-BCR (3907) groups to identify the characteristics of each group.
2.2. BCR Prediction Statistical Analysis
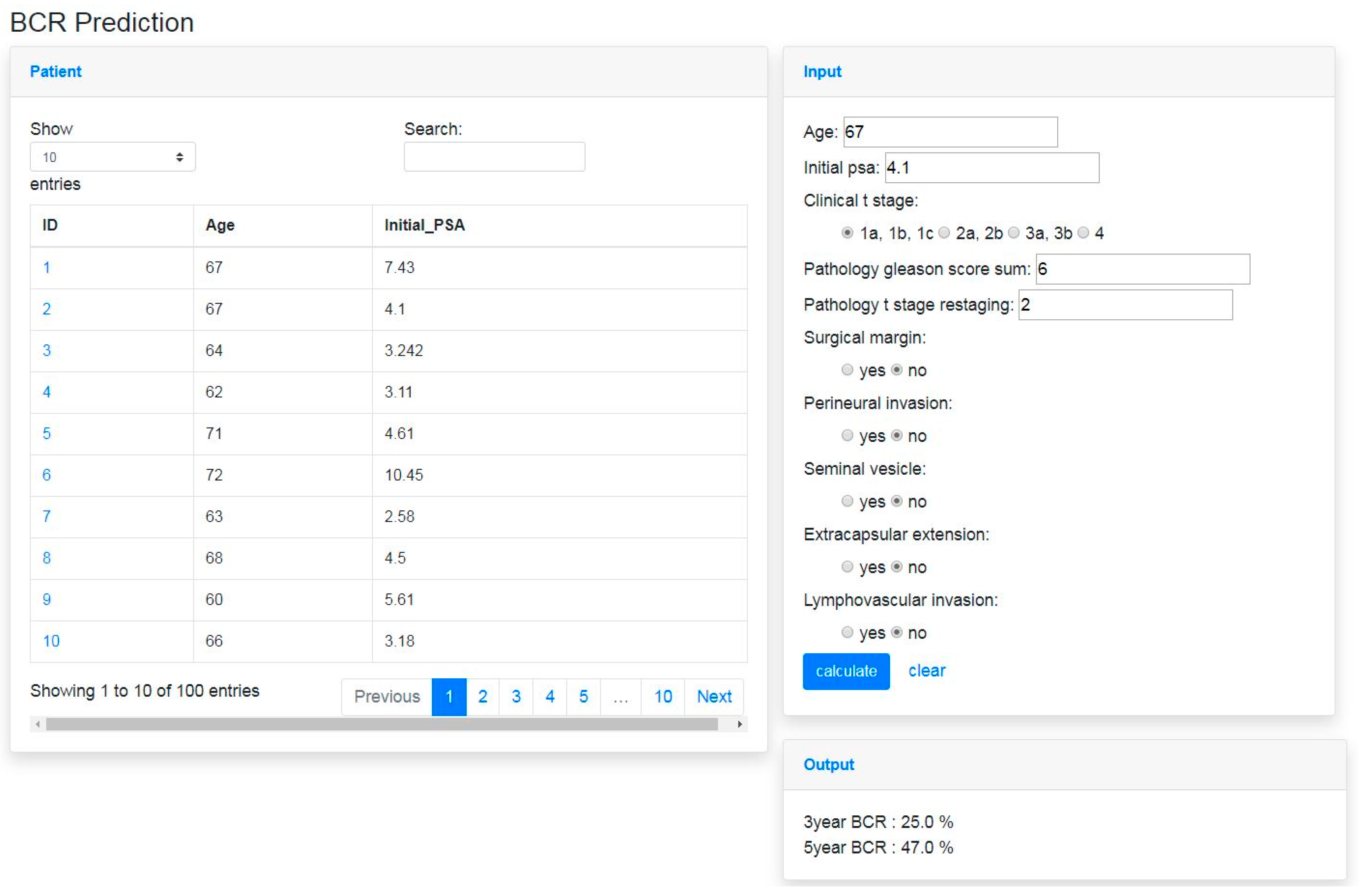
2.3. BCR Prediction System
3. Results
3.1. Patient Characteristics and Distribution
3.2. BCR Prediction Model Analysis
3.3. Development of BCR Prediction System
4. Discussion
4.1. Follow-Up Period for Predicting BCR
4.2. Prostate Cancer Patients Data and Data Provider Characteristics
4.3. The Potential of Misdiagnosis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Cancer Information Center. 2017 Cancer Statistics; Cancer Incidence Rates. Available online: https://www.cancer.go.kr/lay1/S1T639C641/contents.do (accessed on 6 January 2020).
- Lee, J.W.; Cho, K.S.; Han, K.S.; Kim, E.K.; Joung, J.Y.; Seo, H.K.; Chung, J.; Park, W.S.; Lee, K.H. Epidermal growth factor receptor as predicting factor on biochemical recurrence after radical prostatectomy: A prospective study. Korean J. Urol. 2008, 49, 974–980. [Google Scholar] [CrossRef]
- Song, S.H.; Kwak, C. Definition of BCR and further evaluation. Korean J. Urol. Oncol. 2011, 9, 104–111. [Google Scholar]
- Cox, J.D.; Grignon, D.J.; Kaplan, R.S.; Parsons, J.T.; Schellhammer, P.F. Consensus statement: Guidelines for PSA following radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 1035–1041. [Google Scholar]
- Lange, P.H.; Ercole, C.J.; Lightner, D.J.; Fraley, E.E.; Vessella, R. The value of serum prostate specific antigen determinations before and after radical prostatectomy. J. Urol. 1989, 141, 873–879. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, H.M.; Choi, H.Y. The predictors of biochemical recurrence and metastasis following radical perineal prostatectomy in clinically localized prostate cancer. Korean J. Urol. 2005, 46, 1161. [Google Scholar]
- Sa, S. Intelligent heart disease prediction system using data mining techniques. Int. J. Healthc. Biomed. Res. 2013, 1, 94–101. [Google Scholar]
- Paydar, K.; Kalhori, S.R.N.; Akbarian, M.; Sheikhtaheri, A. A clinical decision support system for prediction of pregnancy outcome in pregnant women with systemic lupus erythematosus. Int. J. Med Inform. 2017, 97, 239–246. [Google Scholar] [CrossRef]
- Berner, E.S. (Ed.) Clinical Decision Support Systems; Springer Science + Business Media, LLC: New York, NY, USA, 2007. [Google Scholar]
- Foster, D.; McGregor, C.; El-Masri, S. A survey of agent-based intelligent decision support systems to support clinical management and research. In Proceedings of the Second International Workshop on Multi-Agent Systems for Medicine, Computational Biology, and Bioinformatics, Utrecht, The Netherlands, 25 July 2005; pp. 16–34. [Google Scholar]
- Memorial Sloan Kettering Cancer Center. Prostate Cancer Nomograms. Available online: https://www.mskcc.org/nomograms/prostate (accessed on 6 January 2020).
- National Cancer Information Center. 2017 Cancer Statistics; Global Cancer Incidence. Available online: https://www.cancer.go.kr/lay1/S1T639C644/contents.do (accessed on 6 January 2020).
- Hoffman, R.M.; Gilliland, F.D.; Eley, J.W.; Harlan, L.C.; Stephenson, R.A.; Stanford, J.L.; Albertson, P.C.; Hamilton, A.S.; Hunt, W.C.; Potosky, A.L. Racial and ethnic differences in advanced-stage prostate cancer: The prostate cancer outcomes study. J. Natl. Cancer Inst. 2001, 93, 388–395. [Google Scholar] [CrossRef]
- Hankey, B.F.; Feuer, E.J.; Clegg, L.X.; Hayes, R.B.; Legler, J.M.; Prorok, P.C.; Ries, L.A.; Merrill, R.M.; Kaplan, R.S. Cancer surveillance series: Interpreting trends in prostate cancer—Part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J. Natl. Cancer Inst. 1999, 91, 1017–1024. [Google Scholar] [CrossRef]
- Stanford, J.L.; Stephenson, R.A.; Coyle, L.M.; Cerhan, J.; Correa, R.; Eley, J.W.; Gilliland, F.; Hankey, B.; Kolonel, L.N.; Ross, R. Prostate Cancer Trends 1973–1995; NIH Pub. No. 99-4543; Surveillance Research Program, National Cancer Institute: Bethesda, MD, USA, 1999.
- Ahn, K.I.; Chang, S.G.; Choi, B.K.; Jeon, S.H. The study of biochemical and clinical failure after radical prostatectomy or radiation therapy in localized prostate cancer. Korean J. Urol. Oncol. 2004, 2, 171–177. [Google Scholar]
- De la TaIlle, A.; Rubin, A.A.; Bagiella, E.; Olsson, C.A.; Buttyan, R.; Burchardt, T.; Knight, C.; O’Toole, K.M.; Katz, A.E. Can perineural invasion on prostate needle biopsy predict prostate specific antigen recurrence after radical prostatectomy? J. Urol. 1999, 162, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Blute, M.L.; Bergstralh, E.J.; Iocca, A.; Scherer, B.; Zincke, H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J. Urol. 2001, 165, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Partin, A.W.; Zahurak, M.; Piantadosi, S.; Epstein, J.I.; Walsh, P.C. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J. Urol. 2003, 169, 517–523. [Google Scholar] [CrossRef]
- Pal, M.; Mather, P.M. An assessment of the effectiveness of decision tree methods for land cover classification. Remote Sens. Environ. 2003, 86, 554–565. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Acion, L.; Kelmansky, D.; van der Laan, M.; Sahker, E.; Jones, D.; Arndt, S. Use of a machine learning framework to predict substance use disorder treatment success. PLoS ONE 2017, 12, e0175383. [Google Scholar] [CrossRef]
- Van Houwelingen, H.; Putter, H. Dynamic Prediction in Clinical Survival Analysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Saunders, C.; Gammerman, A.; Vovk, V. Ridge regression learning algorithm in dual variables. In Proceedings of the Fifteenth International Conference on Machine Learning (ICML 1998), Madison, WI, USA, 24–27 July 1998. [Google Scholar]
- Meier, L.; Van De Geer, S.; Bühlmann, P. The group lasso for logistic regression. J. R. Stat. Soc. Ser. B Stat. Methodol. 2008, 70, 53–71. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Ran, R.; Che, Y.; Zhou, Y. A short-term photovoltaic power prediction model based on the gradient boost decision tree. Appl. Sci. 2018, 8, 689. [Google Scholar] [CrossRef]
- Friedman, J.H. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Krauss, C.; Do, X.A.; Huck, N. Deep neural networks, gradient-boosted trees, random forests: Statistical arbitrage on the S&P 500. Eur. J. Oper. Res. 2017, 259, 689–702. [Google Scholar]
- Ohori, M.; Wheeler, T.M.; Scardino, P.T. The new American joint committee on cancer and international union against cancer TNM classification of prostate cancer. Cancer 1994, 74, 104–114. [Google Scholar] [CrossRef]


| BCR (1207 Patients) | Non-BCR (3907 Patients) | ||
|---|---|---|---|
| Age | Average | 65 | 65 |
| Initial PSA (ng/mL) | Average | 17.58 | 9.74 |
| Gleason score (sum) | 2–4 | 0 | 5 |
| 5 | 3 | 19 | |
| 6 | 111 | 994 | |
| 7 | 719 | 2576 | |
| 8–10 | 374 | 313 | |
| Clinical T stage | T1 | 399 | 1753 |
| T2 | 467 | 1416 | |
| T3 | 276 | 677 | |
| T4 | 65 | 61 |
| Analysis | Method | AUC | Accuracy | Sensitivity | Specificity | MCC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-year | 5-year | 3-year | 5-year | 3-year | 5-year | 3-year | 5-year | 3-year | 5-year | ||
| Trees | Decision Tree | 0.8186 | 0.7561 | 0.7817 | 0.7033 | 0.4135 | 0.7009 | 0.9447 | 0.7062 | 0.4448 | 0.4055 |
| Random Forest (ntrees = 20) 1 | 0.8320 | 0.7956 | 0.7817 | 0.7126 | 0.4135 | 0.7308 | 0.9447 | 0.6907 | 0.4448 | 0.4210 | |
| Random Forest (ntrees = 50) | 0.8349 | 0.8047 | 0.7832 | 0.7266 | 0.4279 | 0.7393 | 0.9404 | 0.7113 | 0.4495 | 0.4310 | |
| Random Forest (ntrees = 80) | 0.8362 | 0.8050 | 0.7876 | 0.7196 | 0.4327 | 0.7308 | 0.9447 | 0.7062 | 0.4621 | 0.4360 | |
| Neural Networks | 1hidden, dropout = 0.3 at input | 0.7939 | 0.7895 | 0.7611 | 0.7056 | 0.3606 | 0.7821 | 0.9383 | 0.6134 | 0.4385 | 0.4001 |
| 1hidden dropout = 0.1 at input | 0.8027 | 0.7977 | 0.7699 | 0.6916 | 0.5144 | 0.7393 | 0.8830 | 0.6340 | 0.4089 | −0.0367 | |
| 2hidden, dropout = 0.3 at input | 0.7978 | 0.7941 | 0.6932 | 0.7056 | 0.0000 | 0.7051 | 1.0000 | 0.7062 | 0.3393 | 0.3986 | |
| 2hidden dropout = 0.1 at input | 0.7988 | 0.7967 | 0.7611 | 0.7056 | 0.3702 | 0.8162 | 0.9340 | 0.5722 | 0.3963 | 0.4128 | |
| Survival Regression | Cox PH | 0.7944 | 0.7816 | 0.8025 | 0.7830 | 0.2160 | 0.3333 | 0.9568 | 0.9300 | 0.2894 | 0.3159 |
| Logistic Regression | Ridged Regression (L2) | 0.8288 | 0.8071 | 0.7802 | 0.7290 | 0.4279 | 0.7265 | 0.9362 | 0.7320 | 0.4413 | 0.4210 |
| Lasso (L1) | 0.8319 | 0.7993 | 0.7861 | 0.7243 | 0.4519 | 0.7179 | 0.9340 | 0.7320 | 0.4590 | 0.4481 | |
| GBC | 0.8419 | 0.8031 | 0.7891 | 0.7407 | 0.4567 | 0.7436 | 0.9362 | 0.7371 | 0.4348 | 0.4836 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Yu, S.H.; Kim, Y.; Kim, J.K.; Hong, J.H.; Kim, C.-S.; Seo, S.I.; Byun, S.-S.; Jeong, C.W.; Lee, J.Y.; et al. Prediction System for Prostate Cancer Recurrence Using Machine Learning. Appl. Sci. 2020, 10, 1333. https://doi.org/10.3390/app10041333
Lee SJ, Yu SH, Kim Y, Kim JK, Hong JH, Kim C-S, Seo SI, Byun S-S, Jeong CW, Lee JY, et al. Prediction System for Prostate Cancer Recurrence Using Machine Learning. Applied Sciences. 2020; 10(4):1333. https://doi.org/10.3390/app10041333
Chicago/Turabian StyleLee, Sun Jung, Sung Hye Yu, Yejin Kim, Jae Kwon Kim, Jun Hyuk Hong, Choung-Soo Kim, Seong Il Seo, Seok-Soo Byun, Chang Wook Jeong, Ji Youl Lee, and et al. 2020. "Prediction System for Prostate Cancer Recurrence Using Machine Learning" Applied Sciences 10, no. 4: 1333. https://doi.org/10.3390/app10041333
APA StyleLee, S. J., Yu, S. H., Kim, Y., Kim, J. K., Hong, J. H., Kim, C.-S., Seo, S. I., Byun, S.-S., Jeong, C. W., Lee, J. Y., & Choi, I. Y. (2020). Prediction System for Prostate Cancer Recurrence Using Machine Learning. Applied Sciences, 10(4), 1333. https://doi.org/10.3390/app10041333





