Hypolipidemic Activities of Two Pentapeptides (VIAPW and IRWWW) from Miiuy Croaker (Miichthys miiuy) Muscle on Lipid Accumulation in HepG2 Cells through Regulation of AMPK Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HepG2 Cell Culture
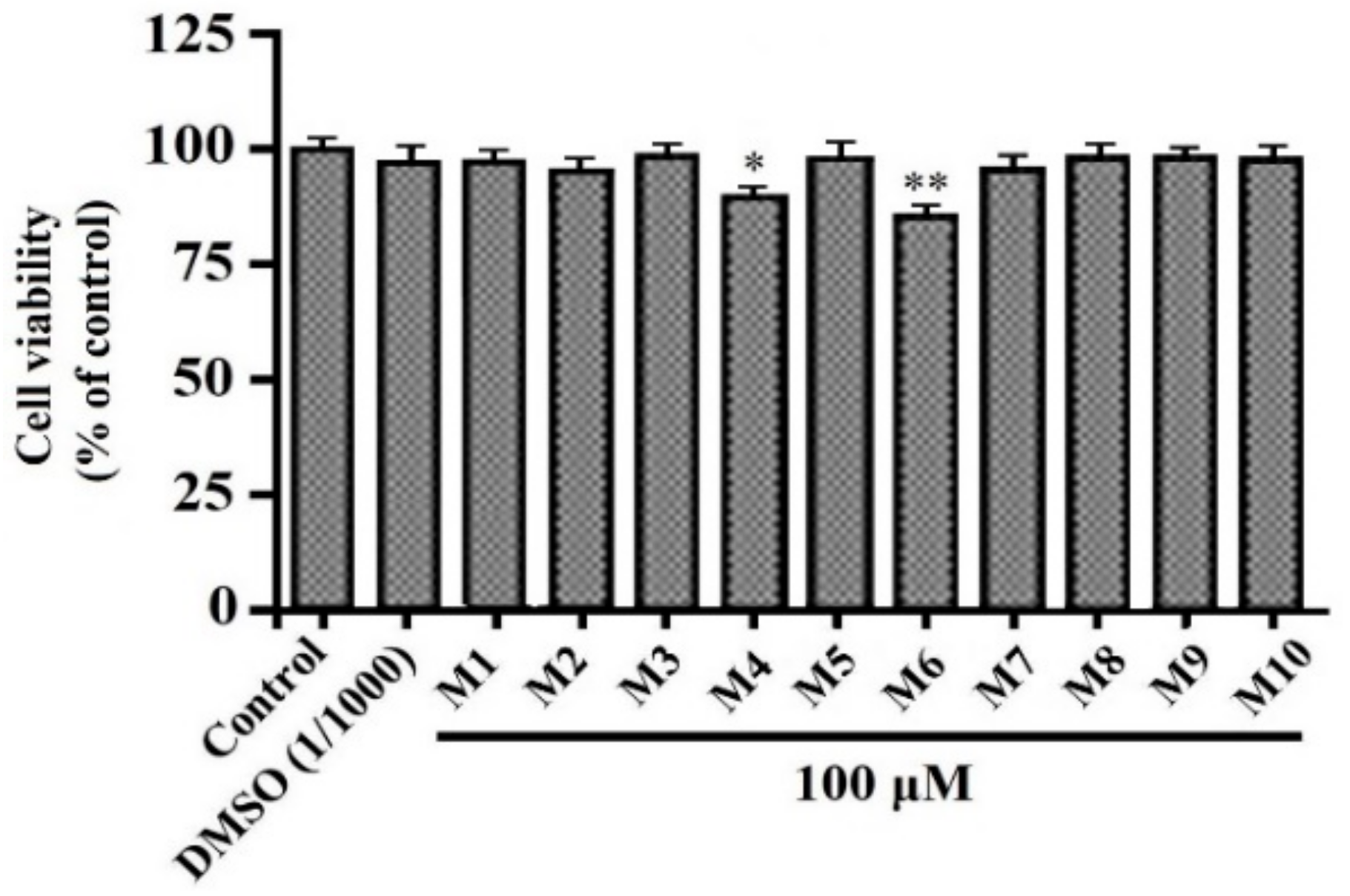
2.3. Cell Viability Assay
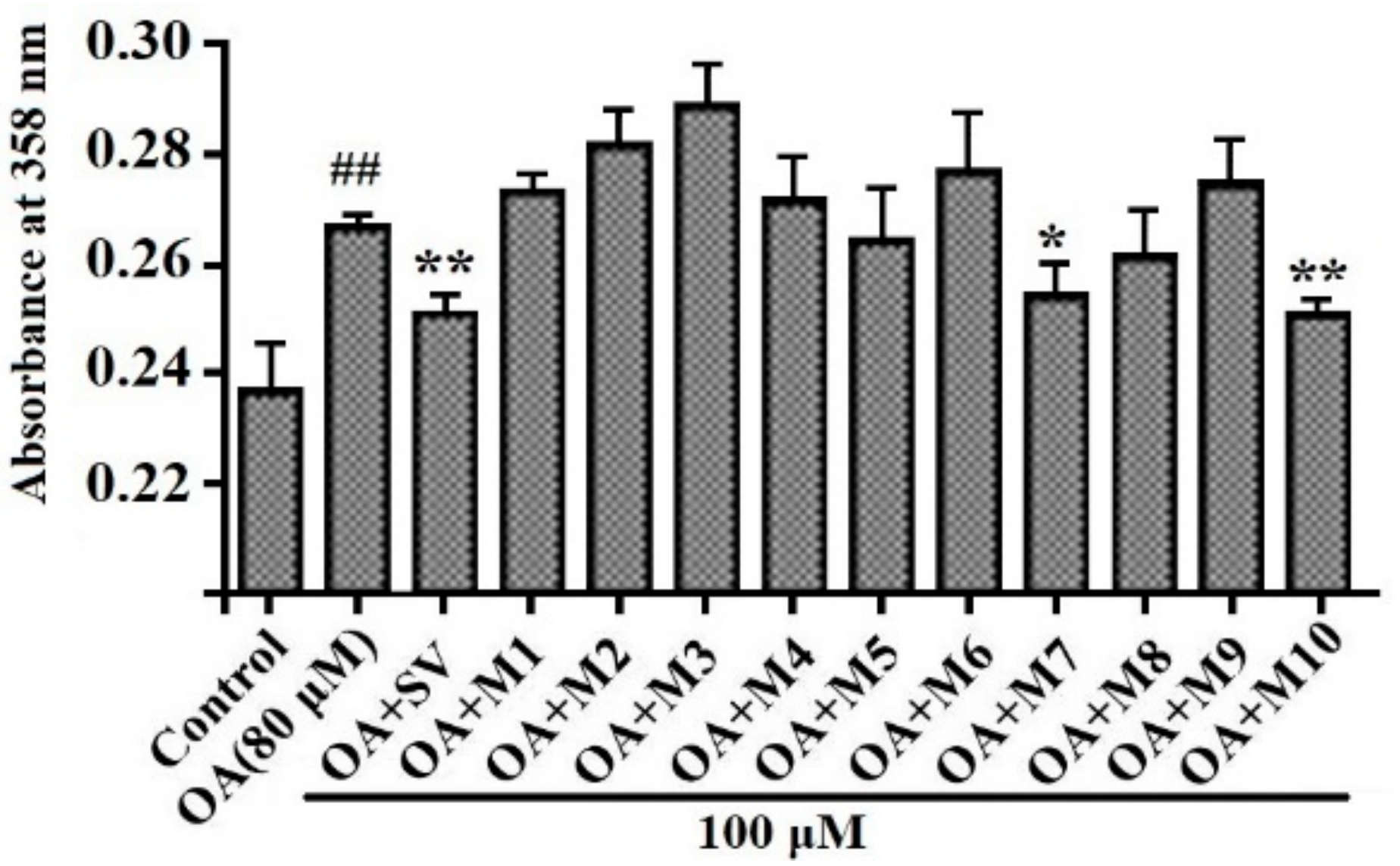
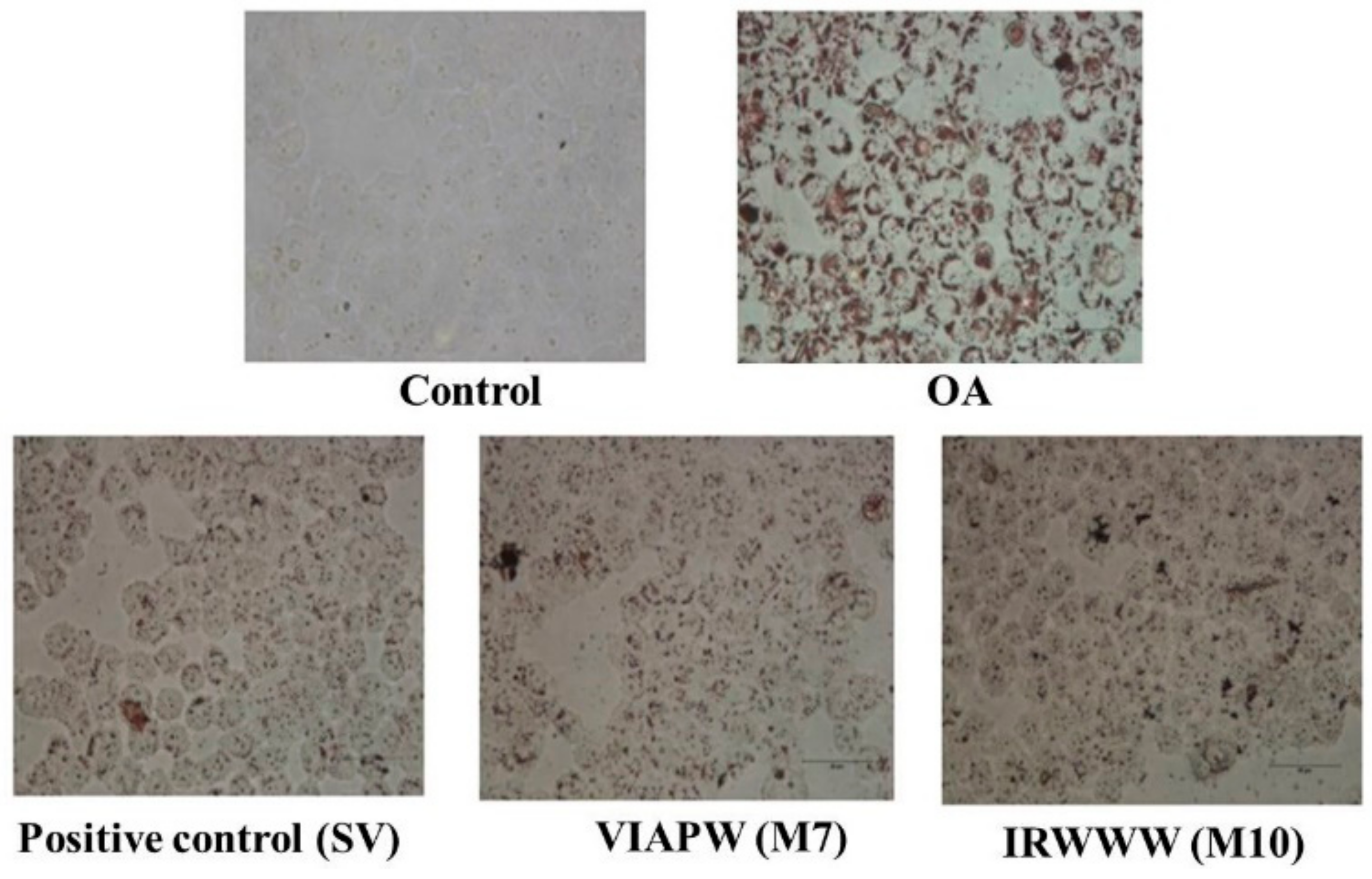
2.4. Oil Red O Staining Assay
2.5. Preparation of Protein Extract of HepG2 Cells
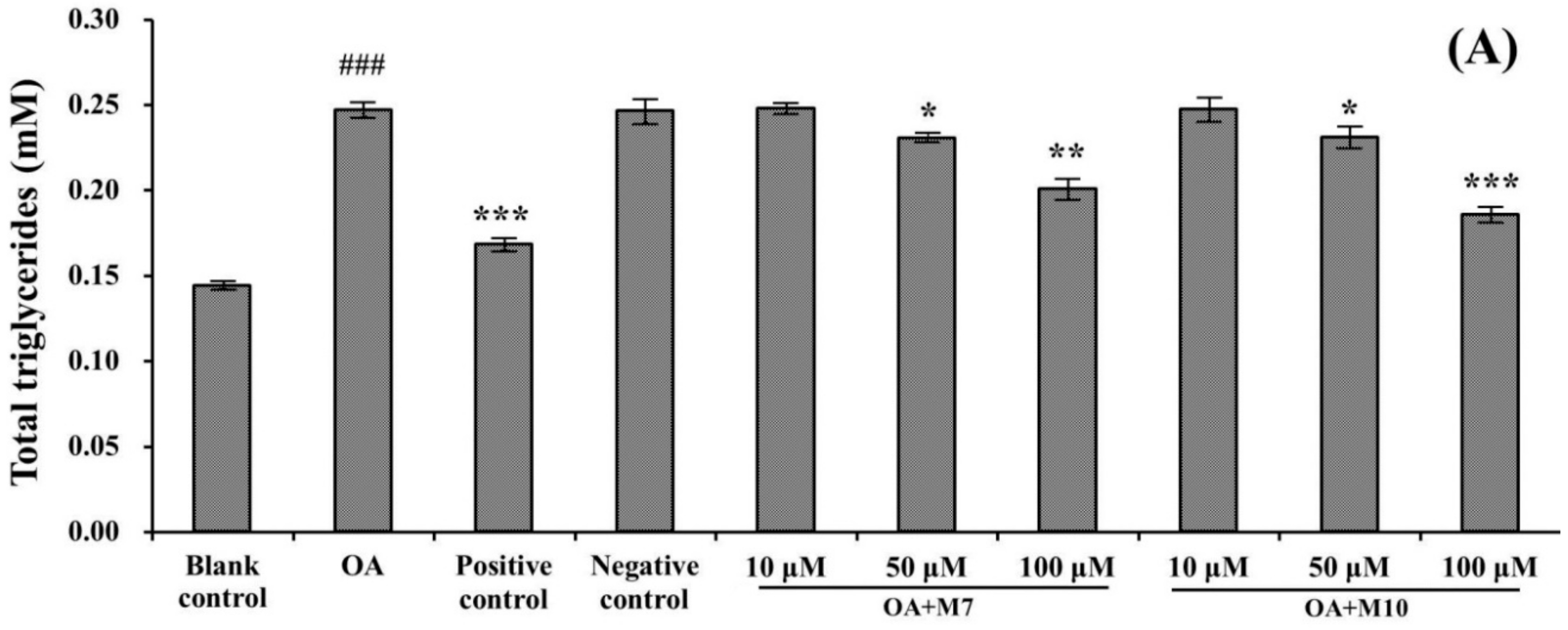
2.6. Cellular TC and TG Contents Analysis
2.7. Fluorescence Quantitative Polymerase Chain Reaction (PCR) Analysis
2.8. Western Blot Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Peptides (M1-M10) on HepG2 Cell Viability
3.2. Effects of VIAPW (M7) and IRWWW (M10) on Lipid Accumulation
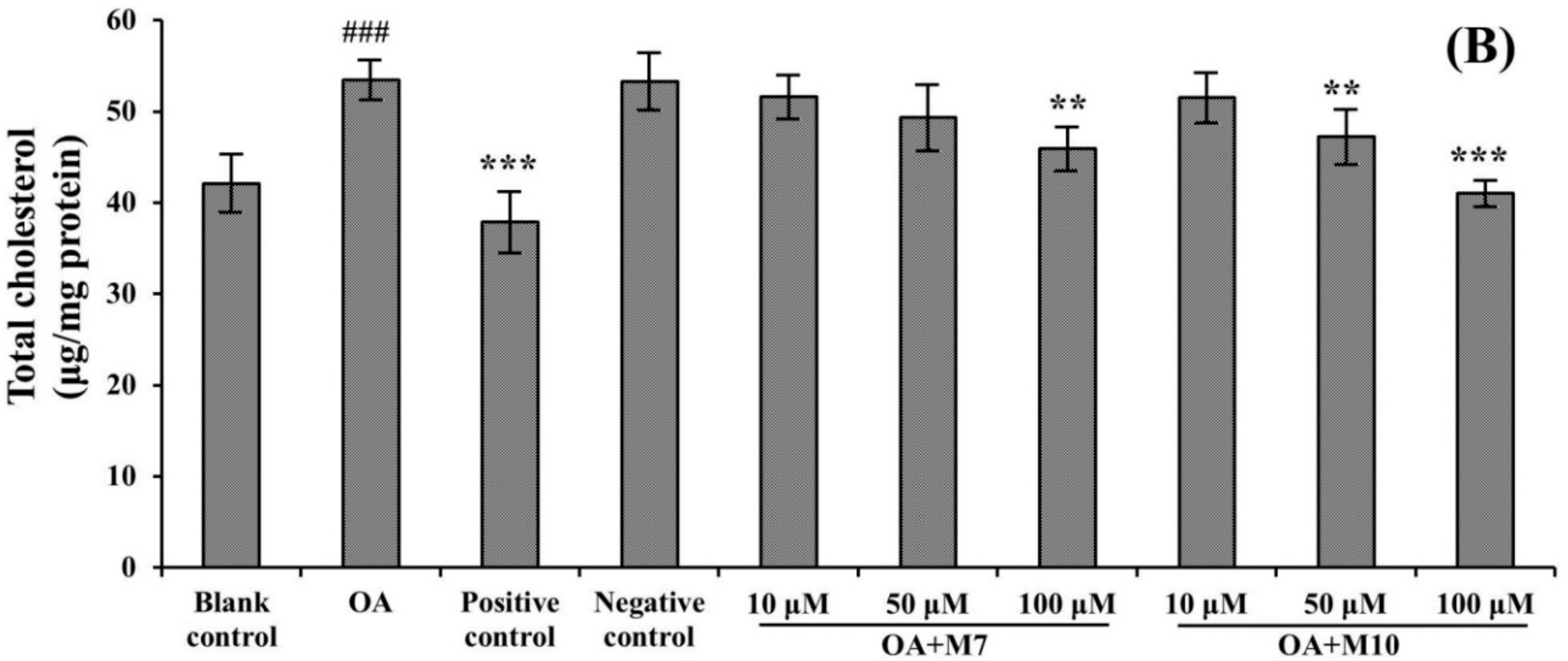
3.3. Effects of VIAPW (M7) and IRWWW (M10) on Lowering Intracellular TG and TC Contents
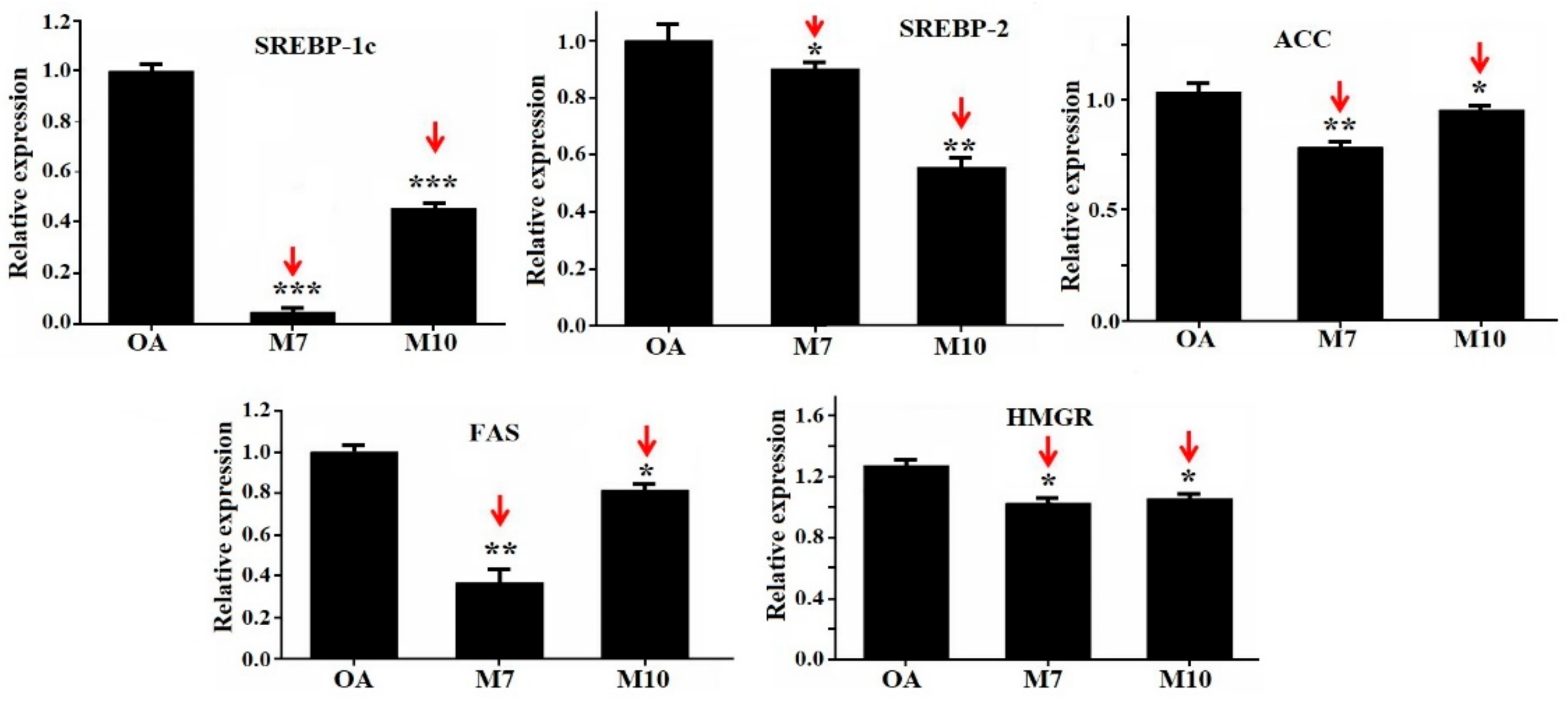
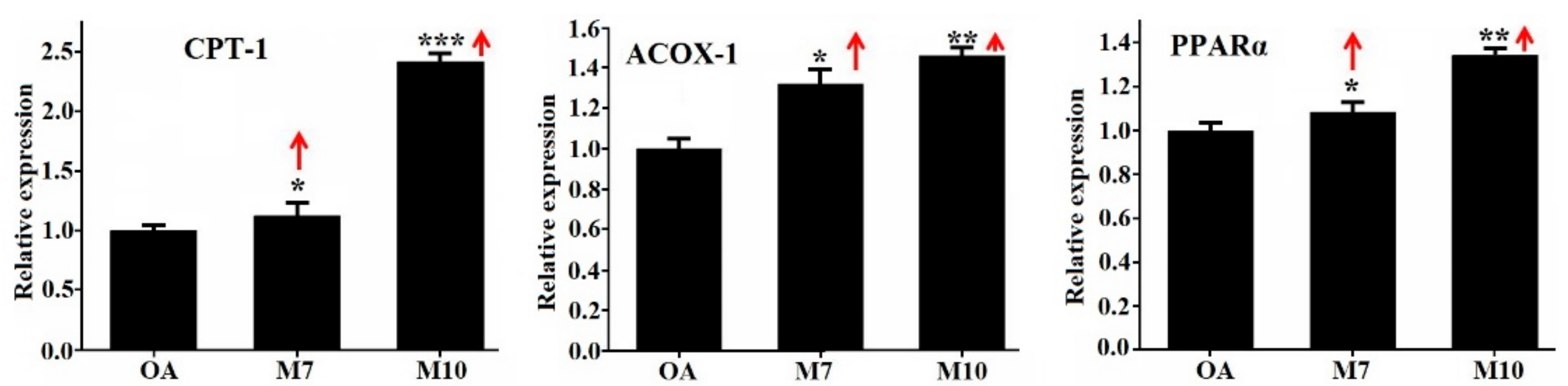
3.4. Effects of VIAPW (M7) and IRWWW (M10) on the Expression Levels of Genes Involved in Lipid Metabolism
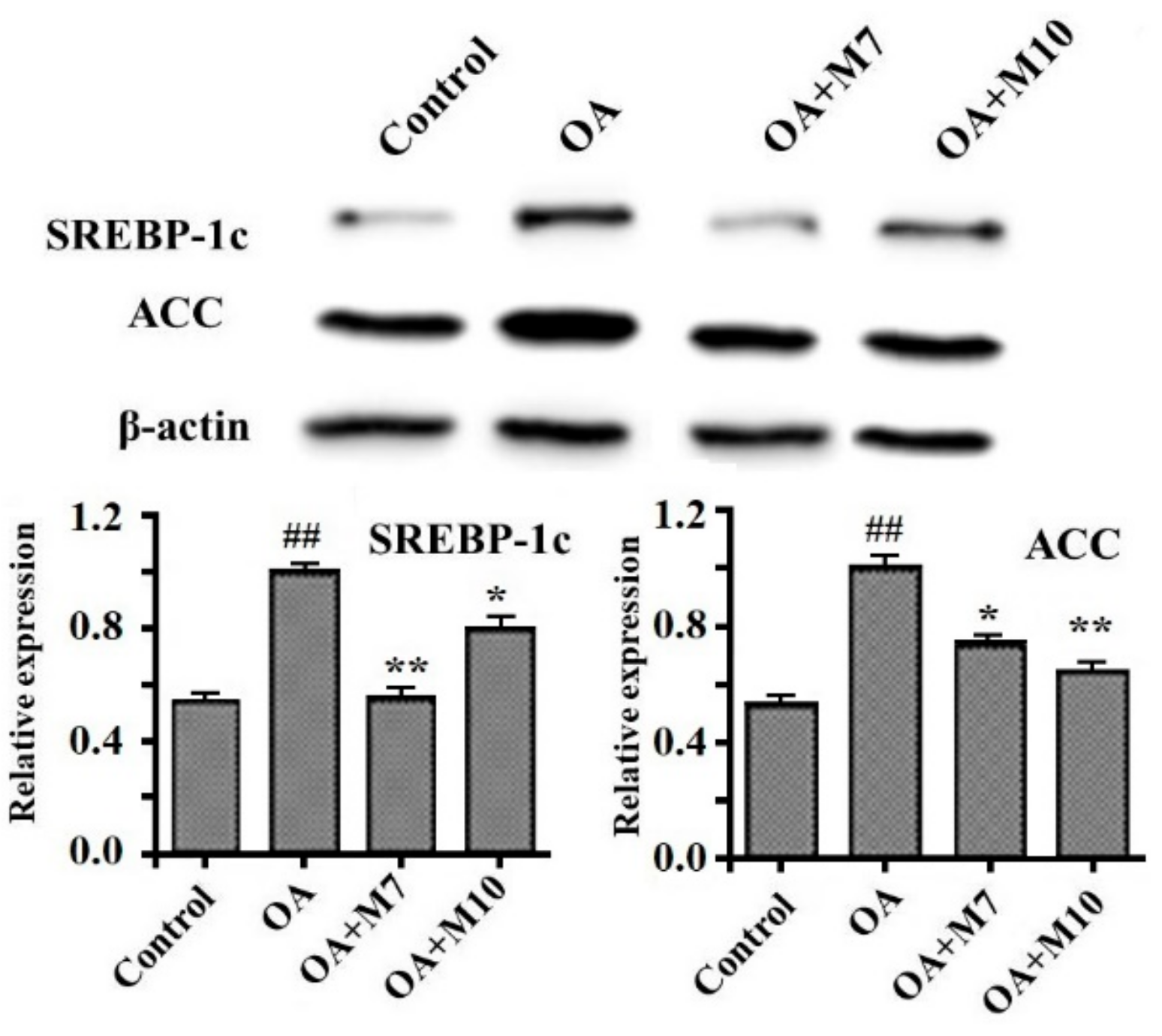
3.5. Effects of VIAPW (M7) and IRWWW (M10) on the Expression Levels of Proteins Related to Lipid Metabolism in HepG2 Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.; Choi, J.M.; Cho, J.Y.; Kim, T.E.; Lee, H.J.; Jung, B.H. Regulation of endogenic metabolites by rosuvastatin in hyperlipidemia patients: An integration of metabolomics and lipidomics. Chem. Phys. Lipids 2018, 214, 69–83. [Google Scholar] [CrossRef]
- Affane, F.; Louala, S.; El Imane Harrat, N.; Bensalah, F.; Chekkal, H.; Allaoui, A.; Lamri-Senhadji, M. Hypolipidemic, antioxidant and antiatherogenic property of sardine by-Products proteins in high-Fat diet induced obese rats. Life Sci. 2018, 199, 16–22. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Gutierrez, E.; Ramos Solano, B.; Seymour, E.M.; Bolling, S.F. The inhibitory potential of Montmorency tart cherry on key enzymes relevant to type 2 diabetes and cardiovascular disease. Food Chem. 2018, 252, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-Obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Tacherfiout, M.; Petrov, P.D.; Mattonai, M.; Ribechini, E.; Ribot, J.; Bonet, M.L.; Khettala, B. Antihyperlipidemic effect of a Rhamnus alaternus leaf extract in Triton-Induced hyperlipidemic rats and human HepG2 cells. Biomed. Pharmacother. 2018, 101, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Prados, I.M.; Marina, M.L.; García, M.C. Isolation and identification by high resolution liquid chromatography tandem mass spectrometry of novel peptides with multifunctional lipid lowering capacity. Food Res. Int. 2018, 111, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A.; Vistoli, G.; Lammi, C. Behavior of three hypocholesterolemic peptides from soy protein in an intestinal model based on differentiated Caco-2 cell. J. Funct. Foods 2018, 45, 363–370. [Google Scholar] [CrossRef]
- Song, D.; Jiang, J. Hypolipidemic Components from Medicine Food Homology Species Used in China: Pharmacological and Health Effects. Arch. Med. Res. 2017, 48, 569–581. [Google Scholar] [CrossRef]
- Jemil, I.; Abdelhedi, O.; Nasri, R.; Mora, L.; Marrekchi, R.; Jamoussi, K.; ElFeki, A.; Hajji, M.; Toldrá, F.; Nasri, M. Hypolipidemic, antiobesity and cardioprotective effects of sardinelle meat flour and its hydrolysates in high-Fat and fructose diet fed Wistar rats. Life Sci. 2017, 176, 54–66. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, L.; Tao, J.; Chi, C.F.; Wang, B. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Res. Int. 2019, 121, 197–204. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Qiu, Y.T.; Wang, Y.M.; Yang, X.R.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Gelatin and antioxidant peptides from gelatin hydrolysate of skipjack tuna (Katsuwonus pelamis) scales: Preparation, identification and activity evaluation. Mar. Drugs 2019, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, G.X.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Identification and active evaluation of antioxidant peptides from protein hydrolysates of skipjack tuna (Katsuwonus pelamis) head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef]
- Fan, X.; Cui, Y.; Zhang, R.; Zhang, X. Purification and identification of anti-Obesity peptides derived from Spirulina platensis. J. Funct. Foods 2018, 47, 350–360. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Kwon, D.Y.; Yun, L. Design of a highly potent inhibitory peptide acting as a competitive inhibitor of HMG-CoA reductase. Amino Acids 2012, 43, 2015–2025. [Google Scholar] [CrossRef]
- Nagaoka, S.; Futamura, Y.; Miwa, K.; Awano, T.; Yamauchi, K.; Kanamaru, Y.; Tadashi, K.; Kuwata, T. Identification of novel hypocholesterolemic peptides derived from bovine milk β-Lactoglobulin. Biochem. Biophys. Res. Commun. 2001, 281, 11–17. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Hwang, E.J.; Jeon, T.I.; Moon, J.H. Glu-Phe from onion (Allium Cepa L.) attenuates lipogenesis in hepatocytes. Biosci. Biotechnol. Biochem. 2017, 81, 1409–1416. [Google Scholar] [CrossRef]
- Shi, W.; Hou, T.; Liu, W.; Guo, D.; He, H. The hypolipidemic effects of peptides prepared from Cicer arietinum in ovariectomized rats and HepG2 cells. J. Sci. Food Agric. 2019, 99, 576–586. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y.F. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef]
- Zhao, W.H.; Luo, Q.B.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy). J. Funct. Foods 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Yang, X.R.; Zhang, L.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Purification and characterization of antioxidant peptides derived from protein hydrolysate of the marine bivalve mollusk Tergillarca granosa. Mar. Drugs 2019, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Ten new pentapeptides from protein hydrolysate of miiuy croaker (Miichthys miiuy) muscle: Preparation, identification, and antioxidant activity evaluation. LWT-Food Sci. Technol. 2019, 105, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wei, C.; Chen, J.; Ye, X. Proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves regulate lipid metabolism and glucose consumption by activating AMPK pathway in HepG2 cells. J. Funct. Foods 2017, 29, 217–225. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Chi, C.F.; Wang, B. Anticancer activity of a hexapeptide from skate (Raja porosa) cartilage protein hydrolysate in HeLa cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef]
- Madushani Herath, K.H.I.N.; Cho, J.; Kim, A.; Eom, T.K.; Kim, J.S.; Kim, J.B.; Doh, Y.H.; Jee, Y. Phenolic acid and flavonoid-Rich fraction of Sasa quelpaertensis Nakai leaves prevent alcohol induced fatty liver through AMPK activation. J. Ethnopharmacol. 2018, 224, 335–348. [Google Scholar] [CrossRef]
- Gerets, H.H.; Tilmant, K.; Gerin, B.; Chanteux, H.; Depelchin, B.O.; Dhalluin, S.; Atienzar, F.A. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol. Toxicol. 2012, 28, 69–87. [Google Scholar] [CrossRef]
- Pardo Andreu, G.L.; Reis, F.H.; Dalalio, F.M.; Nuñez Figueredo, Y.; Cuesta Rubio, O.; Uyemura, S.A.; Curti, C.; Alberici, L.C. The cytotoxic effects of brown Cuban propolis depend on the nemorosone content and may be mediated by mitochondrial uncoupling. Chem.-Biol. Interac. 2015, 228, 28–34. [Google Scholar] [CrossRef]
- Liu, Q.; Bengmark, S.; Qu, S. The role of hepatic fat accumulation in pathogenesis of non-Alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010, 9, 42. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.; Wu, Y.; Chen, Y. Dihydrocurcumin ameliorates the lipid accumulation, oxidative stress and insulin resistance in oleic acid-induced L02 and HepG2 cells. Biomed. Pharmacother. 2018, 103, 1327–1336. [Google Scholar] [CrossRef]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera suppresses SREBP-1c and ACC expression to lower serum triglycerides in high-Fat diet-Induced hyperlipidaemic rats. J. Funct. Foods 2018, 40, 722–728. [Google Scholar] [CrossRef]
- Sozen, E.; Ozer, N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-Review. Redox Bio. 2017, 12, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrin. Met. 2017, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Randy, A.; Kim, M.; Nho, C.W. Ligularia fischeri and its constituent 3,4-Dicaffeoylquinic acid improve obesity-Induced nonalcoholic fatty liver disease by regulating lipid metabolism and activating AMPK. J. Funct. Foods 2016, 27, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.J.; Zhang, L.J.; Zhao, X.; He, X.X.; Li, M.M.; Zhao, X.L.; Wu, J.D.; Qiu, P.J.; Yu, G.L. Activated AMPK explains hypolipidemic effects of sulfated low molecular weight guluronate on HepG2 cells. Eur. J. Med. Chem. 2014, 85, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Ao, N.; Yang, J.; Wang, X.; Du, J. Glucagon-Like peptide-1 preserves non-Alcoholic fatty liver disease through inhibition of the endoplasmic reticulum stress associated pathway. Hepatol. Res. 2016, 46, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Kim, J.H.; Kim, J.H.; Jung, M.H. Protective effects of gomisin N against hepatic steatosis through AMPK activation. Biochem. Biophys. Res. Commun. 2017, 482, 1095–1101. [Google Scholar] [CrossRef]
- Liu, B.; Yang, T.; Luo, Y.; Zeng, L.; Shi, L.; Wei, C.; Nie, Y.; Cheng, Y.; Lin, Q.; Luo, F. Oat β-Glucan inhibits adipogenesis and hepatic steatosis in high fat diet induced hyperlipidemic mice via AMPK signalling. J. Funct. Foods 2018, 41, 72–82. [Google Scholar] [CrossRef]
- Kabirifar, R.; Ghoreshi, Z.; Rezaifar, A.; Binesh, F.; Bamdad, K.; Moradi, A. Curcumin, quercetin and atorvastatin protected against the hepatic fibrosis by activating AMP-Activated protein kinase. J. Funct. Foods 2018, 40, 341–348. [Google Scholar] [CrossRef]
- Li, T.; Wen, L.; Cheng, B. Cordycepin alleviates hepatic lipid accumulation by inducing protective autophagy via PKA/mTOR pathway. Biochem. Biophys. Res. Commun. 2019, 516, 632–638. [Google Scholar] [CrossRef]
| Primer | Sequence (5’–3’) |
|---|---|
| ACC-F | TGATGTCAATCTCCCCGCAGC |
| ACC-R | TTGCTTCTTCTCTGTTTTCTCCCC |
| SREBP-1c-F | CCATGGATGCACTTTCGAA |
| SREBP-1c-R | CCAGCATAGGGTGGGTCAA |
| SREBP-2-F | CTGCAACAACAGACGGTAATGA |
| SREBP-2-R | CCATTGGCCGTTTGTGTCAG |
| FAS-F | CGGTACGCGACGGCTGCCTG |
| FAS-R | GCTGCTCCACGAACTCAAACACCG |
| HMGR-F | GGACCCCTTTGCTTAGATGAAA |
| HMGR-R | CCACCAAGACCTATTGCTCTG |
| CPT1-F | CGTCTTTTGGGATCCACGATT |
| CPT1-R | TGTGCTGGATGGTGTCTGTCTC |
| ACOX1-F | GGGCATGGCTATTCTCATTGC |
| ACOX1-R | CGAACAAGGTCAACAGAAGTTAGGTTC |
| PPARα-F | AAAAGCCTAAGGAAACCGTTCTG |
| PPARα-R | TATCGTCCGGGTGGTTGCT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-M.; Pan, X.; He, Y.; Chi, C.-F.; Wang, B. Hypolipidemic Activities of Two Pentapeptides (VIAPW and IRWWW) from Miiuy Croaker (Miichthys miiuy) Muscle on Lipid Accumulation in HepG2 Cells through Regulation of AMPK Pathway. Appl. Sci. 2020, 10, 817. https://doi.org/10.3390/app10030817
Wang Y-M, Pan X, He Y, Chi C-F, Wang B. Hypolipidemic Activities of Two Pentapeptides (VIAPW and IRWWW) from Miiuy Croaker (Miichthys miiuy) Muscle on Lipid Accumulation in HepG2 Cells through Regulation of AMPK Pathway. Applied Sciences. 2020; 10(3):817. https://doi.org/10.3390/app10030817
Chicago/Turabian StyleWang, Yu-Mei, Xin Pan, Yu He, Chang-Feng Chi, and Bin Wang. 2020. "Hypolipidemic Activities of Two Pentapeptides (VIAPW and IRWWW) from Miiuy Croaker (Miichthys miiuy) Muscle on Lipid Accumulation in HepG2 Cells through Regulation of AMPK Pathway" Applied Sciences 10, no. 3: 817. https://doi.org/10.3390/app10030817
APA StyleWang, Y.-M., Pan, X., He, Y., Chi, C.-F., & Wang, B. (2020). Hypolipidemic Activities of Two Pentapeptides (VIAPW and IRWWW) from Miiuy Croaker (Miichthys miiuy) Muscle on Lipid Accumulation in HepG2 Cells through Regulation of AMPK Pathway. Applied Sciences, 10(3), 817. https://doi.org/10.3390/app10030817







