Abstract
Complex samples such as botanical extracts contain hundreds of compounds. Since we can only identify compounds that are stable, extractable, separable and detectable from complex botanical extracts, minimal sample treatment and different detection methods are essential. A combination of high-performance thin-layer chromatography (HPTLC) with non-targeted screening via bioassays (enzymes), microchemical and biological (microorganisms) detection allows for the fast and quantitative bioprofiling of complex samples. Further hyphenation of HPTLC with spectroscopic methods of identification enables targeted identification of bioactive natural products via Effect Directed Analysis (EDA).
1. Introduction
The plant kingdom is a vast source of secondary metabolites, with many of these compounds possessing therapeutic activity. Although many of these compounds have been characterized and used as therapeutic agents, there are still many more that should be investigated as potential sources of novel, and easy-to-obtain therapeutic agents. Natural products are still a compelling lead in drug discovery. The traditional strategy used to discover therapeutic activity has been challenged in the last few decades, from the laborious and expensive methods under which bioactivity was studied only after the isolation of the components, to an Effect Directed Analysis (EDA) approach. EDA is a combined approach that directly links chromatographic separation with in vitro effect-directed (bio)assays and physico-chemical analysis for the bioactive characterization of detected compounds.
2. Targeted Identification of Bioactive Compounds versus Nontarget Analysis and Effect-Directed Analysis (EDA)
Complex samples such as plant extracts contain hundreds of compounds. In the past, only known target compounds were looked for by target analysis and in this way, most bioactive compounds were left undiscovered.
In nontargeted analysis, many compounds present in the sample are identified by analyzing the peaks identities. Thus, the nontarget identification leads to a huge list of compounds. However, which peaks (present compounds) are responsible for biological activity will not be elucidated. In contrast, the objective of an EDA approach is to identify which compounds are responsible for a certain biological effect.
3. Effect-Directed Analysis (EDA)
EDA is a combined approach, linking biological and chemical analytical methods. The aim is to identify which compounds present in a natural sample are bioactive using a direct chemical analysis, and then identify only those compounds with significant target effect. After a specific biological effect is detected in a bioassay, chemical fractionation (i.e., chromatographic separation) is used to reduce the complexity of the sample (e.g., plant extracts), to bioactive chemical classes and, eventually, to individual compounds. Once fractionation has reduced the sample matrix so that the cause of the biological effect is isolated, the compounds present in the fraction are identified. Chromatographic separation of the sample allows for easier chemical analysis and better identification of the unknown compounds.
EDA is an effect-directed nontargeted analysis, used in screening for natural products with bioactivity from complex samples, like botanical extracts, in drug discovery processes. The chemical characterization of plant components is usually linked to liquid chromatography, column or planar, to enable effective separation and isolation of its constituents. In recent decades the technical developments that have allowed hyphenations of planar chromatography have enabled the parallel biodetection, bioprofiling and chemical characterization of individual bioactive constituents from botanical extracts.
4. High Performance Thin Layer Chromatography/Thin Layer Chromatography (HPTLC/TLC) and Effect-Directed Analysis (EDA)
The most popular separation method for effect-related analysis is thin-layer chromatography (TLC). TLC is a relatively old, well established, chromatographic separation technique, that is ideally suited for the high-throughput screening of highly complex samples, such as plant extracts. The simple concept of TLC allows the parallel analysis of many samples in a single run (on the same plate) in contrast to high performance liquid chromatography (HPLC) or gas chromatography (GC) where samples must be analyzed sequentially. Thus, with TLC, multiple samples can be analyzed in parallel, under identical laboratory conditions, providing a parallel profiling of samples, which allows for enhanced recognition of spot similarity [1]. Profiling samples in this way also allows for the parallel evaluation of different effect-directed assays. TLC is a matrix tolerant method. Crude extracts can be directly applied on the plate with minimal or no sample preparation and possible loss of sample components [2]. Thus, all sample components separated on the plate can be directly evaluated using a bioassay, enabling sample bioactivity to be maintained and accurately assessed. The possibility of multiple detections (UV/Vis absorption and fluorescence measurements) without the need to repeat separation, provides more information about separated compounds. TLC is the only chromatographic method that offers the visual evaluation of the chromatogram. High-performance thin-layer chromatography (HPTLC) is an enhanced form of TLC, which consists of the automatization of instrumentation and increased chromatogram resolution. This advanced form of TLC enables the quantitative analysis of separated analyte components in sample mixtures.
A combination of TLC chromatography with microbial tests and bioassays has led to the rapid and reliable characterization of unknown samples in terms of their mode of action and bioactivity profiles [3]. As an open chromatographic system, HPTLC is compatible with microbial (bacteria and yeast) and biochemical (enzyme) derivatization methods (Table 1). It is possible to run direct in situ bioassays on the plate without much effort, since the mobile phase evaporates from the HPTLC plate before the application of cells or enzymes, thereby improving their effectiveness [4] as cell viability and enzyme inhibitory assays can be affected by organic solvents present in the mobile phase. A combination of HPTLC with bioassays enables the effect-directed, high-throughput screening and bioprofiling of complex botanical extracts. The advantage of HPTLC is that plates/chromatograms can be directly immersed into the enzyme solution (bioassays), incubated for up to several hours, followed by visualization of the (bio)activity profile via an enzyme–substrate reaction.

Table 1.
Effect-directed analysis of plant material.
In contrast to HPTLC, high-performance liquid chromatography (HPLC), while allowing better separation of analytes in mixture, works with solvents that are either toxic to living cells or may inactivate enzymes. The hyphenation of HPLC with bioassays requires the collection of fractions into microtiter plates with the addition of an appropriate bioassay solution [5,6]. A HPLC method has been online hyphenated to a chemical assay (i.e., a DPPH free radical scavenging assay) [7,8], and even to enzymic assays (i.e., a cholinesterase inhibitory assay) [9,10]. In regard to bacterial cell assays, only Aliivibrio fischeri bacteria cells have been introduced directly to HPLC effluent [11], although the cell death increased with the amount of methanol in the eluent. Hyphenation of these methods using HPLC leads to the dilution of analytes with reagents and the use of long reaction coils resulting in extra peak broadening [9]. Moreover, the correct activity of an enzyme in the suboptimal environment of HPLC analysis is also questionable.
5. Hyphenation with Spectroscopic Methods of Identification
The hyphenation of (HP)TLC with spectroscopic and spectrometric techniques makes the characterization of bioactive substances possible. HPTLC combined with bioassays and linked to spectroscopic methods of identification, allows the quantitative detection of bioactive compounds (as bands) in samples and their further chemical characterization by mass spectrometry or other spectroscopic methods (Figure 1). Thus, highly efficient non-targeted screening with bioassays can be used to detect modes of action followed by the targeted chemical identification of newly discovered bioactive compounds. Non-targeted analysis is superior to target analysis, if the whole sample (botanical extract) is of interest.
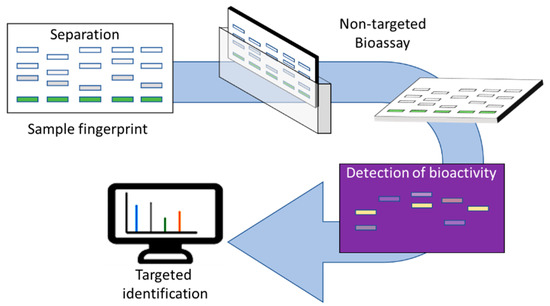
Figure 1.
Overview of the HPTLC-EDA approach.
A broad range of bacterial and fungal strains have been investigated for biological activity using appropriate detection methods with planar chromatography. This is referred to as bioautography [12]. Several HPTLC-enzyme inhibition assays have been developed. For example, α-amylase [13], cholinesterase [14] and glucosidase enzymic inhibition [15] methods have been reported. Hyphenation with mass spectrometry (MS) and, more recently, with nuclear magnetic resonance (NMR) has enabled the rapid characterization of the separated compounds. In the past, unknown substances were scraped off from the plate, eluted into a vial and transferred into a MS. Now elution-based TLC-MS interfaces can be used to extract zones of interest. The manually operated TLC-MS interface is equipped with an elution head, where pump-driven elution solvent directs the eluted zone directly online into an MS or HPLC-MS, or into a vial for the off line structural identification of the organic compound using either HPLC-MS/MS, gas chromatography-MS or NMR [16]. Thus, structure identification methods can be run at the analytical level directly from the detected bioactive zone of interest on the chromatogram. The advantage of this new strategy is that only bioactive components are isolated and characterized.
6. Conclusions
The investigation of plant extracts using these rapid, hyphenated, bioanalytical tools can significantly increase the rate of identification and characterization of bioactive compounds in plant extracts (bioprofiling). This approach is more cost effective, enabling a more streamlined method for identifying compounds in natural products that are suitable candidates for further investigation as potential new drug molecules. More broadly, drug discovery or the analysis of traditional medicinal plants significantly benefits from the effect-directed profiling of samples.
Author Contributions
S.A.-K. Conceptualized and wrote the original draft of the manuscript. D.W.M. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ebrahimi-Najafabadi, H.; Kazemeini, S.S.; Pasdaran, A.; Hamedi, A. A novel similarity search approach for high-performance thin-layer chromatography (HPTLC) fingerprinting of medicinal plants. Phytochem. Anal. 2019, 30, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.; Jesionek, W. Effects-Directed Biological Detection. In Instrumental Thin-Layer Chromatography; Poole, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 279–312. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Ott, P.G.; Yüce, I.; Darcsi, A.; Béni, S.; Morlock, G.E. Effect-directed analysis via hyphenated high-performance thin-layer chromatography for bioanalytical profiling of sunflower leaves. J. Chromatogr. A 2018, 1533, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Skogberg, A.; Maki, A.J.; Mettanen, M.; Lahtinen, P.; Kallio, P. Cellulose nanofiber alignment using evaporation-induced droplet-casting, and cell alignment on aligned nanocellulose surfaces. Biomacromolecules 2017, 18, 3936–3953. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Lagana, G.; Leuzzi, U.; Bellocco, E. C- and O-glycosyl flavonoids in Sanguinello and Tarocco blood orange (Citrus sinensis (L.) Osbeck) juice: Identification and influence on antioxidant properties and acetylcholinesterase activity. Food Chem. 2016, 196, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Riethmuller, E.; Konczol, A.; Szakal, D.; Vegh, K.; Balogh, G.T.; Kery, A. HPLC-DPPH screening method for evaluation of antioxidant compounds in Corylus species. Nat. Prod. Commun. 2016, 11, 641–644. [Google Scholar] [PubMed]
- Bandonienė, D.; Murkovic, M. On-line HPLC-DPPH screening method for evaluation of radical scavenging phenols extracted from apples (Malus domestica L.). J. Agric. Food Chem. 2002, 50, 2482–2487. [Google Scholar] [CrossRef]
- Fabel, S.; Niessner, R.; Weller, M.G. Effect-directed analysis by high-performance liquid chromatography with gas-segmented enzyme inhibition. J. Chromatogr. A 2005, 1099, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, H.; Fu, Q.; An, H.; Liang, Y.; Zhang, B.; Hashi, Y.; Chen, S. Simultaneous separation, identification and activity evaluation of three butyrylcholinesterase inhibitors from Plumula nelumbinis using on-line HPLC-UV coupled with ESI-IT-TOF-MS and BChE biochemical detection. Talanta 2013, 110, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Stolper, P.; Fabel, S.; Weller, M.G.; Knopp, D.; Niessner, R.J.A.; Chemistry, B. Whole-cell luminescence-based flow-through biodetector for toxicity testing. Anal. Bioanal. Chem. 2008, 390, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.; Grzelak, E. Bioautography detection in thin-layer chromatography. J. Chromatogr. A 2010, 1218, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Morton, D.W. High-performance thin-layer chromatography-direct bioautography as a method of choice for alpha-amylase and antioxidant activity evaluation in marine algae. J. Chromatogr. A 2017, 1530, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Simoes-Pires, C.A.; Hmicha, B.; Marston, A.; Hostettmann, K. A TLC bioautographic method for the detection of alpha- and beta-glucosidase inhibitors in plant extracts. Phytochem. Anal. 2009, 20, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Azadniya, E.; Morlock, G.E. Bioprofiling of Salvia miltiorrhiza via planar chromatography linked to (bio)assays, high resolution mass spectrometry and nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2018, 1533, 180–192. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).