Fabrication of Natural Flake Graphite/Ceramic Composite Parts with Low Thermal Conductivity and High Strength by Selective Laser Sintering
Abstract
1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Fabrication Process
2.3. Experiments
2.4. Characterization
3. Results and Discussion
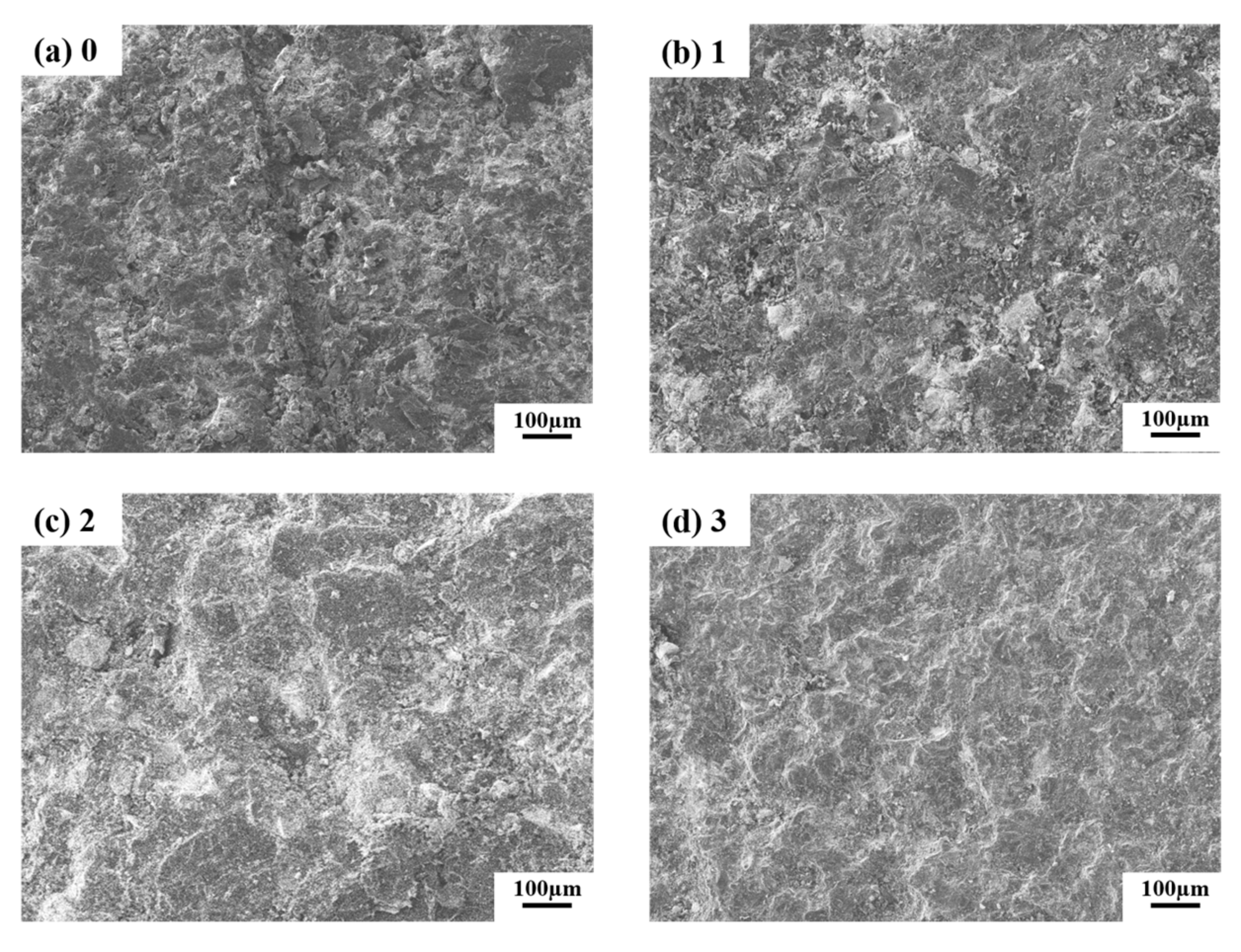
3.1. Effect of Mixture Powder Composition on Properties
3.1.1. Silicon Powder
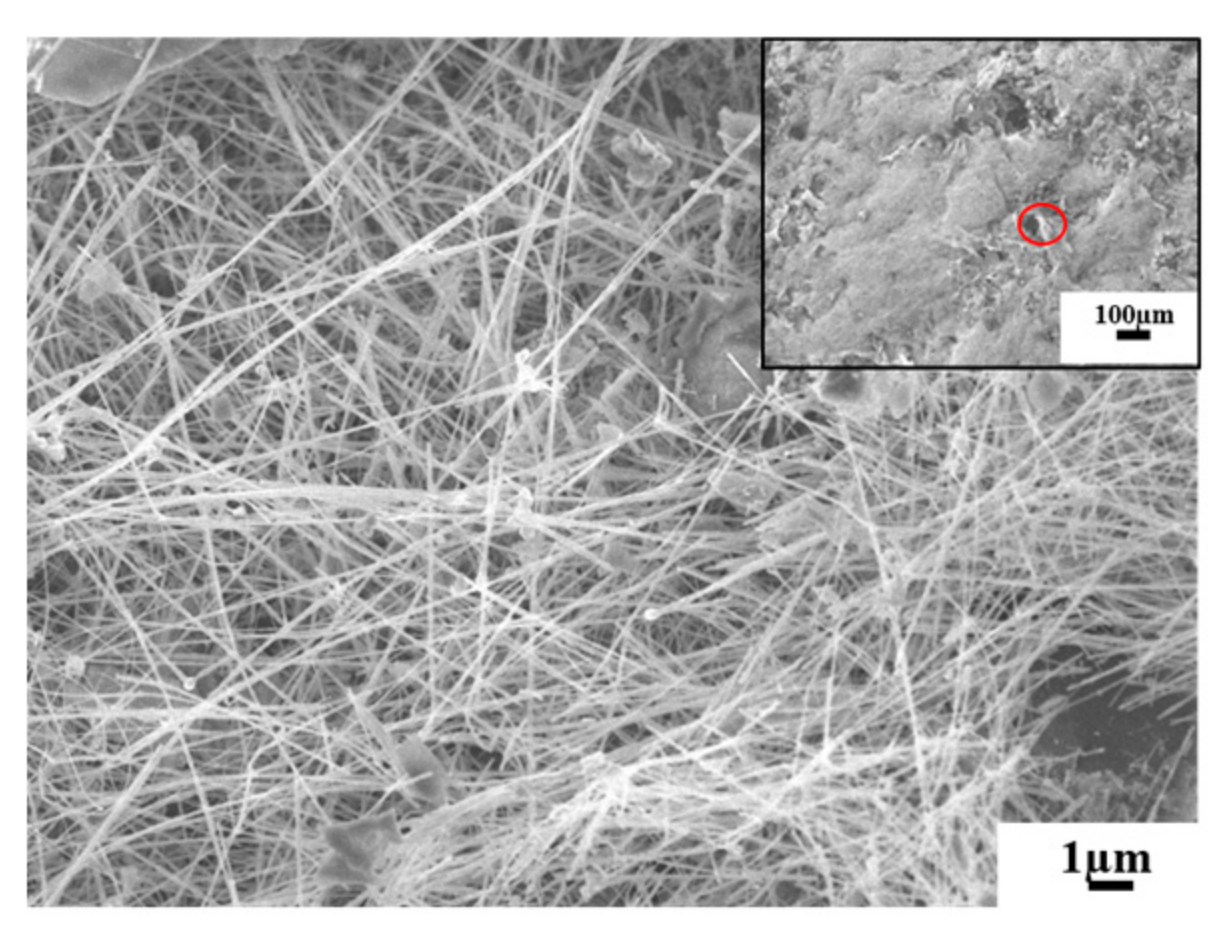
3.1.2. Expanded Graphite
3.2. Effect of Post-Processing on Properties
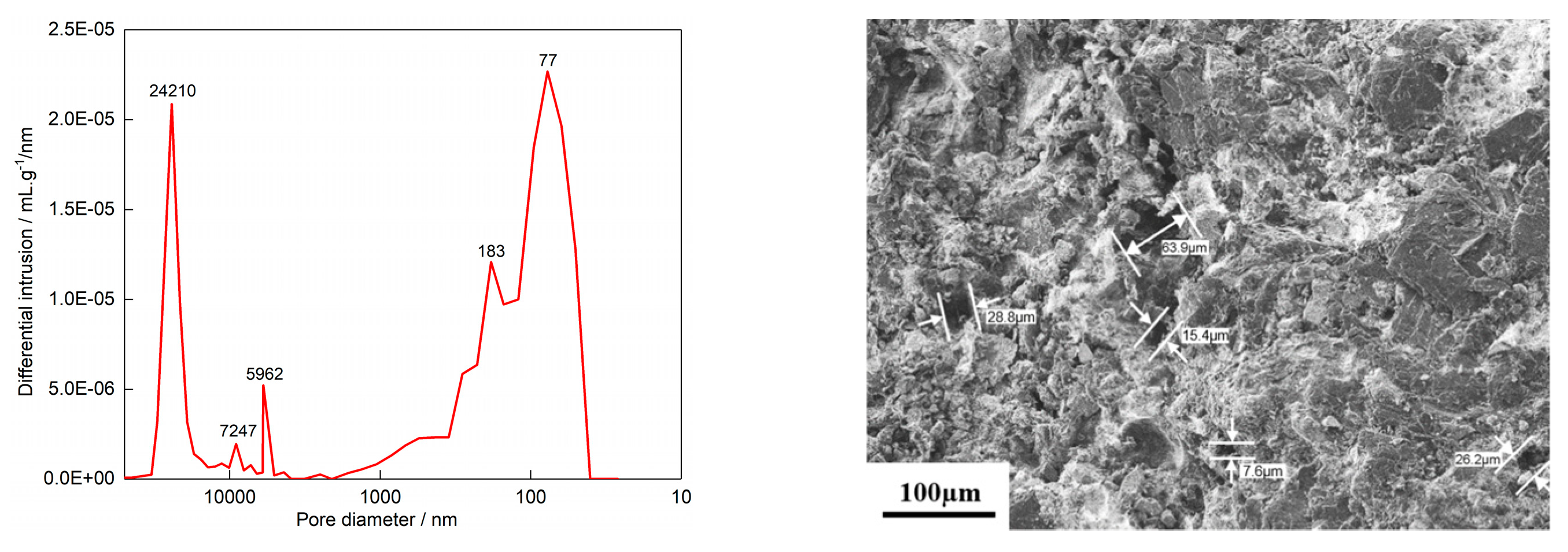
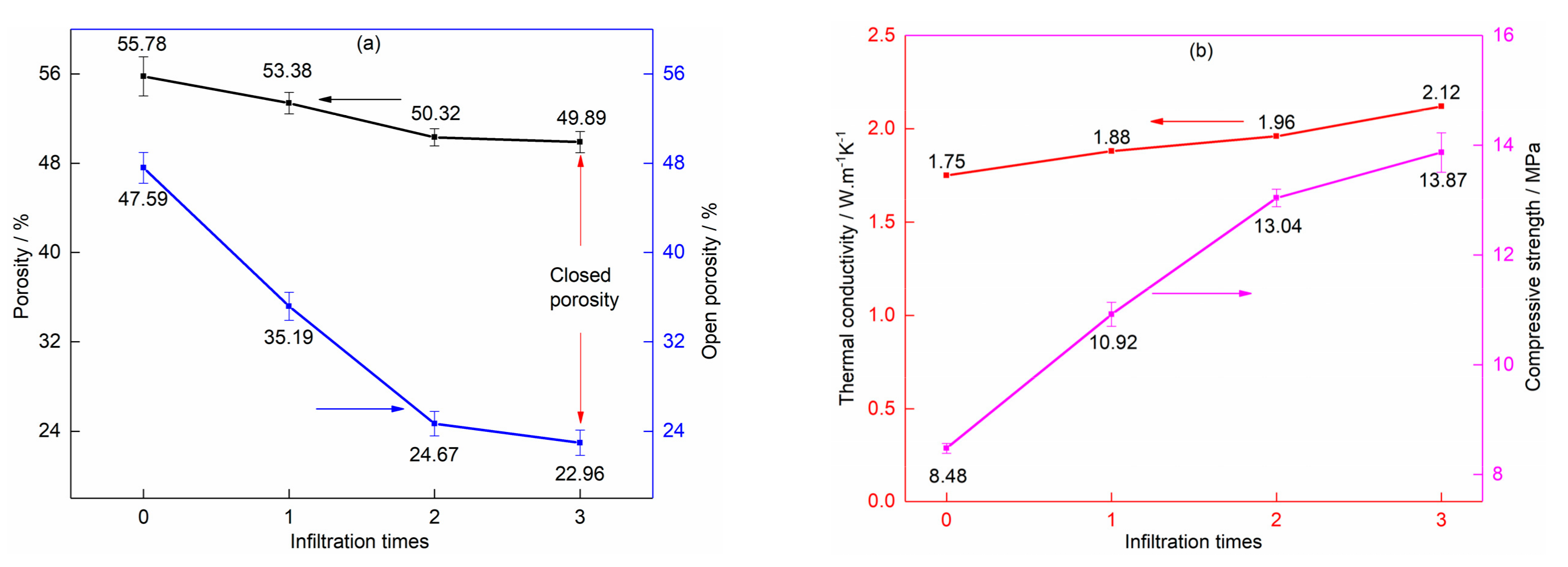
3.2.1. Vacuum Pressure Impregnation of Phenolic Resin Solution and Carbonization
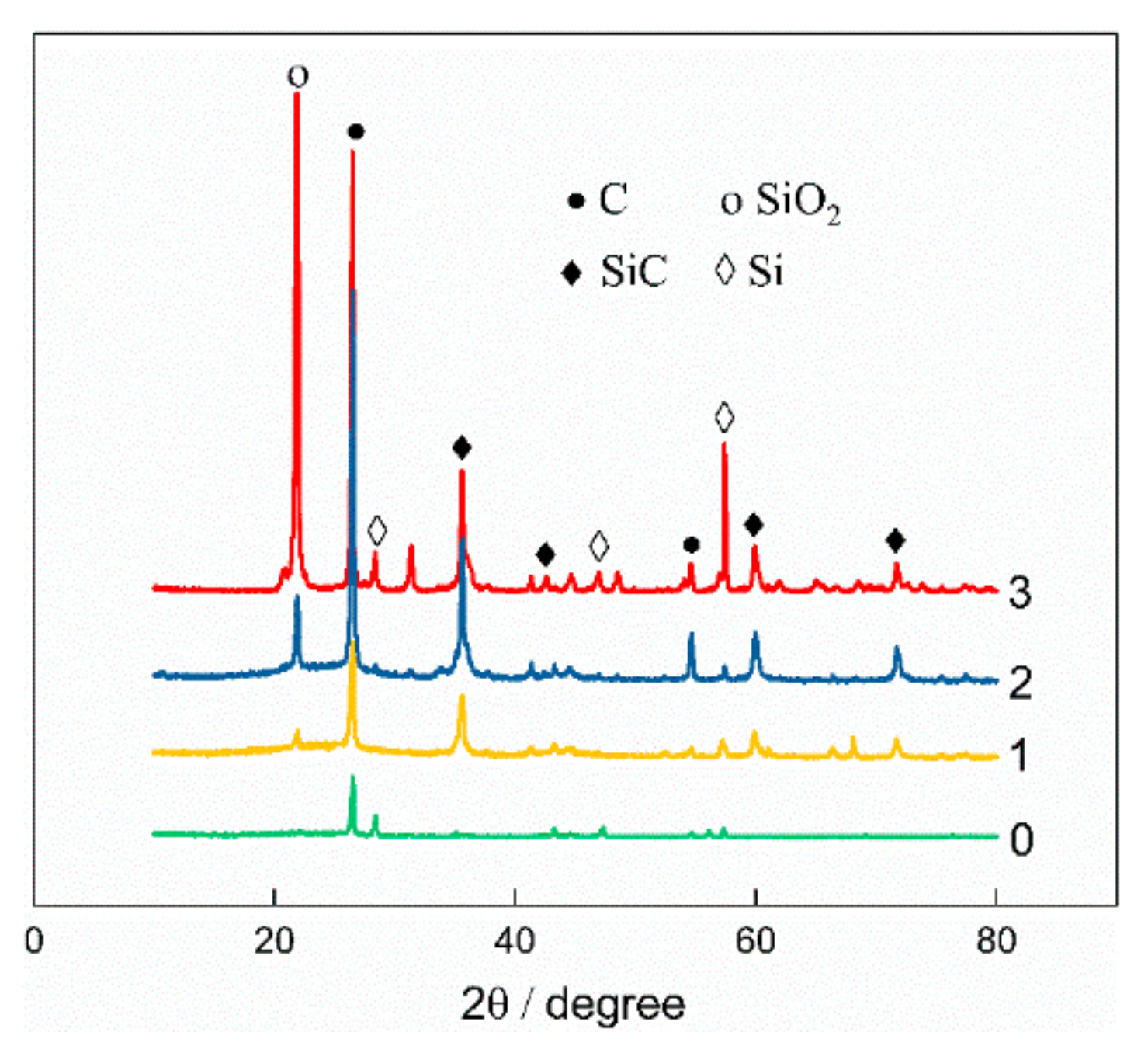
3.2.2. Vacuum Pressure Infiltration of Silica Sol Solution and High-Temperature Sintering
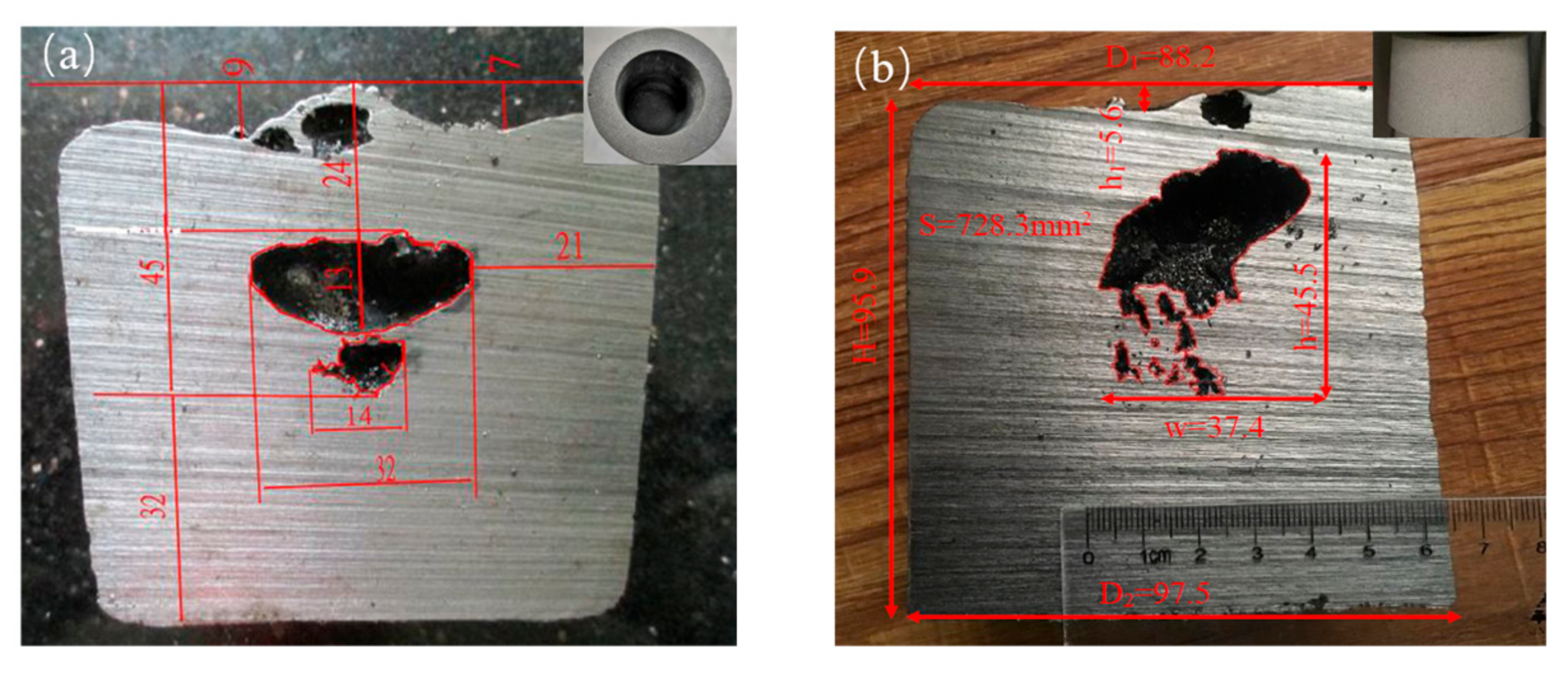
4. Application Examples
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.; Han, X.H.; Sommers, A.; Park, Y.; T’ Joen, C.; Jacobi, A. A review on application of carbonaceous materials and carbon matrix composites for heat exchangers and heat sinks. Int. J. Refrig. 2015, 35, 7–26. [Google Scholar] [CrossRef]
- Jian-Feng, H.; Xie-Rong, Z.; He-Jun, L.; Xin-Bo, X.; Ye-wei, F. Influence of the preparation temperature on the phase, microstructure and anti-oxidation property of a SiC coating for C/C composites. Carbon 2004, 42, 1517–1521. [Google Scholar] [CrossRef]
- Toyoda, M.; Inagaki, M. Heavy oil sorption using exfoliated graphite new application of exfoliated graphite to protect heavy oil pollution. Carbon 2000, 38, 199–210. [Google Scholar] [CrossRef]
- Findlay, J.R.; Laing, T.F. The diffusion of fission products from graphite. J. Nucl. Mater. 1962, 7, 182–191. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; Ershov, A.A. Cracking on the Compaction of a Model Composition Based on Natural Flake Graphite and Binder Pitch. Solid Fuel Chem. 2018, 52, 260–268. [Google Scholar] [CrossRef]
- Mathur, R.B.; Dhakate, S.R.; Gupta, D.K.; Dhami, T.L.; Aggarwal, R.K. Effect of different carbon fillers on the properties of graphite composite bipolar plate. J. Mater. Process. Technol. 2008, 203, 184–192. [Google Scholar] [CrossRef]
- Yang, X.; Yang, W.; Fan, J.; Wu, J.; Zhang, K. Effects of molding on property of thermally conductive and electrically insulating polyamide 6–based composite. J. Thermoplast. Compos. Mater. 2019, 32, 1190–1203. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Wang, H.; Shi, J.; Zhang, J.; Tao, Z.; Guo, Q.; Liu, L. Microstructure and thermal/mechanical properties of short carbon fiber-reinforced natural graphite flake composites with mesophase pitch as the binder. Carbon 2013, 53, 313–320. [Google Scholar] [CrossRef]
- Magampa, P.P.; Manyala, N.; Focke, W.W. Properties of graphite composites based on natural and synthetic graphite powder and a phenolic novolac binder. J. Nucl. Mater. 2013, 436, 76–83. [Google Scholar] [CrossRef]
- Chen, S. Fabrication of PEM Fuel Cell Bipolar Plate by Indirect Selective Laser Sintering. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2006. [Google Scholar]
- Wu, H.; Sun, Y.; Peng, J.; Huang, C.; Ye, X. Effect of processing parameters on dimensional accuracy and bending strength of graphite flake/phenolic resin powder mixture in SLS process. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, 233, 4497–4507. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Effect of different graphite materials on the electrical conductivity and flexural strength of bipolar plates fabricated using selective laser sintering. Int. J. Hydrog. Energy 2012, 37, 3558–3566. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Performance Investigation of Polymer Electrolyte Membrane Fuel Cells Using Graphite Composite Plates Fabricated by Selective Laser Sintering. J. Fuel Cell Sci. Tech. 2013, 11, 011003. [Google Scholar] [CrossRef]
- Alayavalli, K.; Bourell, D.L. Fabrication of modified graphite bipolar plates by indirect selective laser sintering (SLS) for direct methanol fuel cells. Rapid Prototyp. J. 2010, 16, 268–274. [Google Scholar] [CrossRef]
- Rice, R.W. Comparison of stress concentration versus minimum solid area based mechanical property-porosity relations. J. Mater. Sci. 1993, 28, 2187–2190. [Google Scholar] [CrossRef]
- Li, X.; Yan, Q.; Han, Y.; Cao, M.; Ge, C. Fabrication of solid-phase-sintered SiC-based composites with short carbon fibers. Int. J. Miner. Metall. Mater. 2014, 21, 1141–1145. [Google Scholar] [CrossRef]
- Han, Y.; Li, Q.; Yan, Q.; Tang, R. Properties of silicon carbide-reinforced graphite composites prepared by a reactive sintering method. Carbon 2015, 86, 374. [Google Scholar] [CrossRef]
- Argento, C.; Bouvard, D. Modeling the effective thermal conductivity of random packing of spheres through densification. Int. J. Heat Mass Transf. 1996, 39, 1343–1350. [Google Scholar] [CrossRef]
- Liu, R.; Wang, C. Effects of pore-forming agent content on properties of porous 8YSZ ceramics by TBA based gel-casting. Rare Met. Mater. Eng. 2013, 42, 422–425. [Google Scholar]
- Paul, R.M.; Morral, J.E. A 3D-Random pore model for the oxidation of graphite with closed porosity. J. Nucl. Mater. 2018, 509, 425–434. [Google Scholar] [CrossRef]
- Wu, M.; Huang, H.-X.; Tong, J.; Ke, D.-Y. Enhancing thermal conductivity and mechanical properties of poly (methyl methacrylate) via adding expanded graphite and injecting water. Compos. Part A Appl. Sci. Manuf. 2017, 102, 228–235. [Google Scholar] [CrossRef]
- Kim, H.S.; Na, J.H.; Jung, Y.C.; Kim, S.Y. Synergistic enhancement of thermal conductivity in polymer composites filled with self-hybrid expanded graphite fillers. J. Non Cryst. Solids. 2016, 450, 75–81. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Liao, N.; Xu, X.; Sang, S.; Xu, Y.; Wang, G.; Nath, M. Synthesis of boron and nitrogen-doped expanded graphite as efficient reinforcement for Al2O3-C refractories. Ceram. Int. 2017, 43, 16710–16720. [Google Scholar] [CrossRef]
- Choe, C.R.; Lee, K.H.; Yoon, B.I. Effect of processing parameters on the mechanical properties of carbonized phenolic resin. Carbon 1992, 30, 247–249. [Google Scholar] [CrossRef]
- Soto, C.; García-Rosales, C.; Echeberria, J. Production of porous SiC by liquid phase sintering using graphite as sacrificial phase: Influence of SiO2 and graphite on the sintering mechanisms. J. Eur. Ceram. Soc. 2019, 39, 3949–3958. [Google Scholar] [CrossRef]
- Hua, Y.; Bai, S.; Wan, H.; Chen, X.; Hu, T.; Gong, J. Research on controllable synthesis of silicon carbide whiskers and powder on graphite by chemical vapor reaction. J. Mater. Sci. 2019, 54, 2016–2024. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Z.; Zhang, X.; Wang, K.; Zhao, Y.; Xia, H.; Wang, J. Three dimensional AlN skeleton-reinforced highly oriented graphite flake composites with excellent mechanical and thermophysical properties. Carbon 2018, 131, 94–101. [Google Scholar] [CrossRef]
- Li, X.; Zheng, M.; Li, R.; Yuan, G.; Zhou, G.; Zhu, X.; Ren, G. Preparation, microstructure, properties and foaming mechanism of a foamed ceramics with high closed porosity. Ceram. Int. 2019, 45, 11982–11988. [Google Scholar] [CrossRef]
- Li, X.; Zhou, G.; Yang, Q.; Zhu, X.; Ren, G.; Liu, L. Preparation and performance of electromagnetic wave absorbing foamed ceramics with high closed porosity and gradient SiC distribution. Ceram. Int. 2020, 46, 2294–2299. [Google Scholar] [CrossRef]
- Fan, J.; Xu, S. Thermal conductivity and mechanical properties of high density polyethylene composites filled with silicon carbide whiskers modified by cross-linked poly (vinyl alcohol). J. Mater. Sci. Technol. 2018, 34, 185–192. [Google Scholar] [CrossRef]

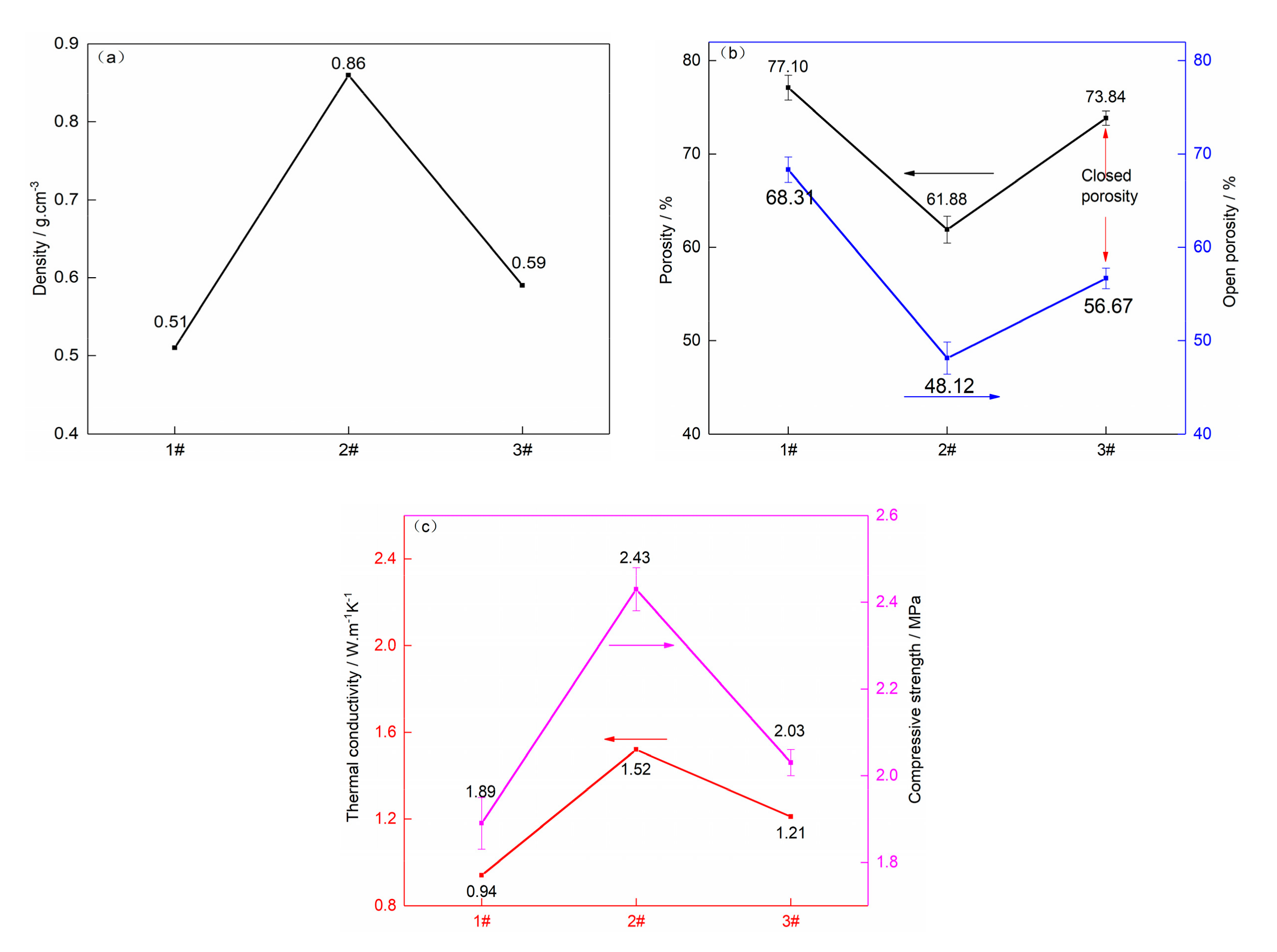
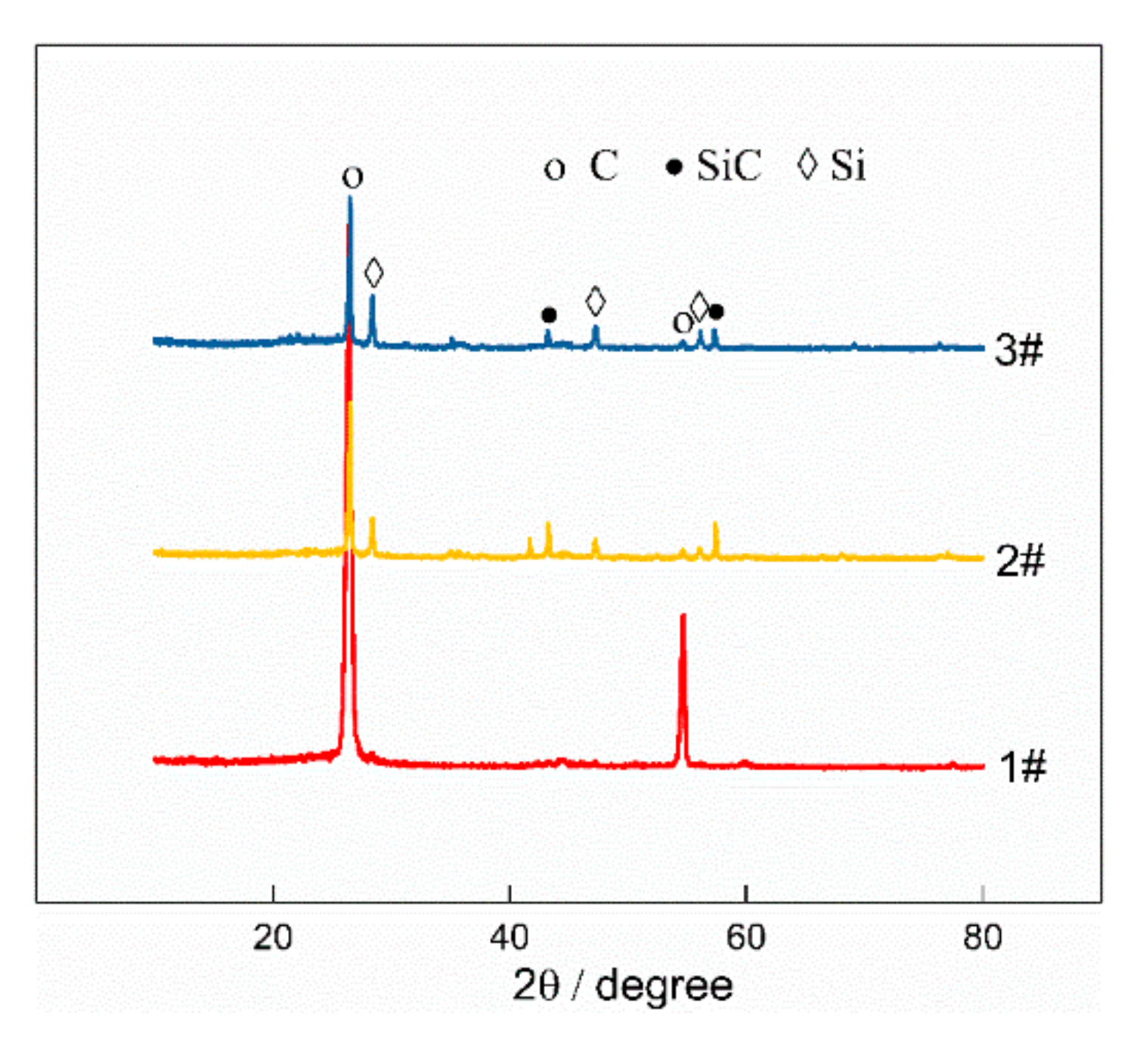

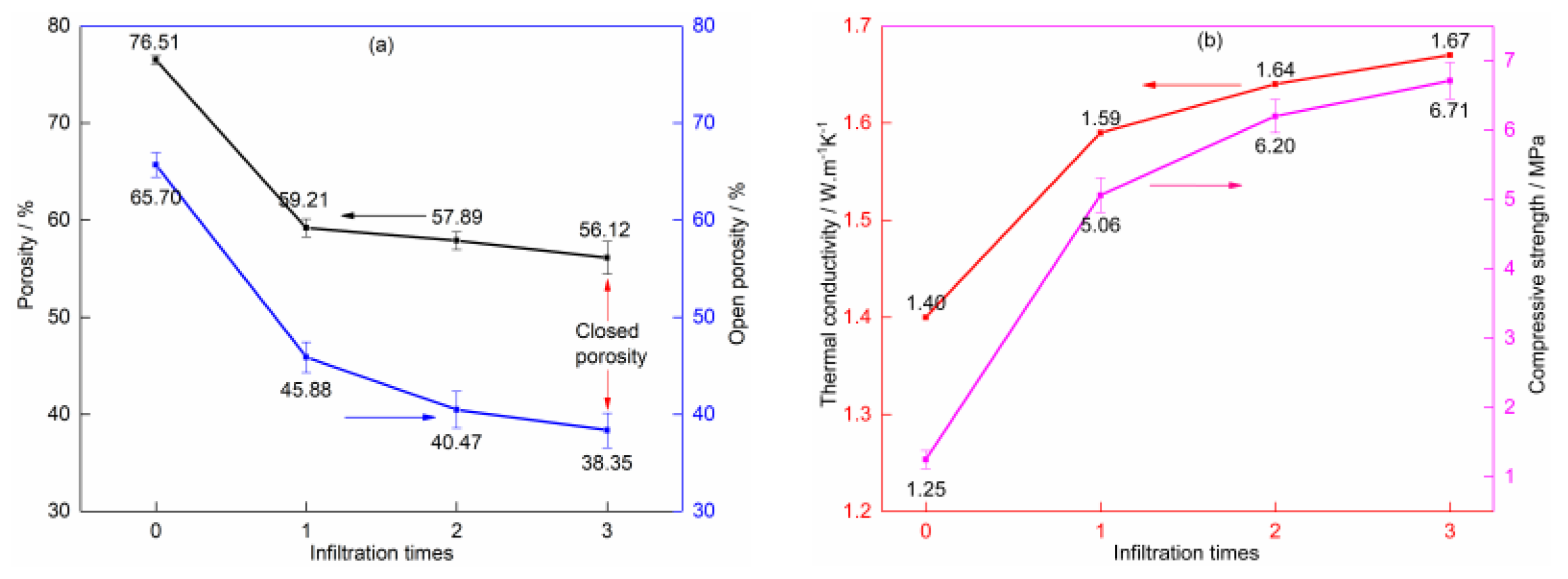
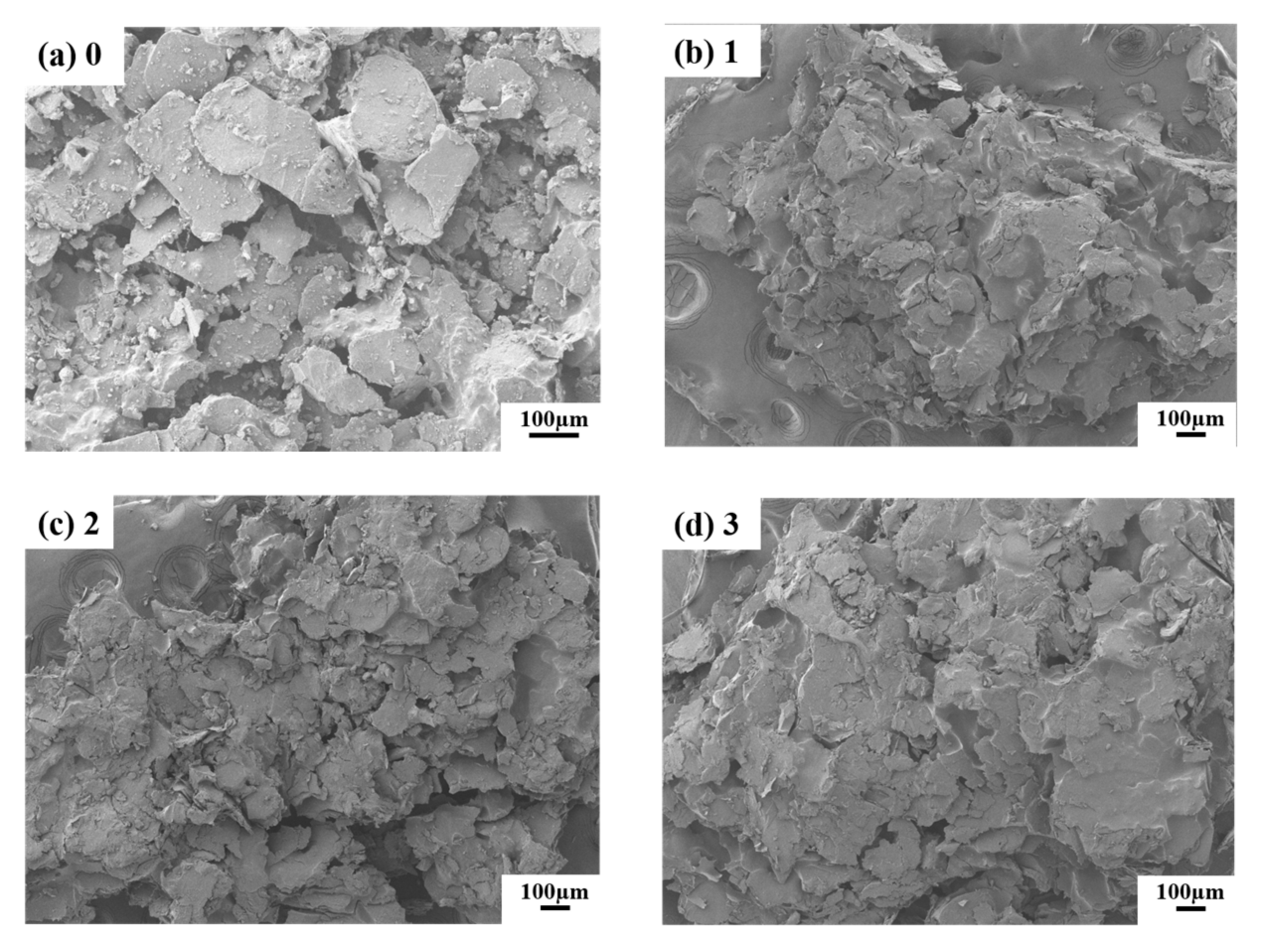


| Sample | Graphite | Phenolic Resin | Silicon Powder | Expanded Graphite |
|---|---|---|---|---|
| 1# | 70 | 30 | 0 | 0 |
| 2# | 46 | 30 | 24 | 0 |
| 3# | 46 | 30 | 24 | 1 |
| Density | Compressive Strength | Thermal Conductivity | Operating Temperature | Repeated use Times | Production Costs | |
|---|---|---|---|---|---|---|
| 3D graphite/ceramic composite mold | 1.05 g·cm−3 | 10.92 MPa | 1.88 W·m−1K−1 | >1550 ℃ | 28 | 6500¥/t |
| 1.11 g·cm−3 | 13.04 MPa | 1.94 W·m−1K−1 | >1550 ℃ | 36 | ||
| Water glass sand mold | 1.60 g·cm−3 | 3.03 MPa | 1.79 W·m−1K−1 | >1100 ℃ | 1 | 570¥/t |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Chen, K.; Li, Y.; Ren, C.; Sun, Y.; Huang, C. Fabrication of Natural Flake Graphite/Ceramic Composite Parts with Low Thermal Conductivity and High Strength by Selective Laser Sintering. Appl. Sci. 2020, 10, 1314. https://doi.org/10.3390/app10041314
Wu H, Chen K, Li Y, Ren C, Sun Y, Huang C. Fabrication of Natural Flake Graphite/Ceramic Composite Parts with Low Thermal Conductivity and High Strength by Selective Laser Sintering. Applied Sciences. 2020; 10(4):1314. https://doi.org/10.3390/app10041314
Chicago/Turabian StyleWu, Haihua, Kui Chen, Yafeng Li, Chaoqun Ren, Yu Sun, and Caihua Huang. 2020. "Fabrication of Natural Flake Graphite/Ceramic Composite Parts with Low Thermal Conductivity and High Strength by Selective Laser Sintering" Applied Sciences 10, no. 4: 1314. https://doi.org/10.3390/app10041314
APA StyleWu, H., Chen, K., Li, Y., Ren, C., Sun, Y., & Huang, C. (2020). Fabrication of Natural Flake Graphite/Ceramic Composite Parts with Low Thermal Conductivity and High Strength by Selective Laser Sintering. Applied Sciences, 10(4), 1314. https://doi.org/10.3390/app10041314




