Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products
Abstract
1. Introduction
- The control of arthropods during the vegetation period.
- The control of pathogens from agricultural crops and in the forest.
- Selective weed control in agricultural crops and forestry.
- The disinsectisation/sanitation of silos, food stores, and greenhouses.
- The treatment of seeds.
2. Started Point: Chemical Plant Protection Products
- Lower impact on human health and a very low toxicity to warm-blooded animals.
- Lower toxicity compared to other alternative pest control products.
- Lesser effects on other organisms in the environment, such as bees, fish, and birds.
- Lower potential for contamination of soil and, implicitly, groundwater.
- Minimum number of required applications.
- Higher efficiency at lower doses and avoidance to pest resistance.
- (a)
- Solid products such as: powdered powders, wettable powders, granules, impregnated strips, solid fertilizer mixtures, etc.
- (b)
- Liquid products, namely: aqueous solutions, mixtures of organic solvents or mineral oils, which can provide a fine spraying and, in the aerosols, emulsifiable concentrates.
- (c)
- Micro-encapsulation and fixation on macromolecular support is a modern conditioning process, with advantages over classical methods, applied in the cases where the structure and properties of active derivatives allow such approach.
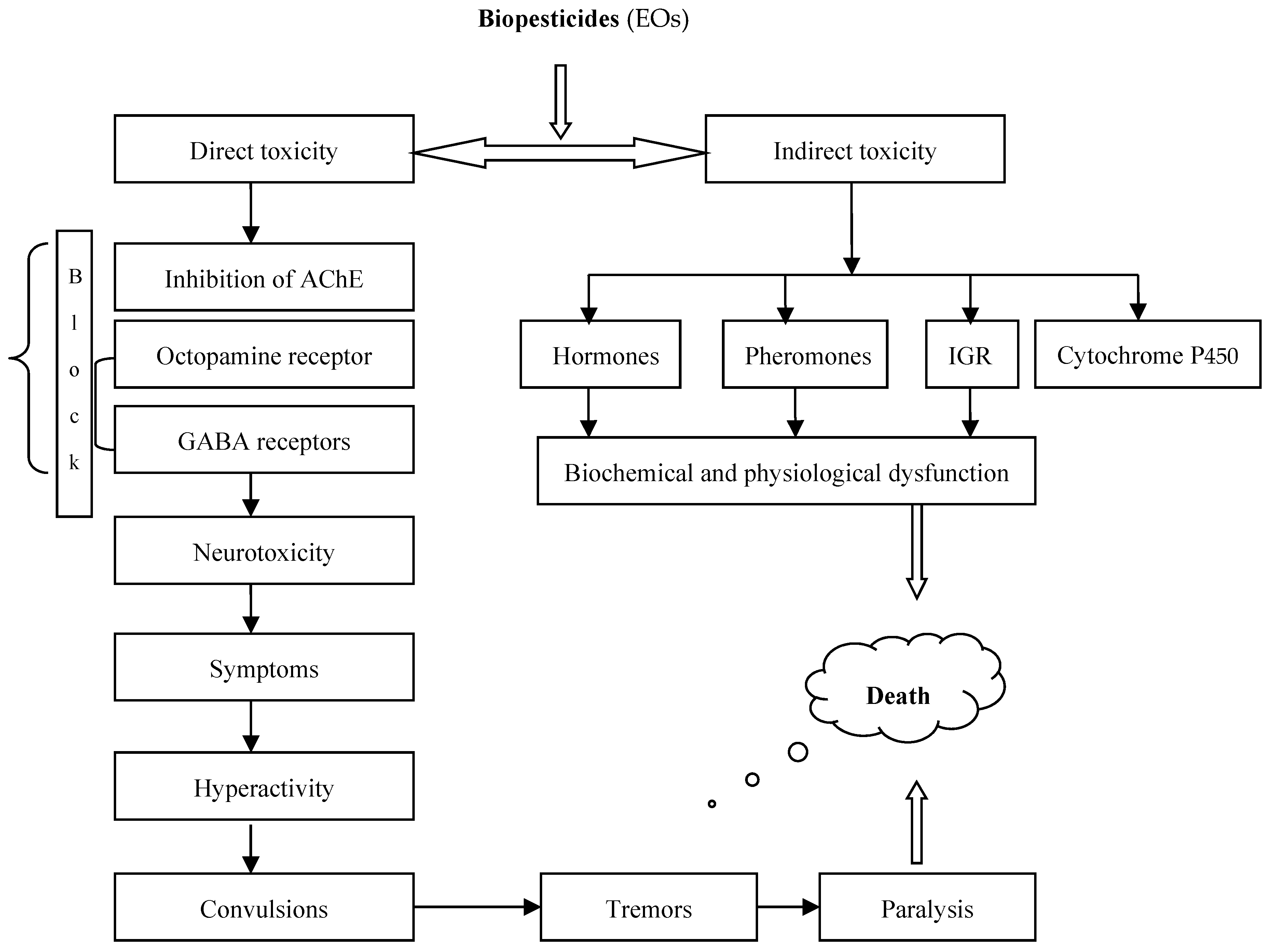
3. Next Level: Natural Plant Protection Products (Biopesticides)
4. Plant Extracts as Natural Plant Protection Products
- High efficacy against a wide range of pests and diseases of agricultural and medical importance.
- Multiple action mechanisms due to the large number of active ingredients in each mix.
- Low toxicity against non-target organisms, including humans.
- Relatively simple and cheap production processes.
- Reducing health risks during application due to low residue toxicity.
4.1. Methods of Vegetal Extracts Preparation
- (1)
- Primary processing consisting of the drying, conditioning, and packaging of plants.
- (2)
- Advanced processing, which involves the transformation of raw materials obtained from primary processing into products that can be marketed: phytotherapeutic products (aqueous extractive solutions, hydroalcoholic extractive solutions, lyophilized powders from extractive solutions), phytosanitation products, cosmetics, nutritional supplements and dietary supplements or food additives.
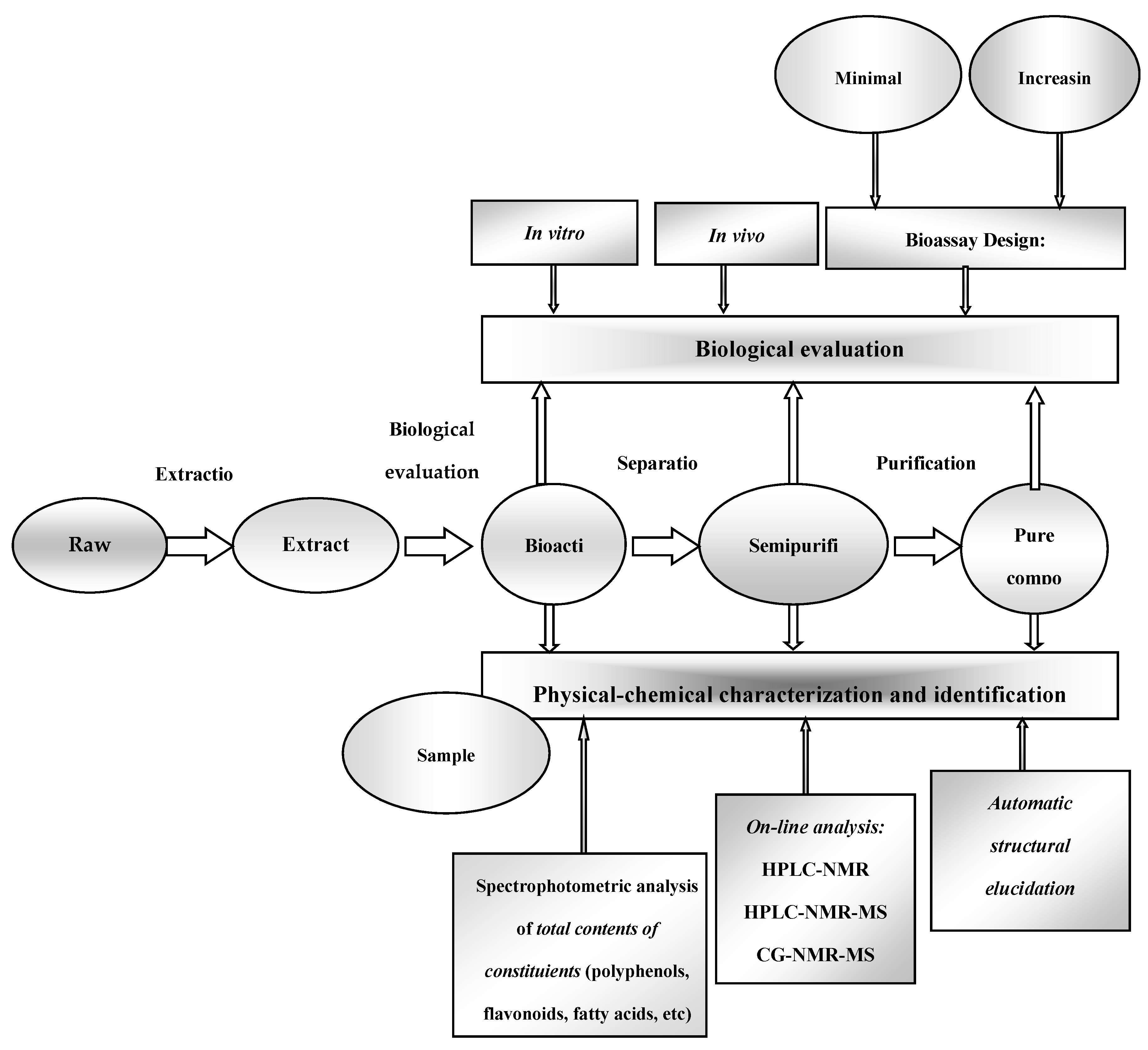
4.2. Methods for Determination of the Chemical Composition of Plant Extracts
5. Bio-Insecticidal Action of Some Spontaneous Flora Species in Moldova (Romania)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gill, H.K.; Garg, H. Pesticide: Environmental Impacts and Management Strategies. In Pesticides—Toxic Effects; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: Rijeka, Croatia, 2014; pp. 187–230. [Google Scholar]
- Saravi, S.S.S.; Shokrzadeh, M. Role of pesticides in human life in the modern age: A review. In Pesticides in the Modern World—Risks and Benefits; Stoytcheva, M., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 3–12. [Google Scholar]
- Singh, B.; Kaur, A. Control of insect pest in crop plants and stored food grains using plant saponins: A review. LWT Food Sci. Technol. 2018, 87, 93–101. [Google Scholar] [CrossRef]
- Valcke, M.; Bourgault, M.H.; Rochette, L.; Normandin, L.; Samuel, O.; Belleville, D.; Blanchet, C.; Phaneuf, D. Human health risk assessment on the consumption of fruits and vegetables containing residual pesticides: A cancer and non-cancer risk/benefit perspective. Environ. Int. 2017, 108, 63–74. [Google Scholar] [CrossRef]
- Comanita, E.; Soldea, C. Chemistry and Technology of Pesticides; Rotaprint: Iasi, Romania, 1982; 20p. (In Romanian) [Google Scholar]
- Kudsk, P.; Jørgensena, L.N.; Ørum, J.E. Pesticide Load—A new Danish pesticide risk indicator with multiple applications. Land Use Policy 2018, 70, 384–393. [Google Scholar] [CrossRef]
- Sabarwala, A.; Kumara, K.; Singha, R.P. Hazardous effects of chemical pesticides on human health—Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Cothran, R.D.; Brown, J.M.; Relyea, R.A. Proximity to agriculture is correlated with pesticide tolerance: Evidence for the evolution of amphibian resistance to modern pesticides. Evol. Appl. 2013, 6, 832–841. [Google Scholar] [CrossRef]
- Rusu, L.; Harja, M.; Suteu, D.; Dabija, A.; Favier, L. Pesticide residues contamination of milk and dairy products. A case study: Bacau district area, Romania. J. Environ. Prot. Ecol. 2016, 17, 1229–1241. [Google Scholar]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Meyers, L.; Bull, J. Fighting change with change: Adaptive variation in an uncertain world. Trends Ecol. Evol. 2002, 17, 551–557. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Concerns of environmental persistence of pesticides and human chronic diseases. Clin. Experim. Pharmacol. 2012, 5, e002. [Google Scholar] [CrossRef]
- Mfarrej, M.F.B.; Rara, F.M. Competitive, Sustainable Natural Pesticides. Acta Ecol. Sin. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Bett, P.K.; Deng, A.L.; Ogendo, J.O.; Kariuko, S.T.; Kamatenesi-Mugisha, M.; Mihale, J.M.; Torto, B. Residual contact toxicity and repellence of Cupressus lusitanica Miller and Eucalyptus saligna Smith essential oils against makor syored product insect pests. Ind. Crop. Prod. 2017, 110, 65–74. [Google Scholar] [CrossRef]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Zoubiri, S.; Baaliouamer, A. Potentiality of plants as source of insecticide principles. J. Saudi Chem. Soc. 2014, 18, 925–938. [Google Scholar] [CrossRef]
- Upasani, S.M.; Kotkar, H.M.; Mendki, P.S.; Maheshwari, V. Partial characterization and insecticidal properties of Ricinus communis L foliage flavonoids. Pest Manag. Sci. 2003, 59, 1349–1354. [Google Scholar] [CrossRef]
- Mocanu, A.M. Pesticide Technology; Matrix Rom: Bucharest, Romania, 2013; pp. 34–56. (In Romanian) [Google Scholar]
- Soldea, C.; Mocanu, A.M. Pesticides—Property—Accessibility; Matrix Rom: Bucharest, Romania, 2009; pp. 24–33. (In Romanian) [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Mossa, A.T.H. Green Pesticides: Essential Oils as Biopesticides in Insect-pest Management. J. Environ. Sci. Technol. 2016, 9, 374–378. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nano-encapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Vimala, P.S.; Devi, P.; Duraimurugan, K.S.V.P.; Chandrika, B.; Gayatri, R.D.; Prasad, R. Nanobiopesticides for Crop Protection. In Nanobiotechnology Applications in Plant Protection; Abd-Elsalam, K.A., Prasad, R., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 145–168. [Google Scholar]
- Lade, B.D.; Gogle, D.P.; Nandeshwar, S.B. Nano Bio Pesticide to Constraint Plant Destructive Pests. J. Nanomed. Res. 2017, 6, 158–167. [Google Scholar] [CrossRef][Green Version]
- Brzozowski, L.; Mazourek, M. Sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability 2018, 10, 2023–2048. [Google Scholar] [CrossRef]
- da Silva, V.C.; Rodrigues, C.M. Natural products: An extraordinary source of value-added compounds from diverse biomasses in Brazil. Chem. Biol. Technol. Agric. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Jimenez-Reyes, M.F.; Carrasco, H.; Olea, A.F.; Silva-Moreno, E. Natural compounds: A sustainable alternative to the phytopathogens control. J. Chil. Chem. Soc. 2019, 64, 4459–4465. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Saudin-Espano, P.; Sevilla-Moran, B.; Lopez-Goti, C.; Alonso-Prados, J.L. Biopesticides from natural products: Curent development, legislative framework and future trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef]
- Gupta, S.; Dikshit, A.K. Biopesticides: An eco-friendly approach for pest control. J. Biopestic. 2010, 3, 186–188. [Google Scholar]
- Zarins, I.; Daugavietis, M.; Halimona, J. Biological activity of plant extracts and their application as ecologically harmless biopesticides. Sci. Work. Lith. Inst. Hortic. Lith. Univ. Agric. 2009, 28, 269–280. [Google Scholar]
- Laznik, Z.; Toth, T.; Lakatos, T.; Vidrih, M. Control of the Colorado potato beetle (Leptinotarsa decemlineata [Say]) on potato under field conditions: A comparison of the efficacy of foliar application of two strains of Steinernema feltiae (Filipjev) and spraying with thiametoxam. J. Plant Dis. Prot. 2010, 117, 129–135. [Google Scholar] [CrossRef]
- Pino, O.; Sánchez, Y.; Rojas, M.M. Plant secondary metabolites as an alternative in pest management. I: Background, research approaches and trends—Review article. Rev. Protección Veg. 2013, 28, 81–94. [Google Scholar]
- Mazid, S.; Kalita, J.C.; Rajkhowa, R.C. A review on the use of biopesticides in insect pest management. Int. J. Sci. Adv. Technol. 2011, 1, 169–178. [Google Scholar]
- Park, D.W.; Kim, K.G.; Choi, E.A.; Kang, G.R.; Kim, T.S.; Yang, Y.S.; Moon, S.J.; Ha, D.R.; Kim, E.S.; Cho, B.S. Pesticide residue sin leafy vegetables, stalk and stem vegetables from South Korea: Along-term study on safety and health risk assessment. Food Addit. Contam. Part A 2016, 33, 105–118. [Google Scholar]
- Bohinc, T.; Horvat, A.; Ocvirk, M.; Košir, I.J.; Rutnik, K.; Trdan, S. The first evidence of the insecticidal potential of plant powders from invasive alien plants against rice weevil under laboratory conditions. Appl. Sci. 2020, 10, 7828. [Google Scholar] [CrossRef]
- Karimi, J.; Dara, S.K.; Arthurs, S. Microbial insecticides in Iran: History, current status, challenges and perspective. J. Invertebr. Pathol. 2018, 165, 67–73. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Rojht, H.; Kosir, I.J.; Trdan, S. Chemical analysis of three herbal extracts and observation of their activity against adults of Acanthoscelides obtectus and Leptinotarsa decemlineata using a video tracking system. J. Plant Dis. Prot. 2012, 119, 59–67. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal Plant Extracts Improve Yield and Reduce Insect Pests on Legume Crops Without Harming Beneficial Arthropods. Front. Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Avramescu, S.M.; Dinu-Pirvu, C.E.; Ionescu, D. Romanian Aromatic and Medicinal Plants: From Tradition to Science. In Aromatic and Medicinal Plants—Back to Nature; El-Shemy, H.A., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 149–173. [Google Scholar]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A Comparative Analysis of the ‘Green’ Techniques Applied for Polyphenols Extraction from Bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complementary Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186–1208. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave Assisted Extraction of Bioactive Compounds from Food: A Review. Int. J. Food Sci. Nutr. Eng. 2017, 7, 19–27. [Google Scholar]
- Yara-Varón, E.; Li, I.Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.S.; Chemat, F. Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Molecules 2017, 22, 1474–1498. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Pirvu, L.; Armatu, A.; Bubueanu, C.; Pintilie, G.; Nita, S. Obtaining and chemical characterization of some vegetal extracts with corrosion-scaling inhibition properties. Part I. Fagus sylvatica and Alii cepae bulbus extracts. Rom. Biotechnol. Lett. 2010, 15, 5683–5689. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction ofanthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsenb, J.; Witkamp, G.J.; Verpoortea, R.; Choia, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S.; Khanuja, S.P.S.; Longo, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants; International Centre for Science and High Technology: Trieste, Italy, 2008; pp. 83–208. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurulm, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Akyıl, S.; İlter, I.; Koç, M.; Ertekin, F.K. Recent Trends in Extraction Techniques for High Value Compounds from Algae as Food Additives. Turkish J. Agric.—Food Sci. Technol. 2018, 6, 1008–1014. [Google Scholar] [CrossRef][Green Version]
- Rasul, M.G. Extraction, Isolation and Characterization of Natural Products from Medicinal Plants. Int. J. Basic Sci. Appl. Comput. 2018, 2, 1–6. [Google Scholar]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols from Aromatic and Medicinal Plants: Overview of the Methods and the Effect of Extraction. In Polyphenols in Plants: Isolations, Purifications and Extract Preparations, 2nd ed.; Watson, R., Ed.; Academic Press: London, UK, 2019; pp. 243–260. ISBN 978-0-12-813768-0. [Google Scholar]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C. Optimization of microwave-assisted extraction based on absorbed microwave power and energy. Chem. Eng. Sci. 2014, 111, 41–47. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Soledispa, P.A.; Gonzalez, J.; Cuellar, A.; Pérez, J.; Monan, M. GC-MS Chemical Characterization of Main Components of Smilax domingensis Wild. in Cuba. J. Agric. Stud. 2018, 6, 79–86. [Google Scholar] [CrossRef][Green Version]
- Saxena, H.O.; Tripathi, Y.C.; Pawar, G.; Kakkar, A. Botanicals as biopesticides: Active chemical constituents and biocidal action. In Familiarizing with Local Biodiversity; Khatri, P.K., Meshram, P.B., Eds.; Tropical Forest Research Institute (ICFRE): Jabalpur, India, 2014; pp. 227–246. [Google Scholar]
- Walia, S.; Saha, S.; Tripathy, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Mocan, A.; Zengin, G.; Mollica, A.; Uysal, A.; Gunes, E.; Crişan, G.; Aktumsek, A. Biological effects and chemical characterization of Iris schachtii Markgr. extracts: A new source of bioactive constituents. Food Chem. Toxicol. 2018, 112, 448–457. [Google Scholar] [CrossRef]
- Besil, M.N.A.; Pequeño, F.; Alonzo, N.; Hladki, R.; Cesio, M.V.; Heinzen, H. Evaluation of different QuEChERS procedures for pesticide residues determination in Calendula officinalis (L.) inflorescences. J. Appl. Res. Med. Aromat. Plants 2017, 7, 143–148. [Google Scholar] [CrossRef]
- García-Beltrán, J.M.; Esteban, M.Á. Properties and Applications of Plants of Origanum Sp. Genus. SM J. Biol. 2016, 2, 1006–1015. [Google Scholar]
- Mockute, D.; Judezentiene, A. Variability of the essential oils composition of Achillea mille-folium ssp. millefolium growing wild in Lithuania. Biochem. Syst. Ecol. 2003, 31, 1033–1045. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Goswami, P.; Singh, V.R.; Chauhan, A.; Verma, S.K.; Iqbal, H.; Verma, R.K.; Chanda, D.; et al. Chemical composition and allelopathic, antibacterial, antifungal and in vitro acetylcholinesterase inhibitory activities of yarrow (Achillea millefolium L.) native to India. Ind. Crop. Prod. 2017, 104, 144–155. [Google Scholar] [CrossRef]
- Zenão, S.; Aires, A.; Dias, C.; Saavedra, M.J.; Fernandes, C. Antibacterial potential of Urtica dioica and Lavandula angustifolia extracts against methicillin resistant Staphylococcus aureus isolated from diabetic foot ulcers. J. Herb. Med. 2017, 10, 53–58. [Google Scholar] [CrossRef]
- Sharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical composition and antifungal activity of Satureja hortensis L. essentiall oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microb. Pathog. 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Abdel-Reheem, M.A.T.; Oraby, M.M. Anti-microbial, cytotoxicity, and necrotic ripostes of Pimpinella anisum essential oil. Ann. Agric. Sci. 2015, 60, 335–340. [Google Scholar] [CrossRef]
- Heydarian, M.; Jooyandeh, H.; Nasehi, B.; Noshad, M. Characterization of Hypericum perforatum polysaccharides with antioxidant and antimicrobial activities: Optimization based statistical modeling. Int. J. Biol. Macromol. 2017, 104, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.; Paraschiv, I.; Colceru-Mihul, S.; Bubueanu, C.; Draghici, C.; Ichim, M. Chemical composition and antioxidant activity of Thymus vulgaris L. volatile oil obtained by two different methods. Rom. Biotechnol. Lett. 2010, 15, 5436–5443. [Google Scholar]
- Judpentiene, A.; Mockute, D. Chemical composition of essential oils of Artemisia absinthium L. (wormwood) growing wild in Vilnius. Chemija 2004, 15, 64–68. [Google Scholar]
- Singh, S.; Kumar, V.; Singh, S.; Dhanjal, D.S.; Datta, S.; Singh, J. Global Scenario of Plant-Microbiome for Sustainable Agriculture: Current Advancements and Future Challenges. In Plant Microbiomes for Sustainable Agriculture, 1st ed.; Yadav, A.N., Singh, J., Rastegari, A.A., Yadav, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 425–443. [Google Scholar] [CrossRef]
- Ke, J.; Wang, B.; Yoshicuni, Y. Microbiome engineering: Synthetic Biology of Plant-Associated Microbiomes in Sustainable Agriculture. Trends Biotechnol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Purcaru, T.; Diguta, C.; Matei, F. Antimicrobial potential of Romanian spontaneous flora—A Minireview. Sci. Pap. Ser. B Hortic. 2018, 62, 667–680. [Google Scholar]
- Brudea, V.; Risca, I.M.; Enea, C.; Tomescu, C.V. Efficacy of some biopesticides and plant secondary metabolites against fall webworm Hyphantria cunea Drury (F. Arctiidae-Lepidoptera) in the lab conditions. Agron. Resour. Mold. 2012, 45, 73–80. [Google Scholar] [CrossRef]
- Coisin, M.; Necula, R.; Grigoras, V.; Gille, E.; Rosenhech, E.; Zamfirache, M.M. Phytochemical evaluation of some Salvia species from Romanian flora. Sci. Ann. UAIC Veg. Biol. 2012, 58, 35–44. [Google Scholar]
- Morar, G.; Sirbu, C.; Oltean, I. Efectul extractelor hidroalcoolice din plante asupra gandacului de Colorado (Leptinotarsa decemlineata Say.) Nota II. ProEnvironment 2008, 2, 101–104. [Google Scholar]
- Daraban, G.; Badeanu, M.; Rusu, L.; Suteu, D. Biopesticides—A new challenge in assuring food quality and sustainable agriculture. USAMV Sci. Pap. Hortic. 2018, 61, 269–274. [Google Scholar]
- Daraban, G.; Badeanu, M.; Rusu, L.; Suteu, D. Researches on the biopesticides obtained by extraction with non-toxic solvents and the insecticide effect on deposit pests. Bull. Inst. Politech. Iasi 2018, 64, 33–41. [Google Scholar]
- Pruteanu, A.; Popescu, C.; Vladut, V.; Gageanu, G. Biochemical analysis of some vegetal extracts obtained from indigenous spontaneous species of Thymus serpyllum L. Rom. Biotechnol. Lett. 2018, 23, 14013–14024. [Google Scholar]
| Ingredient | Oral LD50, [mg/kg] | Dermal LD50, [mg/kg] | Ingredient | Oral LD50, [mg/kg] | Dermal LD50, [mg/kg] |
|---|---|---|---|---|---|
| Nicotine | 50–60 | 50 | Linalool | 2440–3180 | 3578–8374 |
| Rotenone | 60–1500 | 940–3000 | Neem oil | >5000 | >2000 |
| Pyrethrins | 1200–1500 | >5000 | Pongam oil | >4000 | >2000 |
| D-limonene | >4000 | >5000 | Ryania | 750–1200 | 4000 |
| Plant/Common Name | Extraction/Analysis Method | Extract Composition/Pharmacological and Phytosanitary Effects | Ref. |
|---|---|---|---|
| Achillea millefolium L. commonly known as—Yarrow | EOs extraction (0.4–1.1%) was carried out by hydrodistillation of dried plant samples (15–20 g; leaves, flowers and rods) for 2.5 h/. Extract analysis was carried out by GC/MS method (e.g., HP 5890 chromatograph/HP 5971 Mass spectrometer (70 eV ionization), equipped with capillary column (50 m × 0.32 mm, film thickness 0.25 μm). The heater temperature was varied between 70 °C and 250 °C with specific controlled increasing rates and maintained at 250 °C using He as carrier gas (2.0 mL/min). Alcoholic extraction studies (dry plant—ethanol) were also performed (more polar constituents obtained than in aqueous extracts). | Major representative constituents (isolated as ingredients from plant EOs): chamazulene, sabinene, β-pinene, 1,8-cineles, linalool, α-thujone, β-thujone, ocimene, camphor, ascaridole, caryophyllene oxide, β-eudesmol and α-bisabolol/ Pharmacological and phytosanitary effects: antimicrobial action as antiseptic, analgesic, anti-inflammatory and wound healing. | [54,55] |
| Origanum vulgare L., commonly known as—Wild Marjoram | Solid (aerial part of dry plant)—liquid (solvent) extraction with separation of EOs and aqueous, alcoholic (methyl and ethyl alcohol) and organic extracts (in cyclohexane, hexane, dichloromethane, ethyl acetate)/ Extract composition was controlled by GC/MS analysis of representative constituents for each extract or EO. Different conditions such as low pH, temperature or oxygen levels enhanced the antibacterial action of essential oils [4,9,10,11,15]. | Major representative constituents: phenolic compounds such as carvacrol and thymol, γ-terpine [10], linalool, p-cymene, 4-terpinol, β-caryophyllene, other terpenoids and flavonoids, as well as various organic acids such as rosmarinic acid, oleanolic and ursolic acid [20,24,25,30]/ Pharmacological and phytosanitary effects: antibacterial, antifungal, antioxidant, anti-inflammatory, antitumor and antiviral. There are used as infusions [34,40] | [56] |
| Satureja hortensis L., common known as—Thyme | EO extraction was done by hydrodistillation using a Clevenger type device (100 g dry powder plant, 3 h-extraction; water separation from EO by filtration over an anhydrous sodium sulphate layer)/ EOs and aqueous extracts were analyzed by gas chromatography (e.g., HP 6890 chromatograph with HP1/SPB-1/SupelcoWax-10 silicon-based column, thickness 0.25 mm) coupled with mass spectroscopy (e.g., HP 5973 selective mass detector, Agilent technologies). | Major representative constituents: —in EOs: thymol (45.9%), gama-terpinenes (16.71%), carvarol (12.81%) and p-cymene (9.61%); —in aqueous extracts: thymol (63.40%), linalool (42%), α-pinene (27.87%), b-pinene (22.10%), zingiberene (31.79%)/or linalool (42%), thymol (25.10%) and α-terpineol (10%)/or α-pinene (27.87%), 1,8-cineole (20.15%) and linalool (10.26%) | [57] |
| Calendula officinalis, commonly known as—Marigold | Extraction with a solvent (acetonitrile and ethyl acetate or 1% glacial acetic acid) using only the flowering plant, dried, crushed and sieved (<2 mm); extract separation by centrifugation/ Separated extracts were analyzed by liquid chromatography (e.g., LC Agilent 1200 column XDB-C18/4000 QTRAP LC-196) coupled with MS) (LC-MS system) (SANTE guidelines) | Representative constituents for antimicrobial activity in extracts: high content of total polyphenols, flavonoids and tannins as well as various synthetic pesticide residues. | [58] |
| Pimpinella anisum, commonly known as—Anise | EO extraction by hydrodistilation using only the dried and crushed seeds; water separation by filtration over an anhydrous sodium sulfate layer (after stored at 4 °C)/ P. anisum extract and EO were analyzed using UPLC MS Waters Alliance system/USA, with self-collecting and diode waters 2996 | Major representative constituents: —in EOs (LC-MS analysis): phenyl-propenoids, mono- terpene such as trans-anethol (82.1%), cis-anethol (5.8%), estragol (metilcavicol) (2.5%), linalool (2.3%), α-terpineol (1.5%) and metil-eugenol (1.3%) | [59] |
| Urtica dioica, commonly known as—Nettle | EO extraction with alcoholic solution (ethanol and/or methanol)—water (1 g/10 mL alcohol and distilled water (8:2 v/v) using dried leaves (fraction < 14 in); phases separation by centrifugation (3000 rpm, 15 min)/ EOs were analyzed by liquid chromatography coupled with mass spectroscopy (LC-MS) | Major representative constituents: high content of hydroxy-cinnamic acids (chlorogenic acid, caffeic acid, rosmarinic acid) and flavonoids (quercetin) | [60] |
| Hypericum perforatum | Alcoholic extraction (80% ethanol solution) by by reflux distillation (8 h, 60 °C water bath) using only dried leaves (60-mesh); separation of soluble/insoluble extracts by centrifugation (4000 rpm)/filtration/drying/ Extracts/EOs were analyzed by GC-MS system (e.g., Agilent GC-MS system equipped HP-5MS column (30 m × 0.25 m), t = 150–300 °C, 6 °C/min | Main representative constituents: polysaccharides (carbohydrates) and monosaccharides, L-arabinose (40.68%), D-galactose (36.72%), manose (3.58%), ramnose (14.38%), glucose (2.54%), xilose (1.83%), piranose, uronic acid, etc. | [61] |
| Extraction Method | Common Solvents Used (Volume) | Dried Plant Amount/Extraction Time | Characteristics/Advantages/Disadvantages |
|---|---|---|---|
| With solvents | Methanol, ethanol, or mixture of alcohols and water (repetable volume of 50–200 mL; temperature dependent of solvent used) | (5–5000) g/3–4 days |
|
| Distillation | Water—steam system (hydro-distillation), i.e.,
| (5–100) g/3–12 h |
|
| Maceration | Methanol/ethanol, or mixture of alcohols: water, 1:10 (not too much applicable; room temperature) | (0.5–100) g/3–7 days (samples imbibated with solvent processed on rotary evaporator (50 °C, 100 rpm, 120 min) |
|
| Mechanical extraction |
| (5–5000) g/10 min–1–2 h |
|
| Soxhlet extraction method | Methanol/ethanol, or mixture of alcohols and water (150–200 mL; low heating support), other solvents | (10–100) g/(3–18) h |
|
| Enzyme digestion |
| (10–100) g/(3–10) h (70 °C, 450 bar/510 min in pre-treatment of pomegranate peel, or 50–60 °C, 170–260 bar/20.64 min in extraction of lycopene from tomato skin) |
|
| Ultrasound heating (sonication) | Methanol/ethanol, or mixture of alcohol and water (50–100 mL) associated with ultrasonic-assisted installation | (5–100) g/(0.15–1) h (e.g., olive leaves) Solid-liquid oil enrichment (10:1, 20 min/25 °C) with ultrasound (225 W, 50% amplitude, duty cycle 0.5 s) produced edible oils with better quality than nonultra-sonicated oils. |
|
| Microwave heating (with traditional extraction) |
| (5–100) g/(0.15–1) h (e.g., olive leaves) |
|
| Supercritical fluid extraction (SCFE-based method) |
| milli-grams (<0.008 g) to grams (<100 g)/few minutes-often within 1 h (SCF extraction: batch, continuous flow or semi-con-tinuous, 200–1000 W) CO2 has a low critical t = 31.1 °C) and relatively low critical pressure (72.8 bar) |
|
| “Green extraction techniques” (with‚ green solvents’) |
| (5–1000) g/(2–18) h |
|
| Analysis Method | Operating Conditions for Analysis | Advantages/Disadvantages |
|---|---|---|
| HPLC-high performance liquid chromatography |
|
|
| FTIR-Fourier transform infrared spectroscopy |
|
|
| GC-MS |
|
|
| LC-MS/MS |
|
|
| Capillary electrophoresis (CE) |
|
|
| NMR spectroscopy |
|
|
| Thermospray analysis (TSP)/Electro-spray ionisation (ESI)—MS |
|
|
| Paper chromatography (PC) |
|
|
| Thin layer chromatography (TLC)/Bioautography |
|
|
| Spectrophotometric methods for quantification of total phenolics |
|
|
| Type of Extract | Effect on Targets | Observations | Ref. |
|---|---|---|---|
• Hydroalcoholic extracts (20%) of:
| Colorado beetle (Leptinotarsa decemlineata Say.) specific to potato crops Rejective effect for adults who does not attack the crops | Efficiency against larvae: 99.01%, 93.6% and 96.83% Efficiency of 7.14% (poor) till 72.22% (good) | [31,80] |
• Aqueous and alcoholic extracts of:
| Webworm Hyphantria cunea Drury (F. Arctiidae–Lepidoptera). Results: Extracts in ethanol (Sambucus ebulus, Artemisia vulgaris, A. absinthium, Tanacetum vulgare, Urtica dioica, Aristolochia clematidis, Heracleum spondylum, Taxus baccata, Salvia nemorosa) reduced the larvae production; extracts in cold water reduced webworm adults activity (Artemisia vulgaris, A. Dracunculus, Salvia nemorosa, Stachys silvatica). | The extracts were obtained from crushed/milled plants, using 25 g/L of cold water, and under stirring for 24 h. Ethyl alcohol extracts were obtained from 25 g of dry plants/200 mL of alcohol, filled to 1 L with water. The experiments were carried out under laboratory conditions (application on caterpil-lock shoots, placed in growth jar test vessels) | [78] |
• Alcoholic macerates of:
| Leptinotarsa decemlineata say and their larvae at various stages of development. Laboratory results: the most effective extract was that of Primula veris, which demonstrated a 100% killing rate for both adults and larvae in the first 24 h in a 5 L-location space. Hypericum perforatum, Achilleia milefolium and Pimpinella anisum have shown low efficiency for both adult Colorado beetles and their larvae, but these can also reduce the number of Leptinotarsa decemlineatasay in organic crop. | Plants were firstly ground/crushed, than screened and weighted with an analytical balance: 10 g of each plant sample. After these were treated with 100 mL of 97% ethylic alcohol in an Erlenmeyer vessel. The mixtures were periodically stirred and the maceration time was of minimum 90 days. The number of larvaes and adults was periodically counted for determination of its biopesticidal efficiency against larvaes and adults. Different extract dispersion ways were tested for insecticidal effect | [81] |
| Insect beans-Acanthoscelides obsoletus | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. https://doi.org/10.3390/app10248913
Suteu D, Rusu L, Zaharia C, Badeanu M, Daraban GM. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Applied Sciences. 2020; 10(24):8913. https://doi.org/10.3390/app10248913
Chicago/Turabian StyleSuteu, Daniela, Lacramioara Rusu, Carmen Zaharia, Marinela Badeanu, and Gabriel Mihaita Daraban. 2020. "Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products" Applied Sciences 10, no. 24: 8913. https://doi.org/10.3390/app10248913
APA StyleSuteu, D., Rusu, L., Zaharia, C., Badeanu, M., & Daraban, G. M. (2020). Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Applied Sciences, 10(24), 8913. https://doi.org/10.3390/app10248913








