Time-Gated Single-Photon Detection in Time-Domain Diffuse Optics: A Review
Abstract
1. Introduction
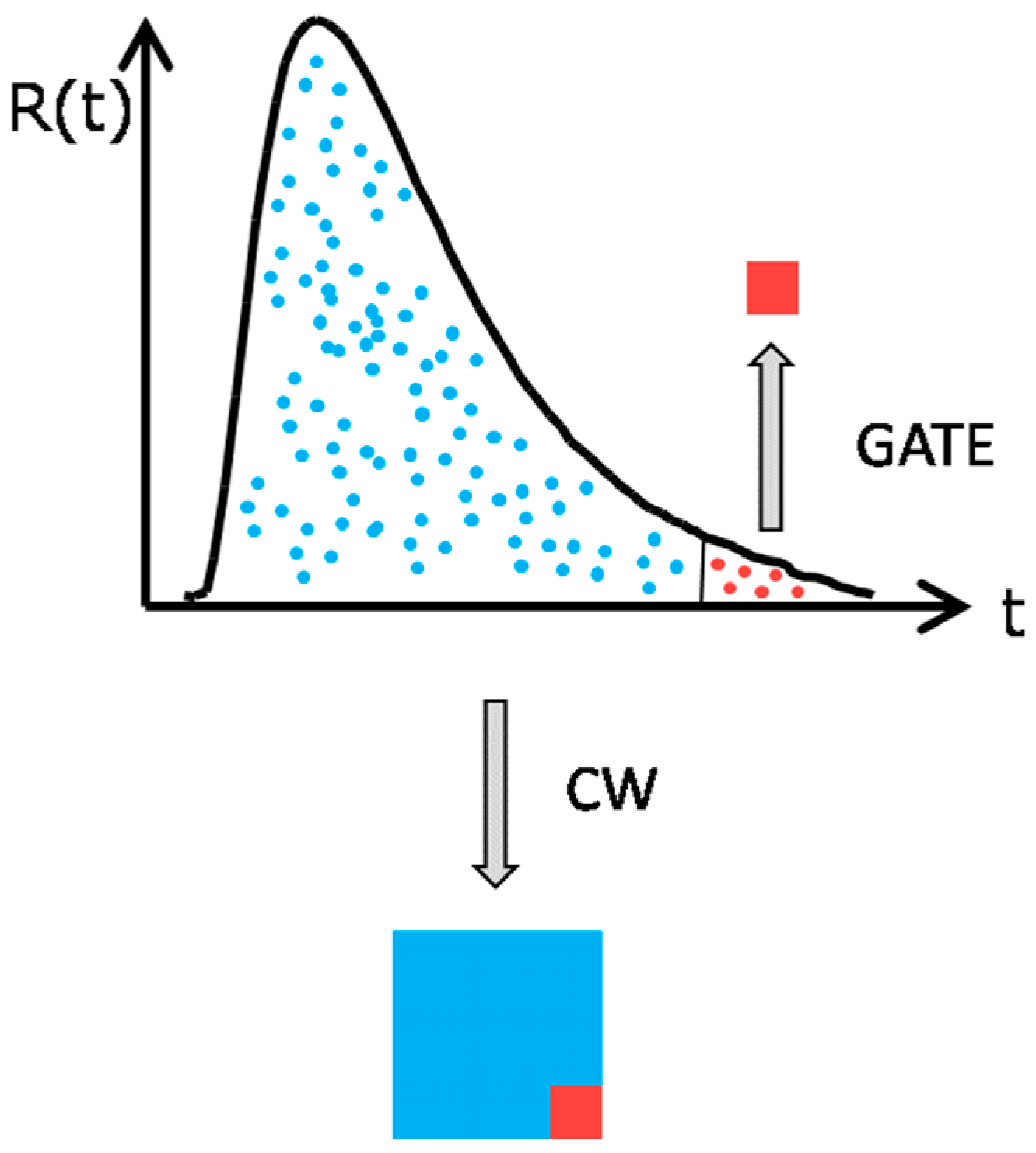
2. Physical Concepts
3. Technology
3.1. Advantages of Single-Photon Avalanche Diodes (SPADs) for TG Measurements
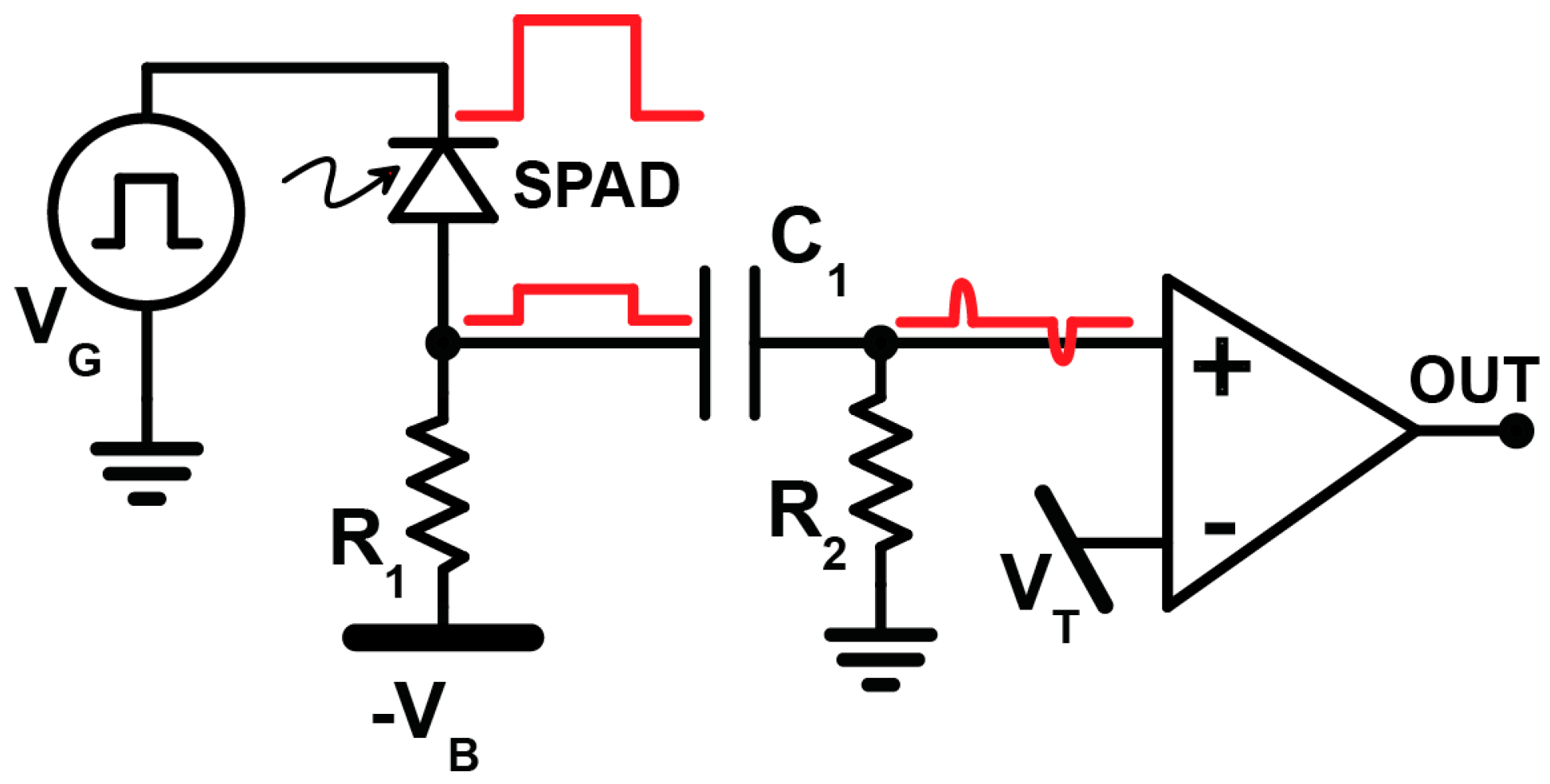
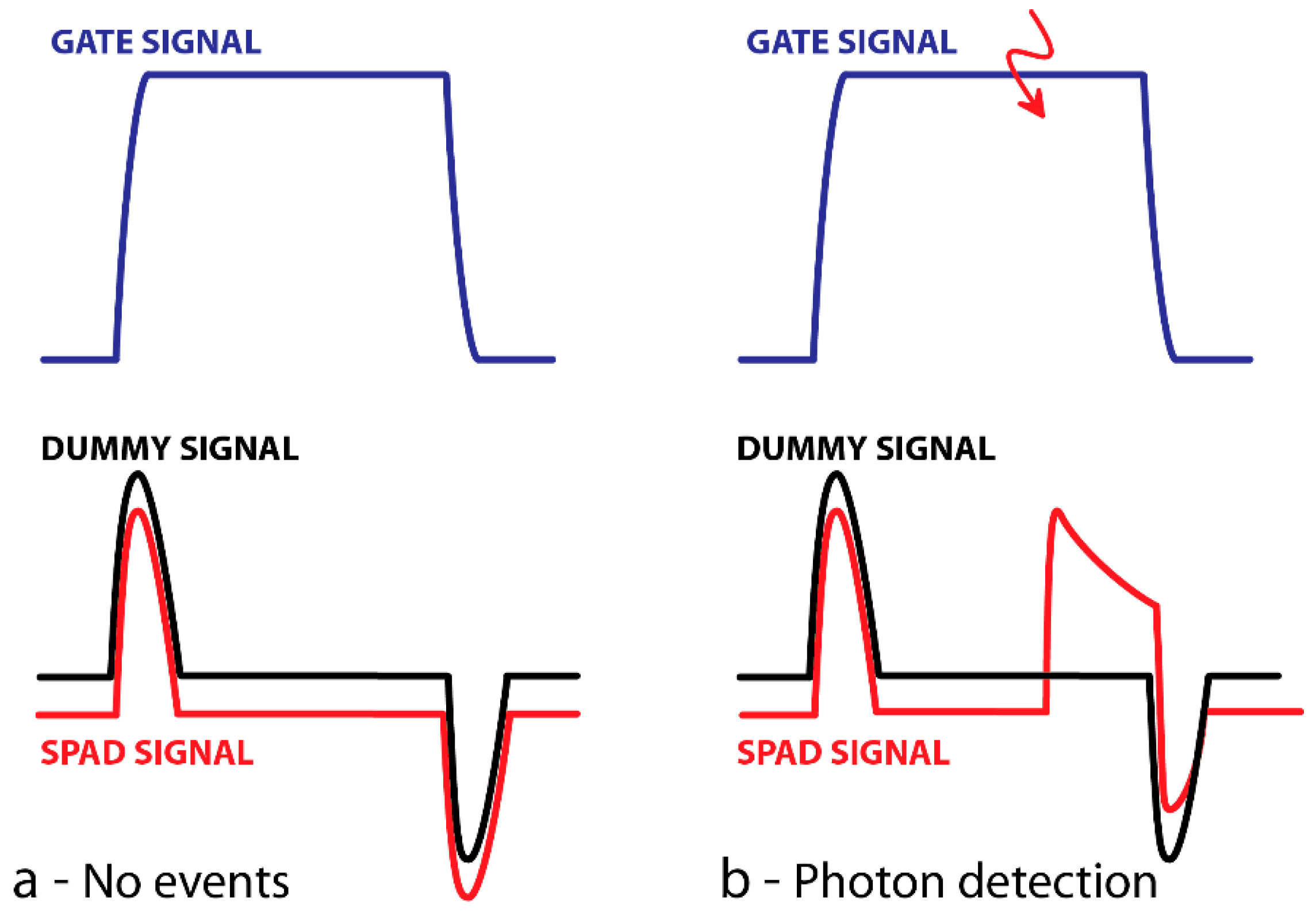
3.2. Electronics Issues for Time-Gated (TG) Operations
3.3. The Path towards First TG-DO Measurements
3.4. General Performances of First TG Prototypes
3.5. Other Solutions for SPADs Time-Gating
3.6. State of the Art
3.7. Current Research Challenges
3.7.1. Detector Active Area
3.7.2. Detector Response Tail
3.7.3. Detector Memory Effect
3.7.4. Non-Time Invariant Detector Response
3.7.5. Impact of the Overall Detector Response Shape
4. Applications
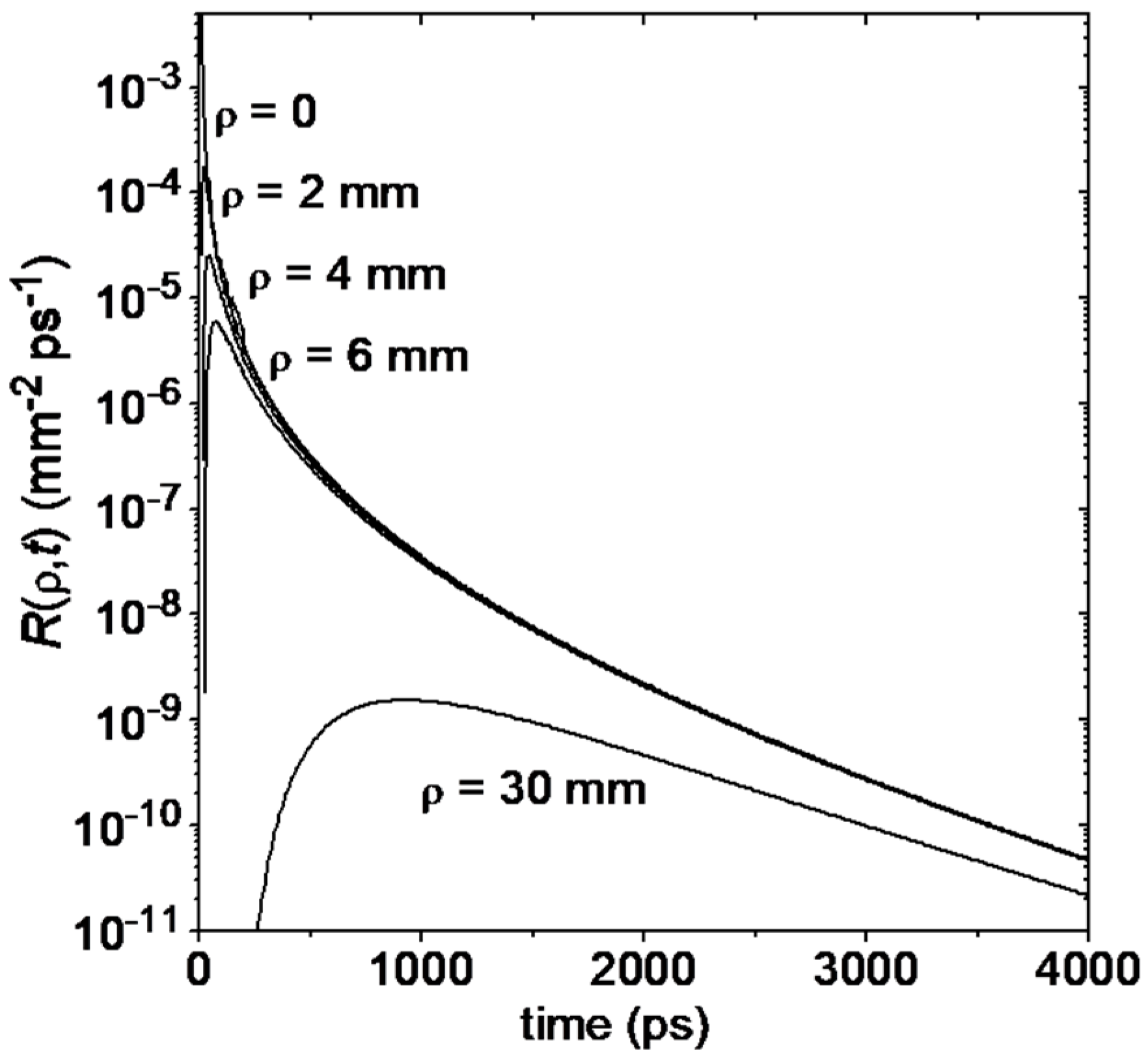
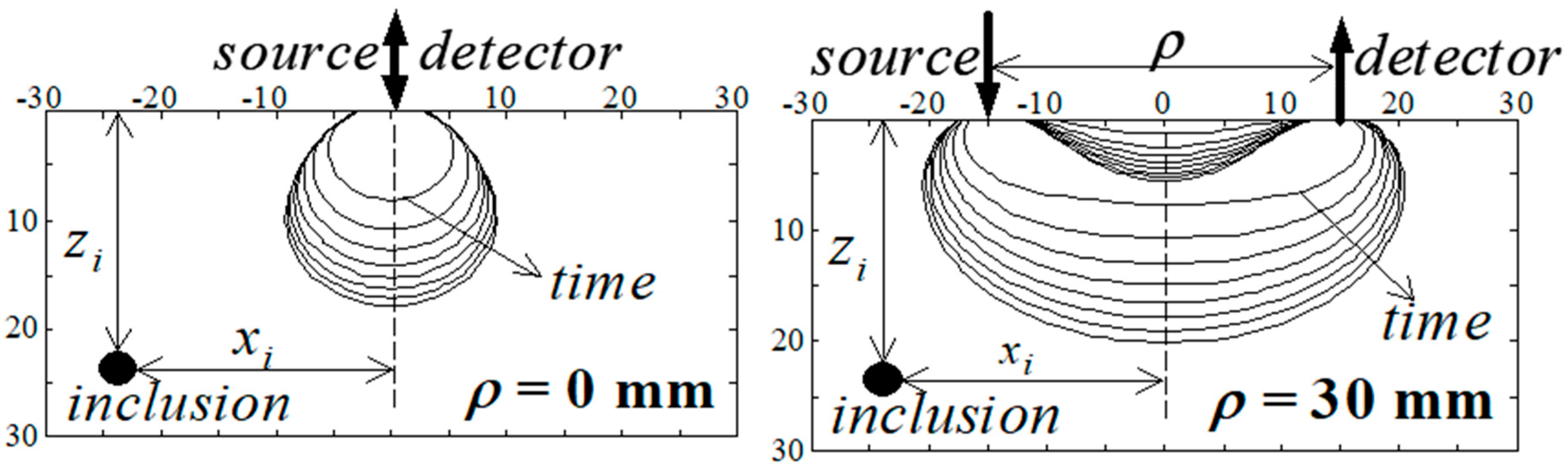
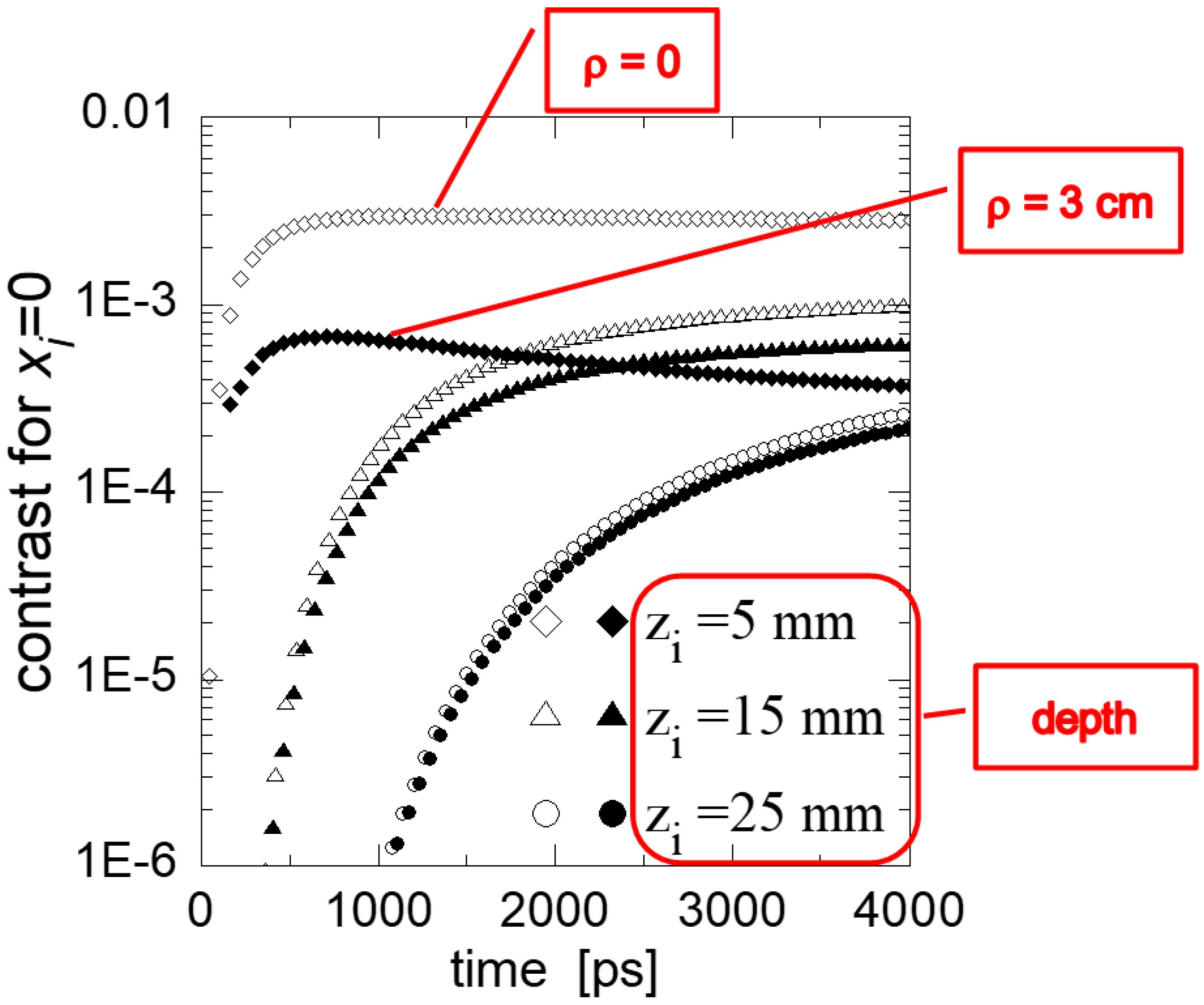
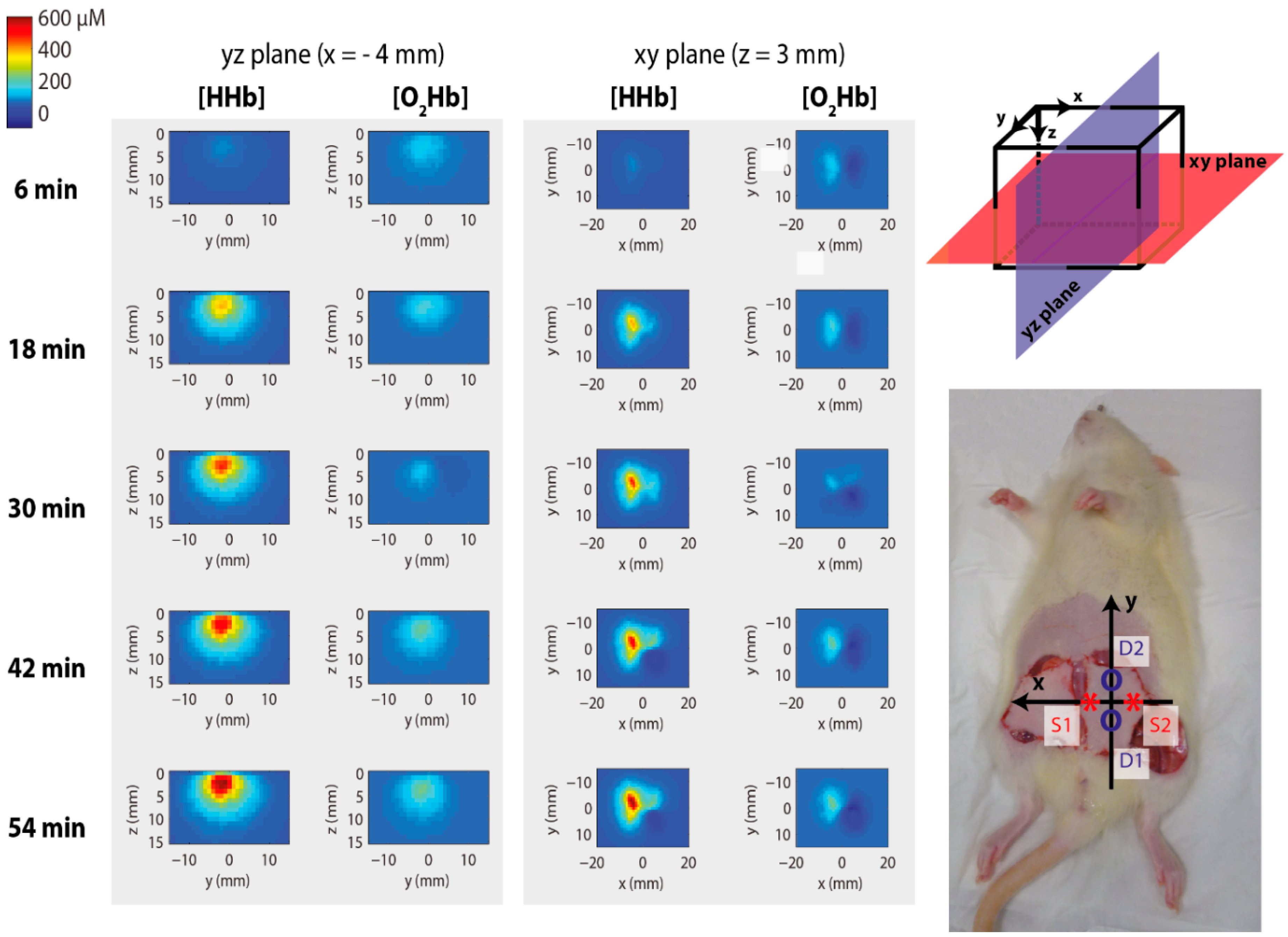
4.1. Functional Imaging
4.2. Non-Contact Imaging
4.3. Single Fiber Spectroscopy
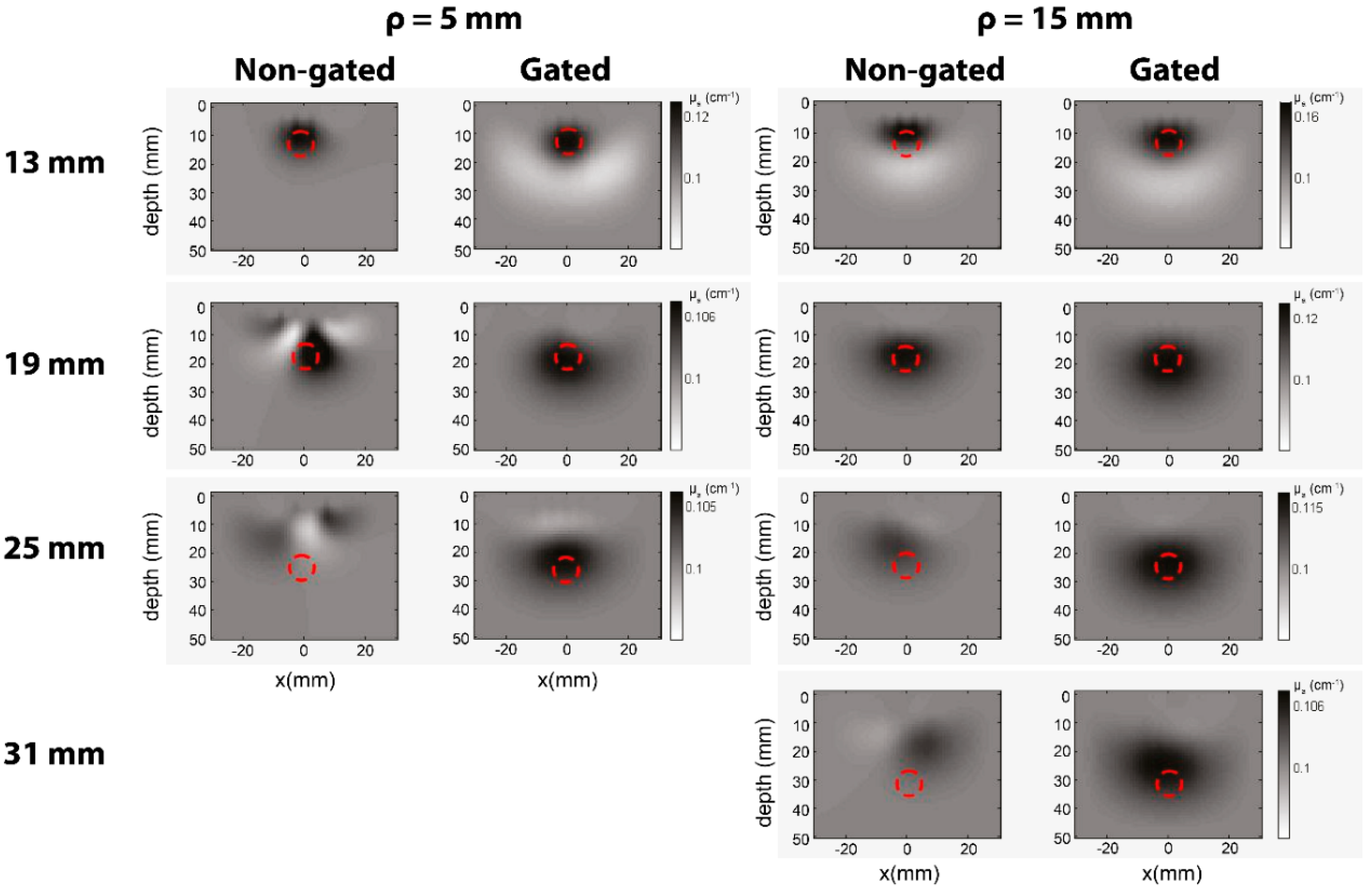
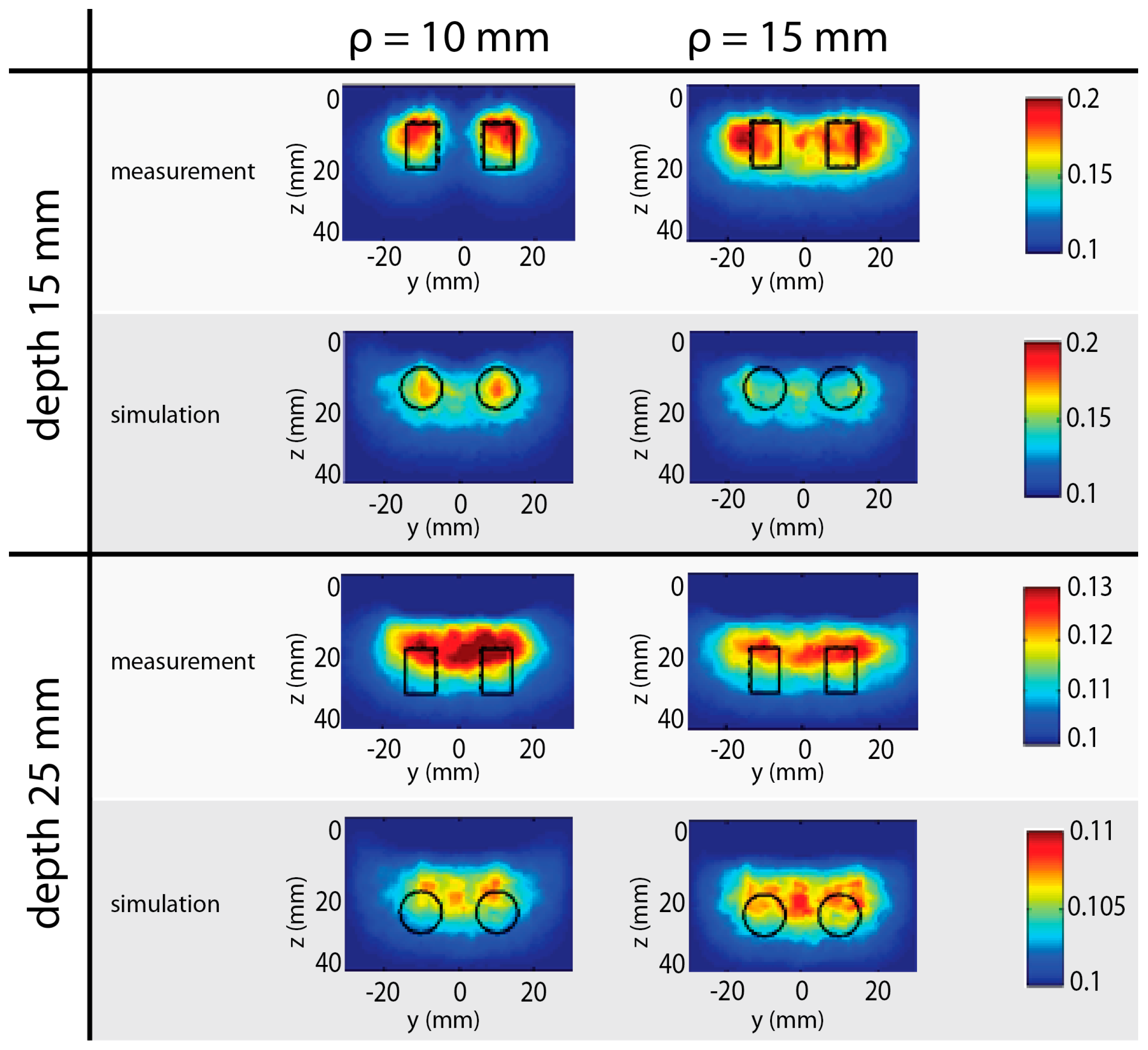
4.4. Tomography
4.5. Time Domain Diffuse Correlation Spectroscopy
4.6. Optical Spectroscopy beyond 1000 nm
4.7. Other Applications
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, A.P.; Hebden, J.C.; Arridge, S.R. Recent advances in diffuse optical imaging. Phys. Med. Biol. 2005, 50, R1–R43. [Google Scholar] [CrossRef]
- Martelli, F.; Del Bianco, S.; Ismaelli, A.; Zaccanti, G. Light Propagation Through Biological Tissue; SPIE: Bellingham, WA, USA, 2010. [Google Scholar] [CrossRef]
- Zude, M. Optical Monitoring of Fresh and Processed Agricultural Crops; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Kienle, A.; D’Andrea, C.; Foschum, F.; Taroni, P.; Pifferi, A. Light propagation in dry and wet softwood. Opt. Express 2008, 16, 9895–9906. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, H.O.; Iriarte, D.I.; Pomarico, J.A.; Ranea Sandoval, H.F.; Macdonald, R.; Voigt, J. Determination of optical properties of slices of turbid media by diffuse CW laser light scattering profilometry. J. Quant. Spectrosc. Radiat. Transf. 2007, 105, 68–83. [Google Scholar] [CrossRef]
- Johansson, J.; Folestad, S.; Josefson, M.; Sparén, A.; Abrahamsson, C.; Andersson-Engels, S.; Svanberg, S. Time-resolved NIR/Vis spectroscopy for analysis of solids: Pharmaceutical tablets. Appl. Spectrosc. 2002, 56, 725–731. [Google Scholar] [CrossRef]
- McCartney, E.J. Optics of the Atmosphere: Scattering by Molecules and Particles; John Wiley and Sons, Inc.: New York, NY, USA, 1976; p. 421. [Google Scholar] [CrossRef]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse optics for tissue monitoring and tomography. Reports Prog. Phys. 2010, 73, 076701. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Optical properties of skin, subcutaneous, and muscle tissues: A review. J. Innov. Opt. Health Sci. 2011, 4, 9–38. [Google Scholar] [CrossRef]
- Torricelli, A.; Contini, D.; Dalla Mora, A.; Pifferi, A.; Re, R.; Zucchelli, L.; Caffini, M.; Farina, A.; Spinelli, L. Neurophotonics: Non-invasive optical techniques for monitoring brain functions. Funct. Neurol. 2014, 29, 223–230. [Google Scholar] [CrossRef]
- Hoshi, Y.; Yamada, Y. Overview of diffuse optical tomography and its clinical applications. J. Biomed. Opt. 2016, 21, 091312. [Google Scholar] [CrossRef]
- Lange, F.; Tachtsidis, I. Clinical brain monitoring with time domain NIRS: A review and future perspectives. Appl. Sci. 2019, 9, 1612. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Mesquita, R.C.; Durduran, T.; Yu, G.; Buckley, E.M.; Kim, M.N.; Zhou, C.; Choe, R.; Sunar, U.; Yodh, A.G. Direct measurement of tissue blood flow and metabolism with diffuse optics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4390–4406. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Suzuki, H.; Yamashita, Y. Time-domain near-infrared spectroscopy and imaging: A review. Appl. Sci. 2019, 9, 1127. [Google Scholar] [CrossRef]
- Torricelli, A.; Contini, D.; Pifferi, A.; Caffini, M.; Re, R.; Zucchelli, L.; Spinelli, L. Time domain functional NIRS imaging for human brain mapping. Neuroimage 2014, 85, 28–50. [Google Scholar] [CrossRef]
- Barstow, T.J. Understanding near infrared spectroscopy (NIRS) and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Grosenick, D.; Rinneberg, H.; Cubeddu, R.; Taroni, P. Review of optical breast imaging and spectroscopy. J. Biomed. Opt. 2016, 21, 091311. [Google Scholar] [CrossRef]
- Pifferi, A.; Torricelli, A.; Taroni, P.; Bassi, A.; Chikoidze, E.; Giambattistelli, E.; Cubeddu, R. Optical biopsy of bone tissue: A step toward the diagnosis of bone pathologies. J. Biomed. Opt. 2004, 9, 474–480. [Google Scholar] [CrossRef]
- Van Veen, R.L.; Sterenborg, H.J.; Pifferi, A.; Torricelli, A.; Chikoidze, E.; Cubeddu, R. Determination of visible near-IR absorption coefficients of mammalian fat using time- and spatially resolved diffuse reflectance and transmission spectroscopy. J. Biomed. Opt. 2005, 10, 054004. [Google Scholar] [CrossRef]
- Taroni, P.; Comelli, D.; Pifferi, A.; Torricelli, A.; Cubeddu, R. Absorption of collagen: Effects on the estimate of breast composition and related diagnostic implications. J. Biomed. Opt. 2007, 12, 014021. [Google Scholar] [CrossRef]
- Torricelli, A.; Contini, D.; Dalla Mora, A.; Martinenghi, E.; Tambornini, D.; Villa, F.; Tosi, A.; Spinelli, L. Recent advances in time-resolved NIR spectroscopy for nondestructive assessment of fruit quality. Chem. Eng. 2015, 44, 43–48. [Google Scholar] [CrossRef]
- Bargigia, I.; Nevin, A.; Farina, A.; Pifferi, A.; D’Andrea, C.; Karlsson, M.; Lundin, P.; Somesfalean, G.; Svanberg, S. Diffuse optical techniques applied to wood characterisation. J. Near Infrared Spectrosc. 2013, 21, 259–268. [Google Scholar] [CrossRef]
- Kamran, F.; Abildgaard, O.H.A.; Sparén, A.; Svensson, O.; Johansson, J.; Andersson-Engels, S.; Andersen, P.E.; Khoptyar, D. Transmission near-infrared (NIR) and photon time-of-flight (PTOF) spectroscopy in a comparative analysis of pharmaceuticals. Appl. Spectrosc. 2015, 69, 389–397. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, C.; Obraztsova, E.A.; Farina, A.; Taroni, P.; Lanzani, G.; Pifferi, A. Absorption spectroscopy of powdered materials using time-resolved diffuse optical methods. Appl. Opt. 2012, 51, 7858–7863. [Google Scholar] [CrossRef] [PubMed]
- Bérubé-Lauzière, Y.; Crotti, M.; Boucher, S.; Ettehadi, S.; Pichette, J.; Rech, I. Prospects on time-domain diffuse optical tomography based on time-correlated single photon counting for small animal imaging. J. Spectrosc. 2016, 2016, 1947613. [Google Scholar] [CrossRef]
- Steinbrink, J.; Wabnitz, H.; Obrig, H.; Villringer, A.; Rinneberg, H. Determining changes in NIR absorption using a layered model of the human head. Phys. Med. Biol. 2001, 46, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Selb, J.; Joseph, D.K.; Boas, D.A. Time-gated optical system for depth-resolved functional brain imaging. J. Biomed. Opt. 2006, 11, 044008. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Contini, D.; Zucchelli, L.; Torricelli, A.; Spinelli, L. Effect of a thin superficial layer on the estimate of hemodynamic changes in a two-layer medium by time domain NIRS. Biomed. Opt. Express 2016, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Gerega, A.; Milej, D.; Weigl, W.; Kacprzak, M.; Liebert, A. Multiwavelength time-resolved near-infrared spectroscopy of the adult head: Assessment of intracerebral and extracerebral absorption changes. Biomed. Opt. Express 2018, 9, 2974. [Google Scholar] [CrossRef]
- Dalla Mora, A.; Tosi, A.; Zappa, F.; Cova, S.; Contini, D.; Pifferi, A.; Spinelli, L.; Torricelli, A.; Cubeddu, R. Fast-gated single-photon avalanche diode for wide dynamic range near infrared spectroscopy. IEEE J. Sel. Top. Quant. 2010, 16, 1023–1030. [Google Scholar] [CrossRef]
- Hamamatsu Photonics K.K. Photomultiplier Tubes, 4th ed.; Hamamatsu Photonics K.K.: Hamamatsu, Japan, 2017. [Google Scholar]
- Dolgoshein, B.; Balagura, V.; Buzhan, P.; Danilov, M.; Filatov, L.; Garutti, E.; Groll, M.; Ilyin, A.; Kantserov, V.; Kaplin, V.; et al. Status report on silicon photomultiplier development and its applications. Nucl. Instruments Methods Phys. Res. Sec. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 563, 368–376. [Google Scholar] [CrossRef]
- Sawosz, P.; Zolek, N.; Kacprzak, M.; Maniewski, R.; Liebert, A. Application of time-gated CCD camera with image intensifier in contactless detection of absorbing inclusions buried in optically turbid medium which mimics local changes in oxygenation of the brain tissue. Opto-Electronics Rev. 2012, 20, 309–314. [Google Scholar] [CrossRef]
- Wiza, J. Microchannel plate detectors. Nucl. Intrum. Meth. 1979, 162, 587–601. [Google Scholar] [CrossRef]
- Hamamatsu Photonics K.K. Guide to Streak Cameras; Hamamatsu Photonics K.K.: Hamamatsu, Japan, 2008. Available online: www.hamamatsu.com/resources/pdf/sys/SHSS0006E_STREAK.pdf (accessed on 20 August 2019).
- Cova, S.; Ghioni, M.; Lacaita, A.; Samori, C.; Zappa, F. Avalanche photodiodes and quenching circuits for single-photon detection. Appl. Opt. 1996, 35, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.; Pifferi, A.; Spinelli, L.; Cubeddu, R.; Martelli, F.; Del Bianco, S.; Zaccanti, G. Time-resolved reflectance at null source-detector separation: Improving contrast and resolution in diffuse optical imaging. Phys. Rev. Lett. 2005, 95, 078101. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, A.; Contini, D.; Dalla Mora, A.; Farina, A.; Spinelli, L.; Torricelli, A. New frontiers in time-domain diffuse optics, a review. J. Biomed. Opt. 2016, 21, 046011. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. Advanced Time-Correlated Single Photon Counting Techniques; Springer: New York, NY, USA, 2005. [Google Scholar]
- Scano, A.; Zanoletti, M.; Pirovano, I.; Spinelli, L.; Contini, D.; Torricelli, A.; Re, R. NIRS-EMG for clinical applications: A systematic review. Appl. Sci. 2019, 9, 2952. [Google Scholar] [CrossRef]
- Grassi, B.; Quaresima, V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A critical review of consumer wearables, mobile applications, and equipment for providing biofeedback, monitoring stress, and sleep in physically active populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Gómez, J.; Oviedo, B.; Zhuma, E. Patient monitoring system based on internet of things. Procedia Comput. Sci. 2016, 83, 90–97. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, L.; Zhang, S.; Kanhere, S.; Sheng, M.; Liu, Y. Internet of things meets brain-computer interface: A unified deep learning framework for enabling human-thing cognitive interactivity. IEEE Internet Things J. 2019, 6, 2084–2092. [Google Scholar] [CrossRef]
- Martini, M.L.; Oermann, E.K.; Opie, N.L.; Panov, F.; Oxley, T.; Yaeger, K. Sensor modalities for brain-computer interface technology: A comprehensive literature review. Neurosurgery 2020, 86, E108–E117. [Google Scholar] [CrossRef]
- Meyerding, S.G.H.; Mehlhose, C.M. Can neuromarketing add value to the traditional marketing research? An exemplary experiment with functional near-infrared spectroscopy (fNIRS). J. Bus. Res. 2020, 107, 172–185. [Google Scholar] [CrossRef]
- Riva, G.; Wiederhold, B.K.; Mantovani, F. Neuroscience of virtual reality: From virtual exposure to embodied medicine. Cyberpsychol. Behav. Soc. Netw. 2019, 22, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, A. Wave Propagation and Scattering in Random Media; Academic Press: New York, NY, USA, 1978; Volume 2, pp. 336–393. [Google Scholar] [CrossRef]
- Tsuchiya, Y. Photon path distribution and optical responses of turbid media: Theoretical analysis based on the microscopic Beer-Lambert law. Phys. Med. Biol. 2001, 46, 2067–2084. [Google Scholar] [CrossRef] [PubMed]
- Sassaroli, A.; Fantini, S. Comment on the modified Beer–Lambert law for scattering media. Phys. Med. Biol. 2004, 49, N255–N257. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical properties. Appl. Optics 1989, 28, 2331–2336. [Google Scholar] [CrossRef]
- Jacques, S.L. Time resolved propagation of ultrashort laser pulses within turbid tissues. Appl. Optics 1989, 12, 2223–2229. [Google Scholar] [CrossRef]
- Madsen, S.J.; Wilson, B.C.; Patterson, M.S.; Park, Y.D.; Jacques, S.L.; Hefetz, Y. Experimental tests of a simple diffusion model for the estimation of scattering and absorption coefficients of turbid media from time-resolved diffuse reflectance measurements. Appl. Optics 1992, 31, 3509–3517. [Google Scholar] [CrossRef]
- Ferrari, M.; Wei, Q.; Carraresi, L.; De Blasi, R.A.; Zaccanti, G. Time-resolved spectroscopy of the human forearm. J. Photoch. Photobiol. B 1992, 16, 141–153. [Google Scholar] [CrossRef]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Ruiz-Altisent, M.; Valero, C.; Ortiz, C.; Dover, C.; et al. Time-resolved reflectance spectroscopy applied to the nondestructive monitoring of the internal optical properties in apples. Appl. Spectrosc. 2001, 55, 1368–1374. [Google Scholar] [CrossRef]
- Del Bianco, S.; Martelli, F.; Zaccanti, G. Penetration depth of light re-emitted by a diffusive medium: Theoretical and experimental investigation. Phys. Med. Biol. 2002, 47, 4131–4144. [Google Scholar] [CrossRef]
- Selb, J.J.; Stott, J.J.; Franceschini, M.A.; Sorensen, A.G.; Boas, D.A. Improved sensitivity to cerebral hemodynamics during brain activation with a time-gated optical system: Analytical model and experimental validation. J. Biomed. Opt. 2005, 10, 011013. [Google Scholar] [CrossRef] [PubMed]
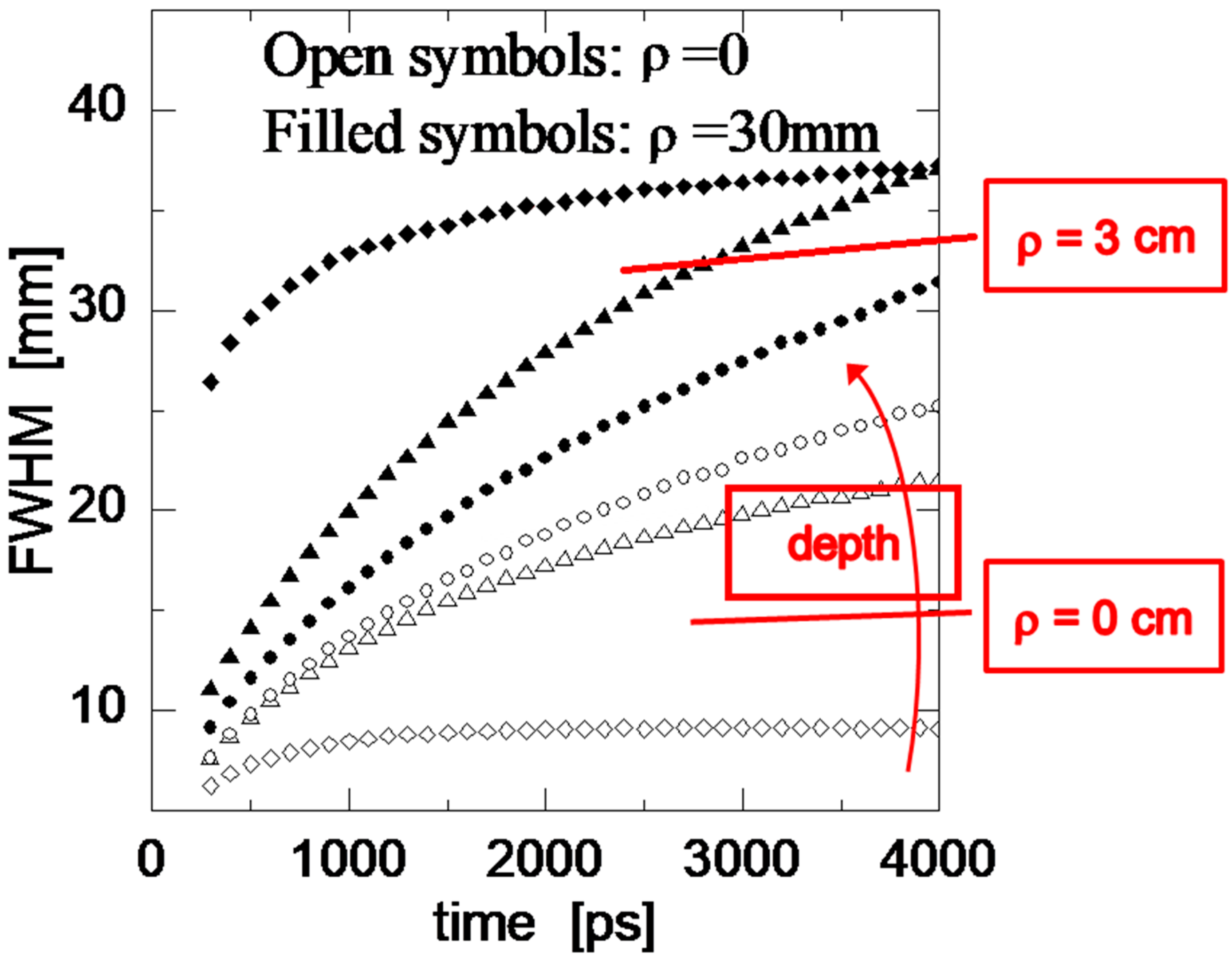
- Martelli, F.; Binzoni, T.; Pifferi, A.; Spinelli, L.; Farina, A.; Torricelli, A. There’s plenty of light at the bottom: Statistics of photon penetration depth in random media. Sci. Rep. 2016, 6, 27057. [Google Scholar] [CrossRef] [PubMed]
- Wabnitz, H.; Jelzow, A.; Mazurenka, M.; Steinkellner, O.; Macdonald, R.; Milej, D.; Zolek, N.; Kacprzak, M.; Sawosz, P.; Maniewski, R.; et al. Performance assessment of time-domain optical brain imagers, part 2: nEUROPt protocol. J. Biomed. Opt. 2014, 19, 86012. [Google Scholar] [CrossRef]
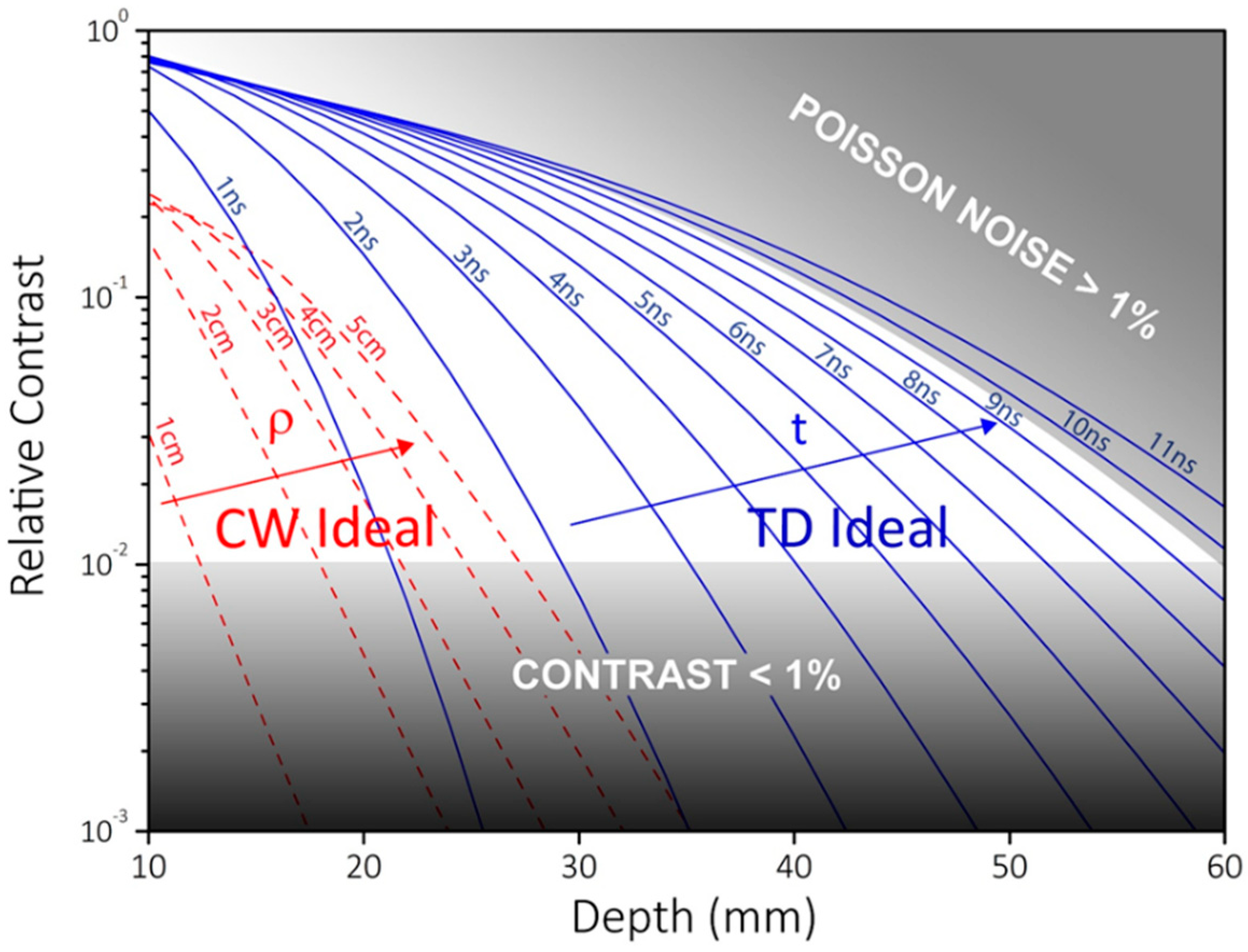
- Dalla Mora, A.; Contini, D.; Arridge, S.; Martelli, F.; Tosi, A.; Boso, G.; Farina, A.; Durduran, T.; Martinenghi, E.; Torricelli, A.; et al. Towards next-generation time-domain diffuse optics for extreme depth penetration and sensitivity. Opt. Express 2015, 6, 1749–1760. [Google Scholar] [CrossRef]
- Becker, W. The bh TCSPC Handbook, 7th ed.; Becker & Hickl GmbH: Berlin, Germany, 2017; Available online: www.becker-hickl.com/wp-content/uploads/2019/01/hb-bh-TCSPC.pdf (accessed on 20 August 2019).
- Jacoby, B.A.; Kotecki, D.E.; Lear, R.D. Direct gating of microchannel plates. IEEE T. Nucl. Sci. 1983, 30, 4624–4627. [Google Scholar] [CrossRef]
- Zipfl, P.; Schneckenburger, H.; Schoch, L. Fast photomultiplier tube gating technique for time-resolved fluorescence measurements. Proc. SPIE 1999, 3600, 158–164. [Google Scholar] [CrossRef]
- Milnes, J.S.; Horsfield, C.J.; Rubery, M.S.; Glebov, V.Y.; Herrmann, H.W. Ultra-high speed photomultiplier tubes with nanosecond gating for fusion diagnostics. Rev. Sci. Instrum. 2012, 83, 10D301. [Google Scholar] [CrossRef]
- Zappa, F.; Tosi, A.; Dalla Mora, A.; Tisa, S. SPICE modeling of single photon avalanche diodes. Sensor. Actuat. A Phys. 2009, 153, 197–204. [Google Scholar] [CrossRef]
- Dalla Mora, A.; Tosi, A.; Tisa, S.; Zappa, F. Single-photon avalanche diode model for circuit simulations. IEEE Photonic. Tech. L. 2007, 19, 1922–1924. [Google Scholar] [CrossRef]
- Tosi, A.; Dalla Mora, A.; Zappa, F.; Cova, S. Single-photon avalanche diodes for the near-infrared range: Detector and circuit issues. J. Mod. Optic. 2009, 56, 299–308. [Google Scholar] [CrossRef]
- Tosi, A.; Acerbi, F.; Dalla Mora, A.; Itzler, M.A.; Jiang, X. Active area uniformity of InGaAs/InP single-photon avalanche diodes. IEEE Photonics J. 2011, 3, 5671447. [Google Scholar] [CrossRef]
- Bethune, D.S.; Risk, W.P. An autocompensating fiber-optic quantum cryptography system based on polarization splitting of light. IEEE J. Quantum Electron. 2000, 36, 340–347. [Google Scholar] [CrossRef]
- Bethune, D.S.; Devoe, R.G.; Kurtsiefer, C.; Retterner, C.T.; Risk, W.P. System for Gated Detection of Optical Pulses Containing a Small Number of Photons Using an Avalanche Photodiode. US Patent US6218657B1, 17 April 2001. [Google Scholar]
- Bethune, D.S.; Risk, W.P.; Pabst, G.W. A high-performance integrated single-photon detector for telecom wavelengths. J. Mod. Opt. 2004, 51, 1359–1368. [Google Scholar] [CrossRef][Green Version]
- Tomita, A.; Nakamura, K. Balanced gated-mode photon detector for quantum-bit discrimination at 1550 nm. Opt. Lett. 2002, 27, 1827–1829. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Kaji, R.; Tsuchida, H. Gated-mode single-photon detection at 1550 nm by discharge pulse counting. Appl. Phys. Lett. 2004, 84, 3606–3608. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, G.; Zeng, H.P. Multigate single-photon detection and timing discrimination with an InGaAs/InP avalanche photodiode. Appl. Opt. 2006, 45, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhou, P.; Liu, X.; Wang, J.; Liao, C.; Guo, J.; Liang, R.; Liu, S. An integral gated mode single photon detector at telecom wavelengths. J. Phys. D Appl. Phys. 2007, 40, 6922–6925. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Kardynal, B.E.; Sharpe, A.W.; Shields, A.J. High speed single photon detection in the near infrared. Appl. Phys. Lett. 2007, 91, 041114. [Google Scholar] [CrossRef]
- Pifferi, A.; Torricelli, A.; Spinelli, L.; Contini, D.; Cubeddu, R.; Martelli, F.; Zaccanti, G.; Tosi, A.; Dalla Mora, A.; Zappa, F.; et al. Time-resolved diffuse reflectance using small source-detector separation and fast single-photon gating. Phys. Rev. Lett. 2008, 100, 138101. [Google Scholar] [CrossRef]
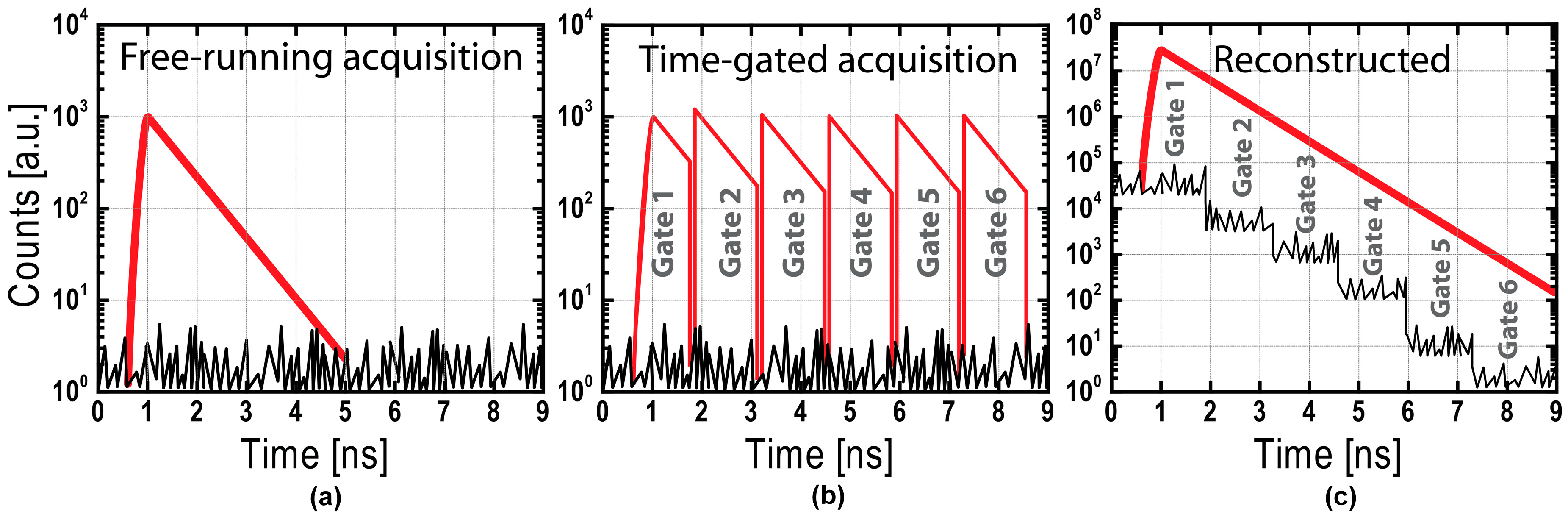
- Tosi, A.; Dalla Mora, A.; Zappa, F.; Gulinatti, A.; Contini, D.; Pifferi, A.; Spinelli, L.; Torricelli, A.; Cubeddu, R. Fast-gated single-photon counting technique widens dynamic range and speeds up acquisition time in time-resolved measurements. Opt. Express 2011, 19, 10735–10746. [Google Scholar] [CrossRef]
- Cominelli, A.; Acconcia, G.; Labanca, I.; Ghioni, M.; Rech, I. Accurate non-invasive measurement of the turn-on transition of fast gated single photon avalanche diodes. Rev. Sci. Instrum. 2019, 90, 033102. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zang, K.; Zheng, T.; Fei, Y.; Huang, M.; Liu, X.; Wang, Y.; Jin, G.; Huo, Y.; Harris, J.S.; et al. Improving characterization capabilities in new single-photon avalanche diode research. Rev. Sci. Instrum. 2019, 90, 043108. [Google Scholar] [CrossRef]
- Portaluppi, D.; Conca, E.; Villa, F. 32 × 32 CMOS SPAD imager for gated imaging, photon timing, and photon coincidence. IEEE J. Quantum Electron. 2018, 24, 8047323. [Google Scholar] [CrossRef]
- Ulku, A.C.; Bruschini, C.; Antolovic, I.M.; Kuo, Y.; Ankri, R.; Weiss, S.; Michalet, X.; Charbon, E. A 512 × 512 SPAD image sensor with integrated gating for widefield FLIM. IEEE J. Quantum Electron. 2019, 25, 8449092. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Connolly, P.W.R.; Halimi, A.; Altmann, Y.; McLaughlin, S.; Gyongy, I.; Henderson, R.K.; Buller, G.S. High-resolution depth profiling using a range-gated CMOS SPAD quanta image sensor. Opt. Express 2018, 5, 5541–5557. [Google Scholar] [CrossRef]
- Gyongy, I.; Calder, N.; Davies, A.; Dutton, N.A.W.; Duncan, R.R.; Rickman, C.; Dalgarno, P.; Henderson, R.K. A 256 × 256, 100-kfps, 61% fill-factor SPAD image sensor for time-resolved microscopy applications. IEEE Trans. Electron. Dev. 2018, 65, 547–554. [Google Scholar] [CrossRef]
- Nissinen, I.; Nissinen, J.; Kostamovaara, J. On the time gating of SPADs in a synchronized time-gated SPAD array in Raman spectroscopy. Proc. IEEE Sensors 2017, 2017, 1–3. [Google Scholar] [CrossRef]
- Prochazka, I.; Blazej, J.; Kodet, J. Effective dark count rate reduction by modified SPAD gating circuit. Nucl. Intrum. Meth. A 2015, 787, 212–215. [Google Scholar] [CrossRef]
- Scarcella, C.; Boso, G.; Ruggeri, A.; Tosi, A. InGaAs/InP single-photon detector gated at 1.3 GHz with 1.5% afterpulsing. IEEE J. Quantum Electron. 2015, 21, 8449092. [Google Scholar] [CrossRef]
- Di Sieno, L.; Dalla Mora, A.; Boso, G.; Tosi, A.; Pifferi, A.; Cubeddu, R.; Contini, D. Diffuse optics using a dual window fast-gated counter. Appl. Optics 2014, 53, 7394–7401. [Google Scholar] [CrossRef]
- Pancheri, L.; Massari, N.; Stoppa, D. SPAD image sensor with analog counting pixel for time-resolved fluorescence detection. IEEE T. Electron. Dev. 2013, 60, 3442–3449. [Google Scholar] [CrossRef]
- Vilella, E.; Alonso, O.; Montiel, A.; Vilà, A.; Diéguez, A. A low-noise time-gated single-photon detector in a HV-CMOS technology for triggered imaging. Sensor. Actuat. A Phys. 2013, 201, 342–351. [Google Scholar] [CrossRef]
- Miyata, T.; Iwata, T.; Nakayama, S.; Araki, T. A nanosecond gate-mode-driven silicon avalanche photodiode and its application to measuring fluorescence lifetimes of Ce-doped YAG ceramics. Meas. Sci. Technol. 2012, 23, 035501. [Google Scholar] [CrossRef]
- Buttafava, M.; Boso, G.; Ruggeri, A.; Dalla Mora, A.; Tosi, A. Time-gated single-photon detection module with 110 ps transition time and up to 80 MHz repetition rate. Rev. Sci. Instrum. 2014, 85, 083114. [Google Scholar] [CrossRef]
- Boso, G.; Dalla Mora, A.; Della Frera, A.; Tosi, A. Fast-gating of single-photon avalanche diodes with 200 ps transitions and 30 ps timing jitter. Sensor. Actuat. A Phys. 2013, 191, 61–67. [Google Scholar] [CrossRef]
- Micro Photon Devices S.r.l. FastGatedSPAD. Available online: http://www.micro-photon-devices.com/Products/SPAD-by-Wavelength/400nm-900nm/FastGATED-SPAD (accessed on 21 August 2019).
- Saha, S.; Lesage, F.; Sawan, M. High-voltage pulse generator with variable delay for ultrafast gating of single photon detector. In Proceedings of the IEEE 7th Latin American Symposium on Circuits & Systems (LASCAS), Florianopolis, Brazil, 28 February–2 March 2016; pp. 131–134. [Google Scholar] [CrossRef]
- Saha, S.; Lu, Y.; Weyers, S.; Sawan, M.; Lesage, F. Time-resolved reflectance using short source-detector separation. In Proceedings of the 2016 IEEE International Symposium on Circuits and Systems (ISCAS), Montreal, QC, Canada, 22–25 May 2016; pp. 333–336. [Google Scholar] [CrossRef]
- Saha, S.; Lu, Y.; Weyers, S.; Sawan, M.; Lesage, F. Compact fast optode-based probe for single-photon counting applications. IEEE Phot. Tech. L. 2018, 30, 1515–1518. [Google Scholar] [CrossRef]
- Saha, S.; Lu, Y.; Weyers, S.; Lesage, F.; Sawan, M. Miniaturized probe for time-domain near-infrared spectroscopy. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 333–336. [Google Scholar] [CrossRef]
- Saha, S.; Burri, S.; Bruschini, C.; Charbon, E.; Lesage, F.; Sawan, M. Time domain NIRS optode based on null/small source-detector distance for wearable applications. In Proceedings of the 2019 IEEE Custom Integrated Circuits Conference (CICC), Austin, TX, USA, USA, 14–17 April 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Di Sieno, L.; Nissinen, J.; Hallman, L.; Martinenghi, E.; Contini, D.; Pifferi, A.; Kostamovaara, J.; Dalla Mora, A. Miniaturized pulsed laser source for time-domain diffuse optics routes to wearable devices. J. Biomed. Opt. 2017, 22, 085004. [Google Scholar] [CrossRef]
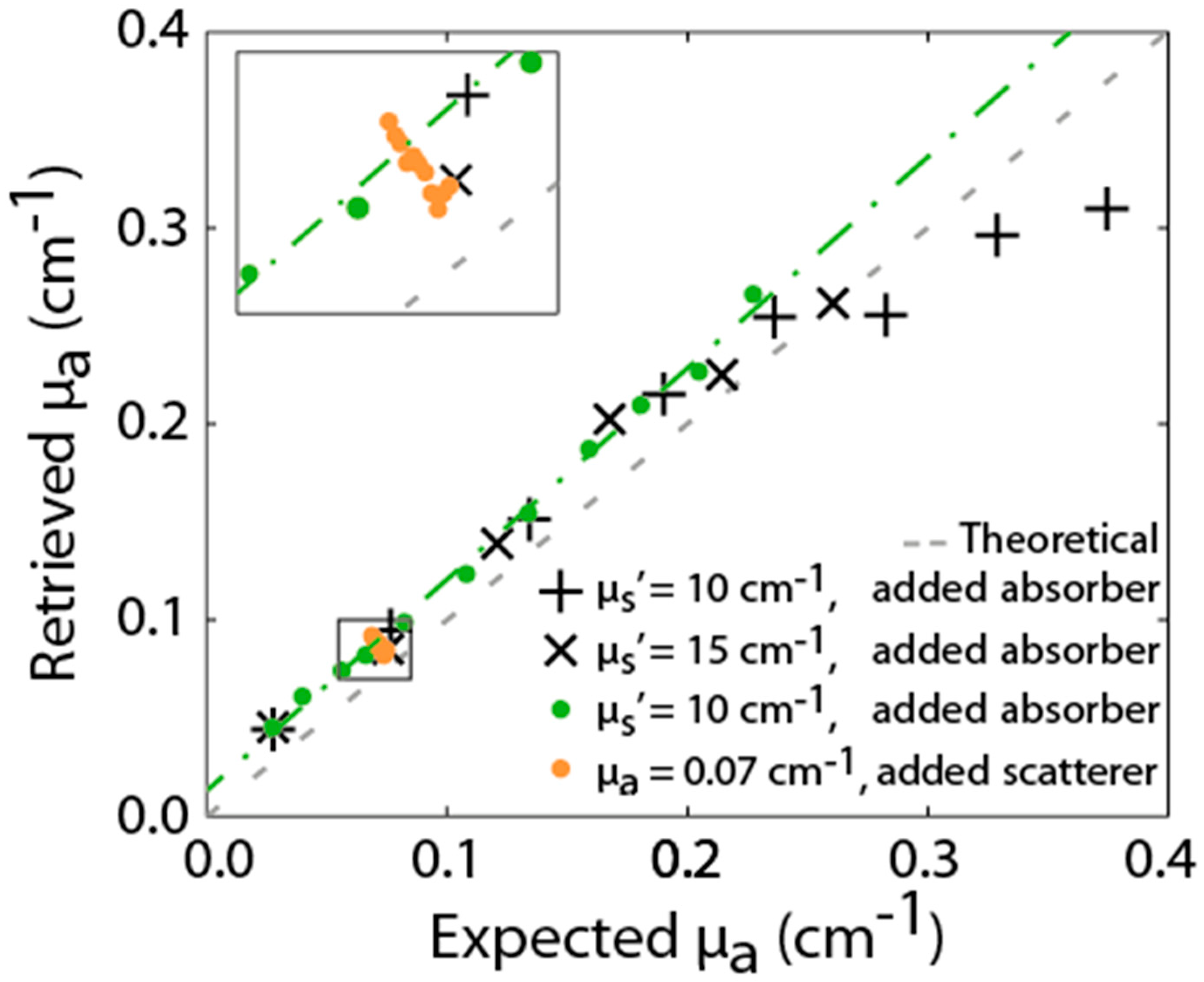
- Behera, A.; Di Sieno, L.; Pifferi, A.; Martelli, F.; Dalla Mora, A. Instrumental, optical and geometrical parameters affecting time-gated diffuse optical measurements: A systematic study. Biomed. Opt. Express 2018, 9, 5524–5542. [Google Scholar] [CrossRef]
- Dalla Mora, A.; Martinenghi, E.; Contini, D.; Tosi, A.; Boso, G.; Durduran, T.; Arridge, S.; Martelli, F.; Farina, A.; Torricelli, A.; et al. Fast silicon photomultiplier improves signal harvesting and reduces complexity in timedomain diffuse optics. Opt. Express 2015, 23, 13937–13946. [Google Scholar] [CrossRef]
- Martinenghi, E.; Dalla Mora, A.; Contini, D.; Farina, A.; Villa, F.; Torricelli, A.; Pifferi, A. Spectrally resolved single-photon timing of silicon photomultipliers for time-domain diffuse spectroscopy. IEEE Photonics J. 2015, 7, 7159013. [Google Scholar] [CrossRef]
- Re, R.; Martinenghi, E.; Dalla Mora, A.; Contini, D.; Pifferi, A.; Torricelli, A. Probe-hosted silicon photomultipliers for time-domain functional near-infrared spectroscopy: Phantom and in vivo tests. Neurophotonics 2016, 3, 045004. [Google Scholar] [CrossRef] [PubMed]
- Buttafava, M.; Martinenghi, E.; Tamborini, D.; Contini, D.; Dalla Mora, A.; Renna, M.; Torricelli, A.; Pifferi, A.; Zappa, F.; Tosi, A. A compact two-wavelength time-domain NIRS system based on SiPM and pulsed diode lasers. IEEE Photonics J. 2017, 9, 7792611. [Google Scholar] [CrossRef]
- Ferocino, E.; Martinenghi, E.; Dalla Mora, A.; Pifferi, A.; Cubeddu, R.; Taroni, P. High throughput detection chain for time domain optical mammography. Biomed. Opt. Express 2018, 9, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Dutton, N.A.W.; Gnecchi, S.; Parmesan, L.; Holmes, A.J.; Rae, B.; Grant, L.A.; Henderson, R.K. A time-correlated single-photon-counting sensor with 14GS/S histogramming time-to-digital converter. Proc. IEEE ISSCS 2015, 58, 204–205. [Google Scholar] [CrossRef]
- Tyndall, D.; Rae, B.; Li, D.; Richardson, J.; Arlt, J.; Henderson, R. A 100Mphoton/s time-resolved mini-silicon photomultiplier with on-chip fluorescence lifetime estimation in 0.13 μm CMOS imaging technology. Proc. IEEE ISSCS 2012, 55, 122–123. [Google Scholar] [CrossRef]
- Contini, D.; Dalla Mora, A.; Spinelli, L.; Farina, A.; Torricelli, A.; Cubeddu, R.; Martelli, F.; Zaccanti, G.; Tosi, A.; Boso, G.; et al. Effects of time-gated detection in diffuse optical imaging at short source-detector separation. J. Phys. D Appl. Phys. 2015, 48, 045401. [Google Scholar] [CrossRef]
- Dalla Mora, A.; Contini, D.; Pifferi, A.; Cubeddu, R.; Tosi, A.; Zappa, F. Afterpulse-like noise limits dynamic range in time-gated applications of thin-junction silicon single-photon avalanche diode. Appl. Phys. Lett. 2012, 100, 241111. [Google Scholar] [CrossRef]
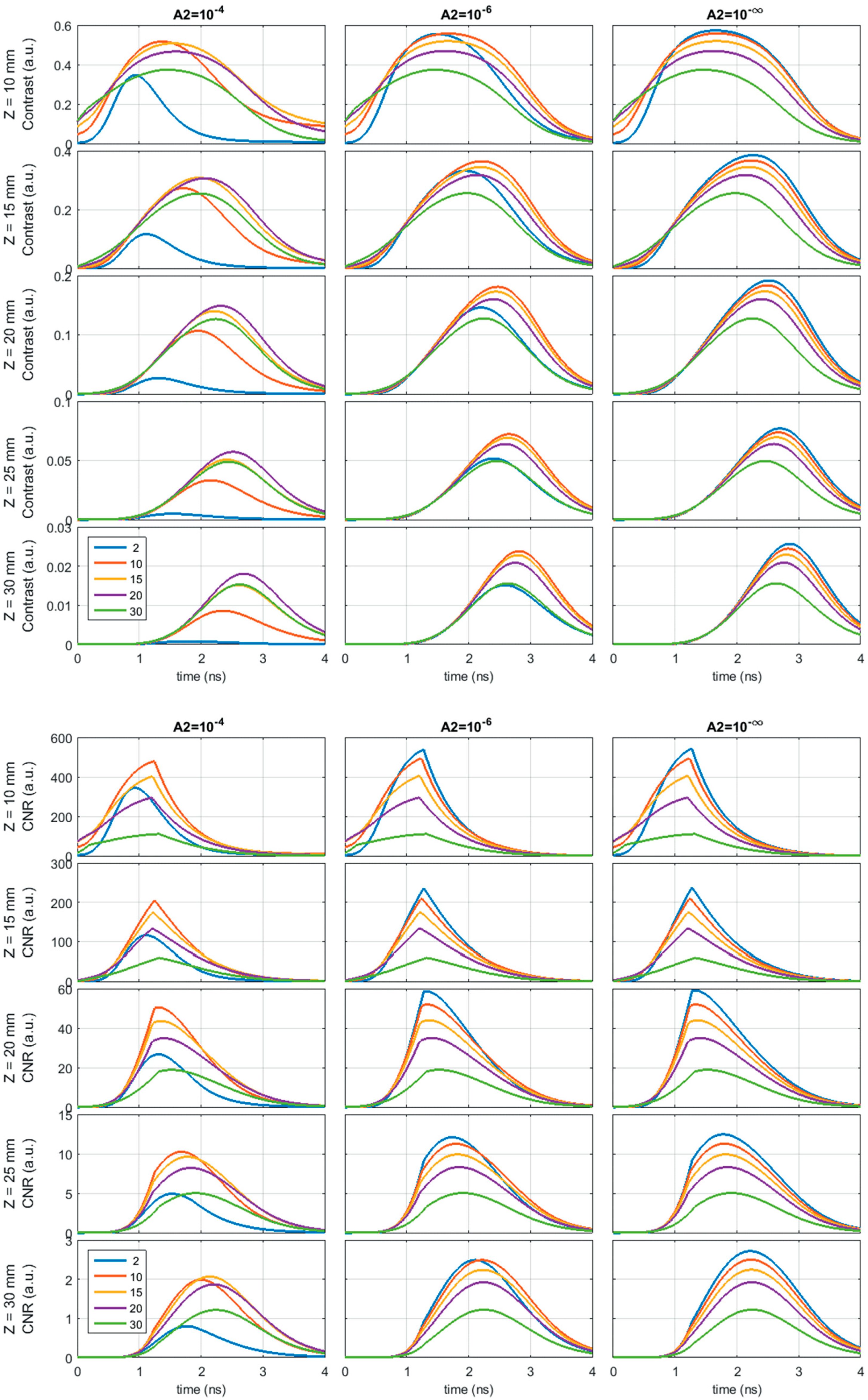
- Dalla Mora, A.; Tosi, A.; Contini, D.; Di Sieno, L.; Boso, G.; Villa, F.; Pifferi, A. Memory effect in silicon time-gated single-photon avalanche diodes. J. Appl. Phys. 2015, 117, 114501. [Google Scholar] [CrossRef]
- Figshare. Dependence of Contrast and CNR on the Memory Tail Amplitude (A2). Available online: https://figshare.com/articles/Dependence_of_contrast_and_CNR_on_the_memory_tail_amplitude_A2_/6736550 (accessed on 21 August 2019).
- Figshare. Dependence of Contrast and CNR on the Optical Responsivity (R). Available online: https://figshare.com/articles/Dependence_of_contrast_and_CNR_on_the_optical_responsivity_R_/6736556 (accessed on 21 August 2019).
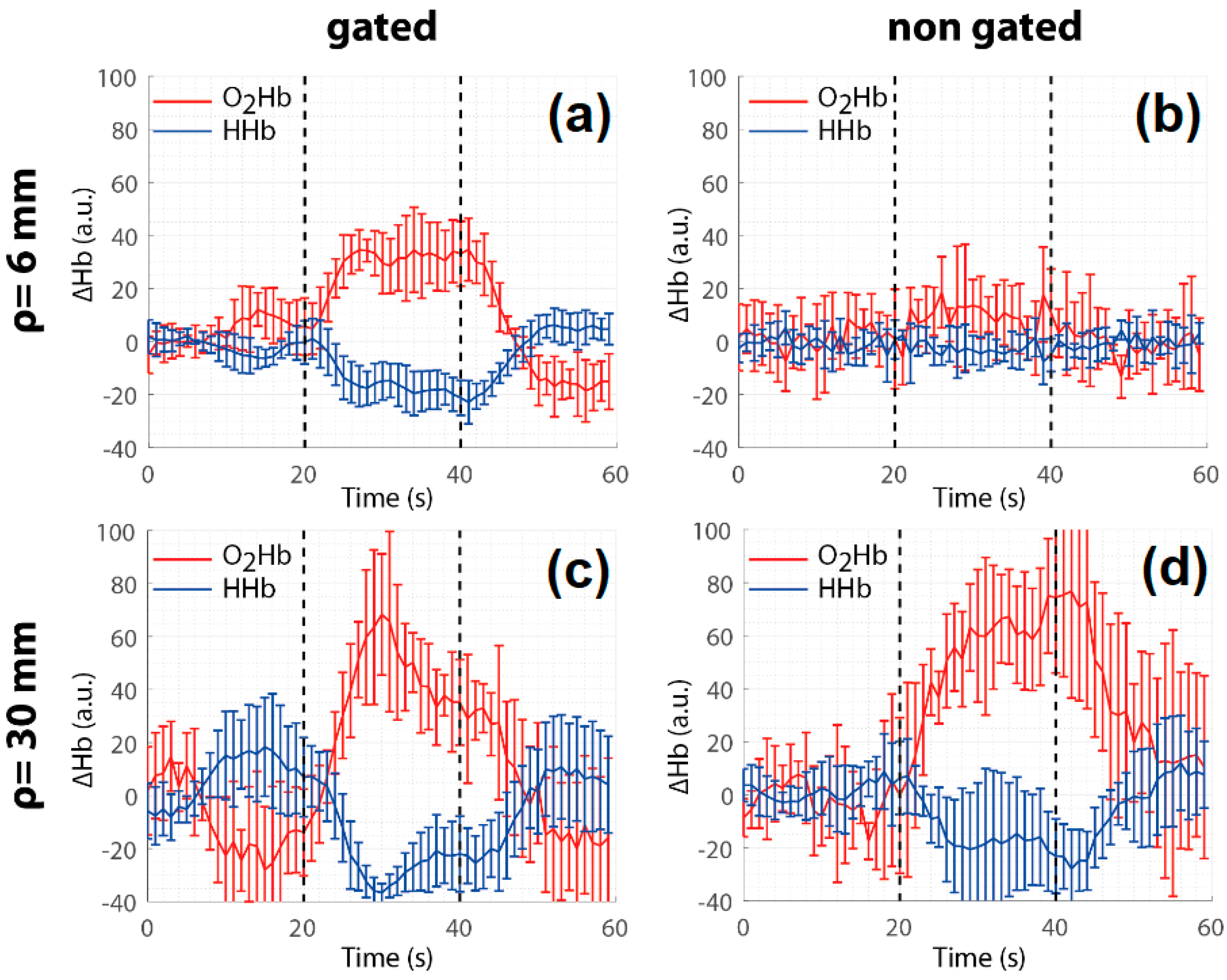
- Di Sieno, L.; Dalla Mora, A.; Torricelli, A.; Spinelli, L.; Re, R.; Pifferi, A.; Contini, D. A versatile setup for time-resolved functional near infrared spectroscopy based on fast-gated SPAD and on four-wave mixing laser. Appl. Sci. Basel 2019, 9, 2366. [Google Scholar] [CrossRef]
- Di Sieno, L.; Contini, D.; Dalla Mora, A.; Torricelli, A.; Spinelli, L.; Cubeddu, R.; Tosi, A.; Boso, G.; Pifferi, A. Functional near-infrared spectroscopy at small source-detector distance by means of high dynamic-range fast-gated SPAD acquisitions: First in-vivo measurements. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 12–16 May 2013. [Google Scholar]
- Cerussi, A.; Siavoshi, S.; Durkin, A.; Chen, C.; Tanamai, W.; Hsiang, D.; Tromberg, B.J. Effect of contact force on breast tissue optical property measurements using a broadband diffuse optical spectroscopy handheld probe. Appl. Opt. 2009, 48, 4270–4277. [Google Scholar] [CrossRef]
- Nakhaeva, I.A.; Zyuryukina, O.A.; Mohammed, M.R.; Sinichkin, Y.P. The effect of external mechanical compression on in vivo water content in human skin. Opt. Spectrosc. 2015, 118, 834–840. [Google Scholar] [CrossRef]
- Sase, I.; Takatsuki, A.; Seki, J.; Yanagida, T.; Seiyama, A. Noncontact backscatter-mode near-infrared time-resolved imaging system: Preliminary study for functional brain mapping. J. Biomed. Opt. 2006, 11, 054006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazurenka, M.; Jelzow, A.; Wabnitz, H.; Contini, D.; Spinelli, L.; Pifferi, A.; Cubeddu, R.; Dalla Mora, A.; Tosi, A.; Zappa, F.; et al. Non-contact time-resolved diffuse reflectance imaging at null source-detector separation. Opt. Express 2012, 20, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wabnitz, H.; Taubert, D.R.; Mazurenka, M.; Steinkellner, O.; Jelzow, A.; Macdonald, R.; Milej, D.; Sawosz, P.; Kacprzak, M.; Liebert, A.; et al. Performance assessment of time-domain optical brain imagers, part 1: Basic instrumental performance protocol. J. Biomed. Opt. 2014, 19, 86010. [Google Scholar] [CrossRef] [PubMed]
- Mazurenka, M.; Di Sieno, L.; Boso, G.; Contini, D.; Pifferi, A.; Dalla Mora, A.; Tosi, A.; Wabnitz, H.; Macdonald, R. Non-contact in vivo diffuse optical imaging using a time-gated scanning system. Biomed. Opt. Express 2013, 4, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Mazurenka, M.; Di Sieno, L.; Boso, G.; Contini, D.; Pifferi, A.; Dalla Mora, A.; Tosi, A.; Wabnitz, H.; MacDonald, R. A non-contact time-domain scanning brain imaging system: First in-vivo results. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 12–16 May 2013. [Google Scholar]
- Kim, J.G.; Liu, H. Variation of haemoglobin extinction coefficients can cause errors in the determination of haemoglobin concentration measured by near-infrared spectroscopy. Phys. Med. Biol. 2007, 52, 6295–6322. [Google Scholar] [CrossRef]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef]
- Lou, P.-J.J.; Jager, H.R.; Jones, L.; Theodossy, T.; Bown, S.G.; Hopper, C.; Jäger, H.R.; Jones, L.; Theodossy, T.; Bown, S.G.; et al. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. Cancer 2004, 91, 441–446. [Google Scholar] [CrossRef]
- Di Sieno, L.; Boetti, N.G.; Dalla Mora, A.; Pugliese, D.; Farina, A.; Konugolu Venkata Sekar, S.; Ceci-Ginistrelli, E.; Janner, D.; Pifferi, A.; Milanese, D. Towards the use of bioresorbable fibers in time-domain diffuse optics. J. Biophotonics 2018, 11, e201600275. [Google Scholar] [CrossRef]
- Dupuis, A.; Guo, N.; Gao, Y.; Godbout, N.; Lacroix, S.; Dubois, C.; Skorobogatiy, M. Prospective for biodegradable microstructured optical fibers. Opt. Lett. 2007, 32, 109–111. [Google Scholar] [CrossRef]
- Huby, N.; Vié, V.; Renault, A.; Beaufils, S.; Lèfevre, T.; Paquet-Mercier, F.; Pézolet, M.; Beche, B. Native spider silk as a biological optical fiber. Appl. Phys. Lett. 2013, 102, 123702. [Google Scholar] [CrossRef]
- Alerstam, E.; Svensson, T.; Andersson-Engels, S.; Spinelli, L.; Contini, D.; Dalla Mora, A.; Tosi, A.; Zappa, F.; Pifferi, A. Single-fiber diffuse optical time-of-flight spectroscopy. Opt. Lett. 2012, 37, 2877–2879. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Brooks, D.H.; Miller, E.L.; Di Marzio, C.A.; Kilmer, M.; Gaudette, R.J.; Zhang, Q. Imaging the body with diffuse optical tomography. IEEE Signal Proc. Mag. 2001, 18, 57–75. [Google Scholar] [CrossRef]
- Mata Pavia, J.; Scandini, M.; Lindner, S.; Wolf, M.; Charbon, E. A 1 × 400 backside-illuminated SPAD sensor with 49.7 ps resolution, 30 pJ/sample TDCs fabricated in 3D CMOS technology for near-infrared optical tomography. IEEE J. Solid-St. Circ. 2015, 50, 2406–2418. [Google Scholar] [CrossRef]
- Di Sieno, L.; Zouaoui, J.; Hervé, L.; Pifferi, A.; Farina, A.; Martinenghi, E.; Derouard, J.; Dinten, J.-M.; Dalla Mora, A. Time-domain diffuse optical tomography using silicon photomultipliers: A feasibility study. J. Biomed. Opt. 2016, 21, 116002. [Google Scholar] [CrossRef]
- Portaluppi, D.; Conca, E.; Villa, F.; Zappa, F. Time-gated SPAD camera with reconfigurable macropixels for LIDAR applications. In Proceedings of the 32nd International Congress on High-Speed Imaging and Photonics, Twente, The Netherlands, 8–12 October 2018. [Google Scholar] [CrossRef]
- Lyons, A.; Tonolini, F.; Boccolini, A.; Repetti, A.; Henderson, R.; Wiaux, Y.; Faccio, D. Computational time-of-flight diffuse optical tomography. Nat. Photonics 2019, 13, 575–579. [Google Scholar] [CrossRef]
- McBride, T.O.; Pogue, B.W.; Gerety, E.D.; Poplack, S.B.; Osterberg, U.L.; Paulsen, K.D. Spectroscopic diffuse optical tomography for the quantitative assessment of hemoglobin concentration and oxygen saturation in breast tissue. Appl. Opt. 1999, 38, 5480–5490. [Google Scholar] [CrossRef]
- McBride, T.O.; Pogue, B.W.; Jiang, S.; Osterberg, U.L.; Paulsen, K.D.; Poplack, S.P. Initial studies of in vivo absorbing and scattering heterogeneity in near-infrared tomographic breast imaging. Opt. Lett. 2001, 26, 822–824. [Google Scholar] [CrossRef]
- Dehghani, H.; Pogue, B.W.; Shudong, J.; Brooksby, B.; Paulsen, K.D. Three-dimensional optical tomography: Resolution in small-object imaging. Appl. Opt. 2003, 42, 3117–3128. [Google Scholar] [CrossRef]
- Zhu, Q. Optical tomography with ultrasound localization: Initial clinical results and technical challenges. Technol. Cancer Res. Treat. 2005, 4, 235–244. [Google Scholar] [CrossRef]
- Vavadi, H.; Mostafa, A.; Zhou, F.; Uddin, K.M.S.; Althobaiti, M.; Xu, C.; Bansal, R.; Ademuyiwa, F.; Poplack, S.; Zhu, Q. Compact ultrasound-guided diffuse optical tomography system for breast cancer imaging. J. Biomed. Opt. 2018, 24, 021203. [Google Scholar] [CrossRef] [PubMed]
- Kavuri, V.C.; Liu, H. Hierarchical clustering method to improve transrectal ultrasound-guided diffuse optical tomography for prostate cancer imaging. Acad. Radiol. 2014, 21, 250–262. [Google Scholar] [CrossRef] [PubMed]
- SOLUS. SOLUS Project Home Page. Available online: http://www.solus-project.eu/ (accessed on 27 November 2019).
- Puszka, A.; Di Sieno, L.; Dalla Mora, A.; Pifferi, A.; Contini, D.; Boso, G.; Tosi, A.; Hervé, L.; Planat-Chrétien, A.; Koenig, A.; et al. Time-resolved diffuse optical tomography using fast-gated single-photon avalanche diodes. Biomed. Opt. Express 2013, 4, 1351–1365. [Google Scholar] [CrossRef]
- Puszka, A.; Di Sieno, L.; Dalla Mora, A.; Pifferi, A.; Contini, D.; Planat-Chrétien, A.; Koenig, A.; Boso, G.; Tosi, A.; Hervé, L.; et al. Spatial resolution in depth for time-resolved diffuse optical tomography using short source-detector separations. Biomed. Opt. Express 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Martelli, F.; Di Ninni, P.; Zaccanti, G.; Contini, D.; Spinelli, L.; Torricelli, A.; Cubeddu, R.; Wabnitz, H.; Mazurenka, M.; Macdonald, R.; et al. Phantoms for diffuse optical imaging based on totally absorbing objects, part 2: Experimental implementation. J. Biomed. Opt. 2014, 19, 76011. [Google Scholar] [CrossRef]
- Zouaoui, J.; Di Sieno, L.; Hervé, L.; Pifferi, A.; Farina, A.; Dalla Mora, A.; Derouard, J.; Dinten, J.-M. Quantification in time-domain diffuse optical tomography using Mellin-Laplace transforms. Biomed. Opt. Express 2016, 7, 4346–4363. [Google Scholar] [CrossRef]
- Di Sieno, L.; Bettega, G.; Berger, M.; Hamou, C.; Aribert, M.; Dalla Mora, A.; Puszka, A.; Grateau, H.; Contini, D.; Hervé, L.; et al. Toward noninvasive assessment of flap viability with time-resolved diffuse optical tomography: A preclinical test on rats. J. Biomed. Opt. 2016, 21, 025004. [Google Scholar] [CrossRef][Green Version]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85, 51–63. [Google Scholar] [CrossRef]
- Buckley, E.M.; Parthasarathy, A.B.; Grant, P.E.; Yodh, A.G.; Franceschini, M.A. Diffuse correlation spectroscopy for measurement of cerebral blood flow: Future prospects. Neurophoton 2014, 1, 011009. [Google Scholar] [CrossRef]
- Selb, J.; Boas, D.A.; Chan, S.-T.; Evans, K.C.; Buckley, E.M.; Carp, S.A. Sensitivity of near-infrared spectroscopy and diffuse correlation spectroscopy to brain hemodynamics: Simulations and experimental findings during hypercapnia. Neurophoton 2014, 1, 015005. [Google Scholar] [CrossRef]
- Diop, M.; Verdecchia, K.; Lee, T.-Y.; St Lawrence, K. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed. Opt. Express 2011, 2, 2068–2081. [Google Scholar] [CrossRef] [PubMed]
- Carp, S.A.; Farzam, P.; Redes, N.; Hueber, D.M.; Franceschini, M.A. Combined multi-distance frequency domain and diffuse correlation spectroscopy system with simultaneous data acquisition and real-time analysis. Biomed. Opt. Express 2017, 8, 3993–4006. [Google Scholar] [CrossRef] [PubMed]
- Sutin, J.; Zimmerman, B.; Tyulmankov, D.; Tamborini, D.; Wu, K.; Selb, J.; Gulinatti, A.; Rech, I.; Tosi, A.; Boas, D.; et al. Time-domain diffuse correlation spectroscopy. Optica 2016, 3, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Pagliazzi, M.; Konugolu Venkata Sekar, S.; Colombo, L.; Martinenghi, E.; Minnema, J.; Erdmann, R.; Contini, D.; Dalla Mora, A.; Torricelli, A.; Pifferi, A.; et al. Time domain diffuse correlation spectroscopy with a high coherence pulsed source: In vivo and phantom results. Biomed. Opt. Express 2017, 8, 5311–5325. [Google Scholar] [CrossRef] [PubMed]
- Pagliazzi, M.; Konugolu Venkata Sekar, S.; Di Sieno, L.; Colombo, L.; Durduran, T.; Contini, D.; Torricelli, A.; Pifferi, A.; Dalla Mora, A. In vivo time-gated diffuse correlation spectroscopy at quasi-null source-detector separation. Opt. Lett. 2018, 43, 2450–2453. [Google Scholar] [CrossRef]
- Taroni, P.; Quarto, G.; Pifferi, A.; Abbate, F.; Balestreri, N.; Menna, S.; Cassano, E.; Cubeddu, R. Breast tissue composition and its dependence on demographic risk factors for breast cancer: Non-invasive assessment by time domain diffuse optical spectroscopy. PLoS ONE 2015, 10, e0128941. [Google Scholar] [CrossRef]
- Tosi, A.; Dalla Mora, A.; Zappa, F.; Cova, S.; Itzler, M.; Jiang, X. InGaAs/InP single-photon avalanche diodes show low dark counts and require moderate cooling. In Proceedings of the Photonics West, San Jose, CA, USA, 25–28 January 2009. [Google Scholar]
- Bargigia, I.; Tosi, A.; Bahgat Shehata, A.; Della Frera, A.; Farina, A.; Bassi, A.; Taroni, P.; Dalla Mora, A.; Zappa, F.; Cubeddu, R.; et al. Time-resolved diffuse optical spectroscopy up to 1700 nm by means of a time-gated InGaAs/InP single-photon avalanche diode. Appl. Spectrosc. 2012, 66, 944–950. [Google Scholar] [CrossRef]
- Konugolu Venkata Sekar, S.; Bargigia, I.; Dalla Mora, A.; Taroni, P.; Ruggeri, A.; Tosi, A.; Pifferi, A.; Farina, A. Diffuse optical characterization of collagen absorption from 500 to 1700 nm. J. Biomed. Opt. 2017, 22, 015006. [Google Scholar] [CrossRef] [PubMed]
- Tosi, A.; Della Frera, A.; Bahgat Shehata, A.; Scarcella, C. Fully programmable single-photon detection module for InGaAsInP single-photon avalanche diodes with clean and sub-nanosecond gating transitions. Rev. Sci. Instrum. 2012, 83, 013104. [Google Scholar] [CrossRef]
- Nachabé, R.; Hendriks, B.H.; Desjardins, A.E.; van der Voort, M.; van der Mark, M.B.; Sterenborg, H.J. Estimation of lipid and water concentrations in scattering media with diffuse optical spectroscopy from 900 to 1600 nm. J. Biomed. Opt. 2010, 15, 037015. [Google Scholar] [CrossRef]
- Martinenghi, E.; Di Sieno, L.; Contini, D.; Sanzaro, M.; Pifferi, A.; Dalla Mora, A. Time-resolved single-photon detection module based on silicon photomultiplier: A novel building block for time-correlated measurement systems. Rev. Sci. Instrum. 2016, 87, 073101. [Google Scholar] [CrossRef] [PubMed]
- Bruschini, C.; Homulle, H.; Antolovic, I.M.; Burri, S.; Charbon, E. Single-photon avalanche diode imagers in biophotonics: Review and outlook. Light Sci. Appl. 2019, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.C.; Buttafava, M.; Boso, G.; Diaspro, A.; Tosi, A.; Vicidomini, G. Gated STED microscopy with time-gated single-photon avalanche diode. Biomed. Opt. Express 2015, 6, 2258–2267. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Halimi, A.; Zhu, F.; Gyongy, I.; Henderson, R.K.; Bowman, R.; McLaughlin, S.; Buller, G.S.; Leach, J. Long-range depth imaging using a single-photon detector array and non-local data fusion. Sci. Rep. 2019, 9, 8075. [Google Scholar] [CrossRef]
- Maccarone, A.; McCarthy, A.; Ren, X.; Warburton, R.E.; Wallace, A.M.; Moffat, J.; Petillot, Y.; Buller, G.S. Underwater depth imaging using time-correlated single-photon counting. Opt. Express 2015, 23, 33911–33926. [Google Scholar] [CrossRef]
- Buttafava, M.; Zeman, J.; Tosi, A.; Eliceiri, K.; Velten, A. Non-line-of-sight imaging using a time-gated single photon avalanche diode. Opt. Express 2015, 23, 20997–21011. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Mora, A.; Di Sieno, L.; Re, R.; Pifferi, A.; Contini, D. Time-Gated Single-Photon Detection in Time-Domain Diffuse Optics: A Review. Appl. Sci. 2020, 10, 1101. https://doi.org/10.3390/app10031101
Dalla Mora A, Di Sieno L, Re R, Pifferi A, Contini D. Time-Gated Single-Photon Detection in Time-Domain Diffuse Optics: A Review. Applied Sciences. 2020; 10(3):1101. https://doi.org/10.3390/app10031101
Chicago/Turabian StyleDalla Mora, Alberto, Laura Di Sieno, Rebecca Re, Antonio Pifferi, and Davide Contini. 2020. "Time-Gated Single-Photon Detection in Time-Domain Diffuse Optics: A Review" Applied Sciences 10, no. 3: 1101. https://doi.org/10.3390/app10031101
APA StyleDalla Mora, A., Di Sieno, L., Re, R., Pifferi, A., & Contini, D. (2020). Time-Gated Single-Photon Detection in Time-Domain Diffuse Optics: A Review. Applied Sciences, 10(3), 1101. https://doi.org/10.3390/app10031101






