Abstract
The color of food is a critical factor influencing its general acceptance. Owing to the effects of chemical colorants on health, current research is directly aimed at producing natural and healthy food colorants from microbial sources. A pigment-producing fungal isolate, obtained from soil samples and selected based on its rapidity and efficiency in producing red pigments, was identified as Monascus ruber OMNRC45. The culture conditions were optimized to enhance pigment production under submerged fermentation. The optimal temperature and pH for the highest red pigment yield were 30 °C and 6.5, respectively. The optimum carbon and nitrogen sources were rice and peptone, respectively. The usefulness of the pigment produced as a food colorant was evaluated by testing for contamination by the harmful mycotoxin citrinin and assessing its biosafety in mice. In addition, sensory evaluation tests were performed to evaluate the overall acceptance of the pigment as a food colorant. The results showed that M. ruber OMNRC45 was able to rapidly and effectively produce dense natural red pigment under the conditions of submerged fermentation without citrinin production. The findings of the sensory and biosafety assessments indicated the biosafety and applicability of the red Monascus pigment as a food colorant.
1. Introduction
In addition to taste, aroma, and texture, the color of food is a main factor that influences its total acceptability. Therefore, since ancient times, people have used multiple colorants in their food to improve its appearance, flavor, taste, preservability, and nutrient quality. Food colorants can be obtained either from natural sources (plants, animals, or microorganisms) or through chemical synthesis [1,2]. Owing to the differences in the origin and composition of the applied food colorants, the degree of health risk and environmental suitability varies widely [3,4]. Chemically synthesized food colorants are not preferred because of their potential toxicity, carcinogenicity, and undesirable side effects on human health and the environment. Natural food pigments are gaining increasing popularity globally, as they are safe to use and have several nutritional benefits and potential medicinal properties [4,5,6].
Microorganisms (bacteria, fungi, and microalgae) are among the most promising natural sources of food colorants, which can be produced both intracellularly and extracellularly. They are characterized by high, sustainable productivity and relatively low costs, as they can be cultured on agricultural and food wastes at any time of the year. In addition, the pigments obtained are varied, easily extractable, and relatively heat-stable, offering several health and nutritional benefits [7,8]. The fungus Monascus was widely used in East Asian countries approximately two thousand years ago, and it continues to be used for the fermentation and coloring of foods, particularly rice (to produce red rice), which is also referred to as “red yeast rice”, “hongqu,”, “red mold rice”, “red koji”, and “anka” in traditional medicine. However, the name “Monascus spp.” was proposed only in 1884 for a versatile group of filamentous fungi comprising Monascus floridanus, Monascus pallens, Monascus pilosus, Monascus purpureus, Monascus ruber, Monascus sanguineus, Monascus eremophilus, Monascus lunisporas, and Monascus argentinensis [9,10,11]. Pigments produced by Monascus by natural fermentation have a high economic value and have attracted considerable attention as coloring agents globally. They have several advantages, such as ease of production using inexpensive substrates, favorable solubility in acidic water and ethanol, presence of numerous bioactive metabolites, and safety when produced under specific conditions [12].
Monascus spp. are able to synthesize different (intracellular and extracellular) polyketide secondary metabolites that protect cells against oxidative stress (antioxidants) and produce different and distinct pigments. The most common pigments produced by Monascus are orange (monascorubrin and rubropunctatin), yellow (monascin and ankaflavin), and hydrosoluble red (monascorubramine and rubropunctamine). The percentage of production of each pigment by Monascus is highly dependent on the species and cultivation conditions (substrate, fermentation mode, dissolved oxygen, pH, and temperature). Of the several pigments produced by Monascus, the red pigment (monascorubramine) is in high demand [4,13,14,15].
Solid-state fermentation of rice and grains is the traditional method for cultivating Monascus to produce high levels of pigments, although it has been linked to scale-up- and contamination-related issues [16]. In addition, traditional solid-state fermentation processes are labor-intensive, time-consuming, and require large cultivation areas [17,18]. Monascus strains usually produce lower levels of pigment in submerged cultivation than in solid-state cultivation [19]. However, the production of pigments by Monascus under submerged fermentation is characterized by controlled cultivation conditions and medium, which helps to control the production of secondary metabolites [10]. During the growth of certain Monascus strains, the mycotoxin citrinin is produced as a secondary metabolite under specific fermentation conditions [20,21]. Citrinin is nephrotoxic, hepatotoxic, and carcinogenic to various animals and humans; therefore, its presence in food products is a critical issue. Previous studies have shown that the citrinin production gene is only harbored by M. purpureus and M. kaoliang (M. kaoliang is a synonym for M. purpureus) and not by M. pilosus, M. ruber, M. floridanus, M. sanguineus, M. barkerior, or M. lunisporas. However, M. ruber has been reported to produce citrinin under certain fermentation conditions [10]. In addition, an “uncommon case of M. ruber invasive gastric infection associated with the consumption of contaminated dried and salted fish” has been reported [22].
In this study, we isolated a fungus that could efficiently produce safe red pigments for use as food colorants, with an emphasis on preventing the production of mycotoxins (e.g., citrinin) as secondary metabolites. Additionally, we attempted to improve pigment production by controlling culture conditions, and the pigment produced was extracted and tested as a food colorant in specific food products for children (jellybeans and lollipops).
2. Materials and Methods
2.1. Samples and Isolation Sources
Nine different samples were collected from various sites in Egypt exposed to spillage of intact or broken rice grains in addition to rice processing residues (wastes). Among the samples, four soil samples were collected from places near rice storage and hulling machines, while two soil samples were collected from rice paddy fields (Kafr El-Sheikh Governorate). In addition, the remaining three samples were collected from wastewater of a local food processing market (in Giza Governorate). The samples were collected in sterilized jars and transferred to the laboratory under aseptic conditions, as previously described by Darwesh et al. [23].
2.2. Isolation of Red Pigment-Producing Fungi
The samples were enriched by culturing with 10% (w/v) sterilized broken rice (1–2 mm diameter) to increase the chances of isolating red pigment-producing fungi. The pigment-producing fungi were isolated on solidified rice agar, which was prepared as described by Wu et al. [16] with certain modifications: the medium contained 15 g/L rice powder, 0.5 g/L KH2PO4, 1.0 g/L K2HPO4, 0.1 g/L NaCl, 0.2 g/L MgSO4∙7H2O, and 1.0 g/L NH4NO3, and the pH was adjusted to 5.5–6. After 7 days of culture on rice agar plates at 28 ± 2 °C, the fungal colonies that developed and produced red pigments were selected and re-cultured on potato dextrose agar (PDA) for purification and preservation. The selected fungal isolates were screened to assess the intense and rapid production of red pigments (morphologically). For screening, individual fungal discs (9.5 mm in diameter) were cut from PDA Petri dishes and placed at the center of the surface of rice agar plates and incubated for 7 days at 28 ± 2 °C. For evaluating the production of red pigments, the fungal discs that produced the most intense and widely dispersed pigments rapidly were selected for subsequent studies.
2.3. Identification of the Target Fungal Strain
The selected fungal isolate that exhibited satisfactory red pigment production was identified and characterized morphologically as well as by using molecular biology techniques. The morphological characteristics were evaluated using a light microscope (model cx41, Olympus, Tokyo, Japan) after 3 days of culturing on PDA plates [24]. Molecular identification of the fungal isolate was performed after culturing for 3 days on potato dextrose broth (PDB) and harvesting using a filter paper (Whatman No. 1, Sigma-Aldrich). Total genomic DNA was extracted using the CTAB protocol after drying and grinding of the mycelium using liquid nitrogen [25]. DNA from the fungal isolate was amplified by polymerase chain reaction (PCR) using internal transcribed spacer 1 (ITS1) (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) primers designed for sequencing. The sequence was identified by comparing the contiguous DNA sequence with data from the reference and type strains available in GenBank using the BLAST program (National Centre for Biotechnology Information). The obtained sequences were aligned using the Jukes Cantor Model [26].
2.4. Optimization of Extracellular Red Pigment Production
Various environmental and nutritional parameters, such as temperature, pH, and carbon and nitrogen sources, were evaluated to improve and optimize the production of red pigments from the fungal strain under study. The optimization experiments were performed in 250 mL conical flasks containing 100 mL of modified rice broth medium (submerged fermentation), with incubation for 7 days (unless otherwise indicated) in an orbital incubator shaker. Red pigment production was indirectly evaluated by measuring the absorbance of the culture filtrate at 500 nm using a spectrophotometer (after centrifugation at 4000 rpm for 5 min) [27,28].
Instead of using the carbon or nitrogen sources present in the original medium, different components were added to the fermentation medium (rice broth medium) as carbon and nitrogen sources. The carbon sources included rice powder, wheat flour, corn flour, corn cob powder, and glucose at a concentration of 10 g/L, whereas the nitrogen sources included (NH4)2SO4, NH4NO3, KNO3, peptone, and urea (quantities were calculated to be equivalent to 100 mg/L). As important environmental parameters, the incubation temperature (20, 25, 28, 30, 35, and 40 °C) and initial pH values (4.5, 5.5, 6.0, 6.5, 7.5, and 8.5) in the medium were modulated to study their effect on the production of red pigments by the fungal strain. In addition, another experiment was performed to evaluate the production of red dyes under different incubation periods (4, 6, 8, 10, 12, 14, 16, 18, and 20 days) at the optimum temperature and pH with the highest pigment yield.
2.5. Extraction of Pigments and Secondary Metabolites
The optimized carbon and nitrogen sources from the previous experiment were used for fermentation of the selected fungal strain under submerged conditions to study the secondary metabolites produced. Static submerged fermentation was initiated by inoculating five activated fungal agar discs in a 500 mL flask containing 350 mL of modified liquid rice medium (pH 6.0). After incubation for 5 days at 28 ± 2 °C, the mycelia were harvested by filtration and washed twice with distilled water. The mycelium-free supernatant and wash mixture were collected and centrifuged at 4000 rpm for 5 min (to remove impurities) after volume adjustment for quantitative calculation. The washed mycelia were extracted by vigorous stirring for 12 h in 200 mL of 95% ethanol followed by centrifugation. Both aqueous and ethanolic extracts were subjected to spectrophotometric analysis at 500 nm for quantifying the red pigments produced.
2.6. Citrinin Analysis
The presence of citrinin in the rice products fermented using the selected fungal strain was analyzed in 10-day-old cultures containing broken rice, under solid or liquid fermentation conditions. Citrinin extraction was performed using 100 mL of chloroform per 50 g of culture-mycelium mixture after homogenization in a high-speed mixer (Overhead Stirrer NOHS-100, Labnics, London, UK) at 16,000 rpm for 5 min. The extraction process was repeated three times, following which the chloroform extracts were combined, washed, filtered, and concentrated until near dryness. Next, thin-layer chromatography (TLC) was performed to detect citrinin [29].
2.7. Biosafety Evaluation
The biological toxicity of the produced red pigment was evaluated in mice (according to the appropriate and recognized ethics) as follows: Young adult male mice (white mice, Mus musculus domesticus) weighing 25–30 g were obtained from the animal house at King Saud University, Riyadh, Saudi Arabia. The animals were housed in polycarbonate boxes at room atmosphere in the laboratory and acclimated for 1 week before the experiments commenced. The mice were divided into 13 groups, and the control and treatment groups comprised six mice each. The animals were fed a basal diet containing 20% casein, 10% sugar cane, 50% corn starch, 10% corn oil, 1% vitamin mixture, 5% cellulose, and 4% salt mixture [30]. The animals were provided with free access to water. Next, two types of pigments—natural (fungal) and synthetic red pigments (carmoisine E122; Sigma-Aldrich, India) —were fed by oral dosing for comparison. An acute toxicity test was performed to determine the oral lethal dose (LD50) of the two different colorants separately. The male white mice were randomly divided into natural- and carmoisine-fed groups. Each group was divided into six subgroups consisting of six animals that received six different doses of each pigment orally (10, 100, 1000, 1600, 2900, and 5000 mg/kg body weight). The mice in the control group received distilled water instead of pigments. The animals were observed thoroughly for the onset of any immediate toxic signs as well as during the observation period of 1 week. Motor activity, tremors, convulsions, aggressiveness, sedation, muscle relaxation, hypnosis, analgesia, ptosis, lacrimation, paralysis, diarrhea, and skin color were evaluated in the first 1 h, 24 h, and 1 week of pigment administration. The death rate was also recorded in each group. For hematological and serum biological analyses, at the end of the observation period, blood samples were collected and centrifuged to separate the serum. The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and blood urea nitrogen (BUN) were measured in the serum samples using kits from Biomed Diagnostics (Egypt). At the end of the experimentation, the animals used for blood sampling were sacrificed and autopsied. The liver, heart, spleen, and kidney were harvested, and the weight of each organ, along with the total body weight, was measured. The organs were also observed for major changes in appearance before weighing. The weight of each organ relative to the total body weight was calculated. The hepatosomatic index (HIS) was calculated as the percentage of liver weight upon body weight [31].
2.8. Application of the Obtained Red Pigment as a Food Colorant
As sample food products, lollipops were prepared by mixing sucrose (242.4 g), corn syrup (129.5 mL), water (126.6 mL), and citric acid (0.75 g) and heating the mixture to 157 °C. The mixture was rapidly cooled to 110 °C, and 1.05 g of a flavoring agent as well as the natural fungal red pigment was added to produce red-colored lollipops. Jellybeans were prepared by mixing the ingredients using the traditional procedure (15 g of gelatin mixed with 85 g of purified sucrose, 0.2% citric acid, flavoring agent, and a mixture of sodium benzoate and potassium citrate at 0.1%). The concentrated fungal red pigment (0.1%) was added after mixing the ingredients, following which 0.1% (w/w) of ascorbic acid was added to the jelly. The jellies were placed in molds in a refrigerator until they solidified and then were packed in a special foil. The control was prepared using 0.1% carmoisine E122. Sensory evaluation was performed by ten panelists. The panelists were asked to evaluate the taste, color, texture, odor, and overall acceptability of the prepared food products.
2.9. Statistical Analyses
The experiments were performed in triplicate, and the results are expressed as the mean ± standard deviation (SD). Statistical significance was evaluated using analysis of variance (SAS Studio 3.8, SAS Institute Inc., Cary, NC, USA) followed by the determination of the least significant difference (LSD) at 0.05.
3. Results and Discussion
3.1. Isolation and Identification of Pigment-Producing Fungal Strains
Owing to the hazards associated with chemical colorants, in the current study, we attempted to directly produce natural and environmentally safe food colorants from fungal sources. To this end, nine samples suspected to contain pigment-producing fungi (based on their history of exposure to rice residues) were used as isolation sources of the target fungi. Six soil samples and three wastewater samples collected from food processing facilities were enriched using broken rice as the preferred carbon source to isolate pigment-producing fungi. Following the initial isolation process, twenty pigment-producing fungal isolates were obtained (Figure 1), which were subsequently screened to select the most efficient strain in terms of pigment production.
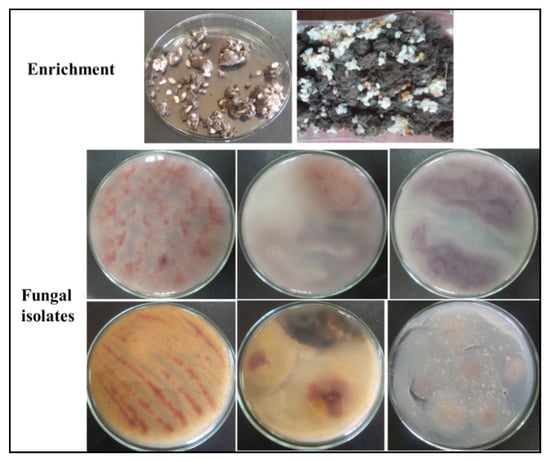
Figure 1.
Enrichment and purification of pigment-producing fungi.
The isolated fungi were screened for their ability to produce pigments on rice agar medium plates. The rice medium used in this experiment was selected based on previous reports of its use as a preferred carbon source for multiple edible/pigment-producing fungi [6,32]. Pigmentation of various colors (red, yellow, orange, and violet) was observed on the incubated rice agar medium plates (Figure 1). However, the red pigment was observed to be the most highly dispersible pigment, which was produced by fungal isolate No. 2 (isolated from soil samples enriched with broken rice). For confirmation, the fungal isolates were re-cultured in rice broth medium to confirm the production of pigments. It was clearly observed in both broth and solid cultures that fungal isolate No. 2 could rapidly produce an intense red pigment (Figure 2). The produced pigment showed an absorption maximum at a wavelength of 500 nm, as shown in Figure 2. Accordingly, fungal isolate No. 2 was selected for the subsequent studies.
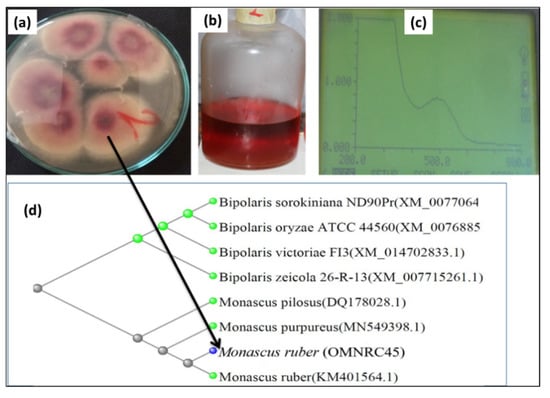
Figure 2.
Red pigment-producing fungal isolate (No. 2) with the highest potency: (a) its growth on rice agar medium, (b) pigment produced in liquid medium, (c) wavelength scanning using a UV spectrophotometer, and (d) phylogenetic tree identification as Monascus ruber OMNRC45.
The fungal isolate was characterized and identified based on its morphological, culture, and molecular biology properties. In the microscopic observation of the fungal isolate, the asexual form with a chain of conidia and the sexual form with thin-walled ascoscarps containing oval ascospores were observed. These characteristics, in addition to the culture characteristics, indicated that the obtained isolate was of M. ruber [33]. Moreover, molecular biology techniques were applied to validate the results. Genomic DNA was isolated and purified according to a previously described protocol using isopropyl alcohol [34,35]. The ITS genes were sequenced after amplification using PCR. The obtained sequences were compared with related sequences available in GenBank using BLAST. The phylogenetic tree of the fungal isolate is shown in Figure 2, where its sequence was observed to show 99.45% similarity with the reference strain M. ruber in GenBank. Accordingly, it was named Monascus ruber OMNRC45 and registered and deposited under the same name with the National Research Centre (NRC), Egypt.
3.2. Optimization of Red Pigment Production by M. ruber OMNRC45
Pigment productivity can be influenced by the microbial strain, medium composition, and fermentation conditions [36]. Hence, to maximize the yield of red pigments produced by M. ruber OMNRC45, the environmental and culture conditions were improved. Carbon is an essential nutrient for the biosynthesis of cellular components, such as carbohydrates, proteins, and fats, and through oxidation, it provides the energy necessary for cells. For Monascus spp., carbon plays a critical role in cell growth, metabolism, and pigment production [37,38]. In the present study, different carbon sources (i.e., rice powder, wheat flour, corn flour, corn cob powder, and glucose) were used to improve the growth of M. ruber OMNRC45 (in liquid medium) and its red pigment yield. The results presented in Figure 3a show that under the experimental conditions, rice powder was the best source of carbon for biomass and red pigment production. These results were consistent with the findings reported by Carvalho et al. [29], who obtained the highest pigment yield using rice powder as a carbon source. Through the results obtained, the higher yield of red pigments in the case of using rice flour as a carbon source can be linked to its higher content of starch compared to other carbon sources used. This is supported by the results obtained by Long et al. [39], who indicated that the increase in the production of Monascus pigments is directly related to the efficiency in α-amylase production and starch degradation by M. ruber. Conversely, crushed corn cob did not lead to high growth or pigment yield, which could be attributed to the need for additional pre-treatment when using this substance to match the growth requirements of the fungal strain.
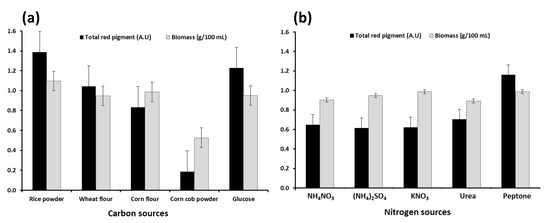
Figure 3.
Effect of carbon (a) and nitrogen (b) sources on biomass and total red pigment production by Monascus ruber OMNRC45 in liquid medium.
For similar reasons, the source of nitrogen in the culture medium is an essential factor influencing microbial growth as well as the production of primary and/or secondary bioactive metabolites [40]. As secondary metabolites, the production of both intracellular and extracellular pigments is expected to be influenced (quantitatively and qualitatively) by the nitrogen source used. We investigated the effects of organic (peptone–urea) and inorganic (NH4NO3-(NH4)2SO4-KNO3) nitrogen sources on the growth of M. ruber OMNRC45 and extracellular red pigment productivity. As shown in Figure 3b, M. ruber OMNRC45 cultured using peptone as the nitrogen source (in cultivation medium) showed the highest pigment yield. However, the increase in fungal biomass production using the same nitrogen source was not significant compared to the results obtained using ammonium sulfate and potassium nitrate. Gunasekaran and Poorniammal [41] reported that the inclusion of peptone in the culture medium led to the highest red pigment yield by Penicillium sp. and various other pigment-producing fungi.
In this study, temperature and pH were considered important environmental and culture parameters for improving the yield of red dyes from M. ruber OMNRC45. Cultivation temperature plays an important role in the rate of all microbial biochemical activities, including nutrient absorption, enzyme synthesis, and pigment production [42]. The optimum temperature for growth and production of dyes by Monascus spp. has a wide range (from 25 to 37 °C) and primarily depends on the species [29]. Therefore, an experiment was conducted to determine the effect of different incubation temperatures (20–40 °C) on the biomass and red pigment yield from M. ruber OMNRC45. The data obtained and presented in Figure 4a showed that 30 °C was the optimum temperature for maximum biomass and red pigment production. The production of the red pigment increased gradually with a rise in the fermentation temperature from 20 °C to 25 °C and then to 30 °C. Subsequently, pigment production decreased as the incubation temperature increased from 30 °C to 35 °C and then to 40 °C. Similar findings were reported by Jeon et al. [43] on the optimum temperature for pigment production by M. purpureus MMK2, M. ruber KCTC6122, and M. purpureus P-57. More recently, Padmavathi and Prabhudessai [44] reported that optimum mycelium growth and pigment production by M. anguineus and M. purpureus MTCC410 were observed at a fermentation temperature of 30 °C. However, these results were slightly different from those reported by Babitha et al. [45], who found that the maximum pigment yield was obtained between 32 °C and 35 °C.
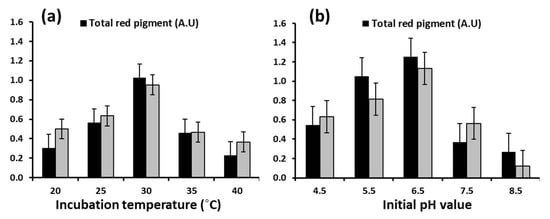
Figure 4.
Effect of incubation temperature (a) and initial pH (b) on biomass and total red pigment production by Monascus ruber OMNRC45.
It is well known that the initial pH of the culture medium has a significant effect on enzyme and pigment production by Monascus spp. [46]. Accordingly, an experiment was designed to study the effect of different pH values on biomass and red pigment production by M. ruber OMNRC45. When the fungal isolate was cultured in a liquid medium under different initial pH values (4.5–8.5), pH 6.5 was found to be optimal for pigment production, as shown in Figure 4b. Evidently, an alkaline pH (8.5) strongly inhibited both biomass and extracellular pigment production. Joshi et al. [47] reported that pH values ranging from 5.5 to 6.5 were ideal for the production of pigments by Monascus spp., while Babitha et al. [48] reported (for M. purpureus) a wider optimal pH range (4.5–7.5) for the same.
3.3. Biosafety Evaluation of the Monascus Pigments
In addition to pigments, the mycotoxin citrinin is also produced under certain conditions as a byproduct of fermentation by Monascus spp. Citrinin has hepatotoxic properties and causes functional and structural damage to the kidneys, in addition to altering liver metabolism [49,50,51]. Several toxicity studies on Monascus pigments have confirmed the safety of their consumption in specific quantities. Conversely, several studies have confirmed the potential toxicity of some Monascus strains owing to citrinin contamination, whereas some strains have been reported to be devoid of citrinin production ability. In addition to its dependence on the type of fungal strain, citrinin production is also markedly influenced by the environmental and culture conditions during fungal growth [11,52,53,54]. In the present study, we aimed to evaluate the biosafety of the red pigments produced by M. ruber OMNRC45. The biosafety assessment was performed by citrinin detection (using TLC) in the extracted red pigments in parallel with the measurement of its toxicity in mice.
The results of TLC analyses revealed that no citrinin (nephrotoxic or hepatotoxic) was detected in the chloroform extract or the filtrate of M. ruber OMNRC45 cultured in potato dextrose broth (PDB), PDB plus glycine, or rice media (Figure 5). In TLC, the reference sample (citrinin) formed a yellow fluorescence band under UV light, whereas similar bands were not observed in the target samples. This result is consistent with those reported by Chen et al. [20] and Moharram et al. [28], who confirmed the presence of genes associated with citrinin biosynthesis in only 18 out of 30 Monascus strains studied. Notably, heat treatment of citrinin (140 °C) leads to the production of a novel compound, citrinin H2, which exerts weaker cytotoxic effects than citrinin [55]. Hence, if present in food materials, citrinin-contaminated pigments should be subjected to heat treatment or degradation via other strategies. Based on the aforementioned results, it can be concluded that M. ruber OMNRC45 cultured under the conditions described in the present study can be considered safe and devoid of citrinin contamination.
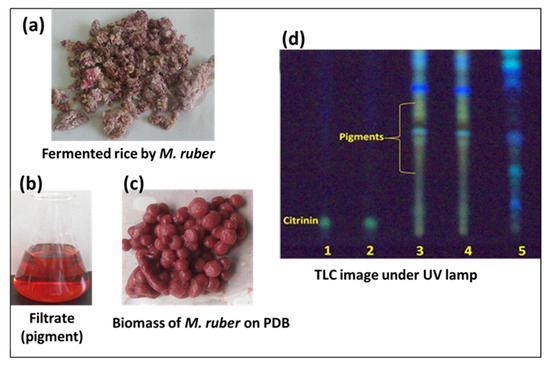
Figure 5.
Fermentation of Monascus ruber OMNRC45 in rice (a), cultural filtrate (b), and biomass (c) on potato dextrose broth (PDB) media. TLC analysis (d) for citrinin detection: lanes 1 and 2—reference citrinin; lanes 3, 4, and 5—chloroform extract of M. ruber cultivated on PDB, PDB plus glycine, and rice media, respectively.
The proximate composition of red Monascus rice was determined. It contained 9.26% moisture, 72.67% carbohydrate, 11.89% protein, 1.34% lipid, and 0.42% ash. These values were similar to those reported by Kumari et al. [31]. The biotoxicity of the red pigments obtained from M. ruber OMNRC45 was evaluated in a mouse feeding experiment. Mice were fed with fungal pigments at oral doses of 0, 10, 100, 1000, 1600, 2900, and 5000 mg/kg body weight in distilled water. The artificial dye carmoisine E122 was used as a positive control for comparison. At the end of the experiment, the following organs were harvested: the heart, liver, kidney, and spleen. Changes in organ appearance were examined, and the organs were weighed. The hepatosomatic index (HIS), mortality percentage, and toxicity signs (abnormal signs) were evaluated (Table 1). Body and organ weights and the ratio of liver to body weight are indicators of organ damage or abnormalities resulting from the provision of treatment [56]. The results showed that there were significant differences between the control and Monascus and carmoisine pigment-fed mice at different concentrations in terms of liver, kidney, heart, and spleen weight. In addition, visual examination of the organs after dissection showed that organs harvested from mice treated with the highest concentrations of Monascus pigment showed normal signs, similar to the organs harvested from control mice. However, this was not the case for the mice fed carmoisine, especially at 5000 mg/kg. At this concentration, marginal changes were observed in liver color and size. Moreover, no mortality was observed in mice fed the Monascus pigment. According to Masango et al. [57], mortality with characteristic enlarged liver (liver weight/body mass >7%) is used to measure hepatotoxicity. There was no hepatotoxicity observed in mice treated with different concentrations of the pigment, since the ratio ranged between 5.4 and 5.7; however, the HIS value increased, especially in mice treated with carmoisine at different concentrations. Further, aggressive cases were observed in male mice treated with 5000 mg/kg of carmoisine, while cases of diarrhea and deaths were observed in both females and males. The LD50 value of carmoisine (determined via a simple toxicity assessment) was found to be 4166 mg/kg body weight.

Table 1.
Effect of Monascus pigment and carmoisine E122 at different concentrations on mean organ weight (g), hepatotoxicity, mortality, and other observations.
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) activities are indicators of hepatic health. Our study demonstrated that the Monascus pigment exerted no significant effect on AST, ALT, and ALP levels compared to the control group (Table 2). Blood urea nitrogen (BUN) is an indicator of kidney damage resulting from the enzymatic hydrolysis of urea to ammonia by urease [58]. The current study showed that there were no differences in BUN levels in mice fed the Monascus pigment compared to the levels in control mice. The results for blood enzyme levels in mice fed the red Monascus pigments from M. ruber OMNRC45 were similar to those obtained by Kumari et al. [31]. However, the carmoisine-fed mice showed significant differences in the levels of AST, ALT, and ALP compared to the Monascus pigment-fed and control mice, possibly owing to the effect of the synthetic dye. Although the values of most parameters evaluated in our study were within the normal range, similar to findings reported by Serfilippi et al. [59], the values increased significantly at high concentrations of carmoisine.

Table 2.
Effects of Monascus pigment and carmoisine E122 at different concentrations on the biological activities of mice.
Based on the results of the biosafety evaluation and findings reported by Serfilippi et al. [59], the natural red pigment obtained from M. ruber OMNRC45 can be considered a promising natural colorant. Conversely, the application of the synthetic dye carmoisine can induce several abnormal signs in mice, especially at high concentrations, and it must be replaced with a safe natural product.
3.4. Sensory Evaluation of Food Products
Two food products were evaluated as models for food industries, and the results of sensory scores are presented in Table 3. The food products (lollipops and jellybeans) acquired an intense and persistent red color, and their organoleptic properties were enhanced upon the addition of red pigments from M. ruber OMNRC45. The appeal, color, appearance, and overall acceptability of the lollipops and jellybeans were considered to have improved owing to the use of the natural fungal colorant. As stated in previous studies, the application of natural dyes can be potentially beneficial for protecting consumer health because it allows the manufacturing of completely natural foods devoid of artificial additives [60,61].

Table 3.
Mean sensory scores of food samples colored with the red pigment from Monascus ruber OMNRC45.
4. Conclusions
The fungal strain M. ruber OMNRC45 was shown to effectively produce a natural red pigment under submerged fermentation conditions without producing the harmful mycotoxin citrinin. The production of fungal pigments could be maximized by controlling fermentation conditions such as carbon and nitrogen sources and the pH value. Sensory and biosafety evaluation of the red Monascus pigment produced by M. ruber OMNRC45 indicated its biosafety and applicability as a food colorant compared to unsafe artificial colorants. Further studies should be conducted to better understand the sources, production, and biosafety of microbial pigments as promising alternatives to hazardous artificial colorants.
Author Contributions
All authors conceived and planned the study; O.M.D. and I.A.M. performed the microbiological experiments; H.S.A. and S.A.A. performed the sensory and biosafety evaluation. All authors contributed equally to data analysis and discussion of results and commented on the manuscript. O.M.D. and I.A.M. wrote the manuscript in consultation with all authors; I.A.M. reviewed and edited the manuscript; Y.-K.O. revised and consolidated the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The research team expresses their gratefulness to King Saud University (project number: RSP-2020/283), Riyadh, Saudi Arabia, and the National Research Centre, Cairo, Egypt, for the financial support of this work. Y.-K. Oh would also like to acknowledge the support of the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT, Republic of Korea (NRF-2019R1A2C1003463).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barakat, K.M.; Mattar, M.Z.; Sabae, S.Z.; Darwesh, O.M.; Hassan, S.H. Production and characterization of bioactive pyocyanin pigment by marine Pseudomonas aeruginosa OSh1. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 933–943. [Google Scholar]
- Darwesh, O.M.; Barakat, K.M.; Mattar, M.Z.; Sabae, S.Z.; Hassan, S.H. Production of antimicrobial blue green pigment Pyocyanin by marine Pseudomonas aeruginosa. Biointerface Res. Appl. Chem. 2019, 9, 4334–4339. [Google Scholar]
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J.S.; Lemmens, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M.; Van Loey, A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015, 171, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Ogbodo, U.O.; Ugwuanyi, J.O. Production, Use, and Prospects of Microbial Food Colorants. In Microbial Production of Food Ingredients and Additives; Elsevier Inc.: Academic Press: London, UK, 2017; Volume 2017, pp. 189–216. [Google Scholar]
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Bühler, R.M.M.; de Carvalho, J.C.; de Oliveira, D.; Moritz, D.E.; Schmidell, W.; Ninow, J.L. Monascus: A reality on the production and application of microbial pigments. Appl. Biochem. Biotechnol. 2016, 178, 211–223. [Google Scholar] [CrossRef]
- Malik, K.; Tokkas, J.; Goyal, S. Microbial pigments: A review. Int. J. Microbial. Res. Technol. 2012, 1, 361–365. [Google Scholar]
- Kim, Y.E.; Matter, I.A.; Lee, N.; Jung, M.; Lee, Y.C.; Choi, S.A.; Lee, S.Y.; Kim, J.R.; Oh, Y.K. Enhancement of astaxanthin production by Haematococcus pluvialis using magnesium aminoclay nanoparticles. Bioresour. Technol. 2020, 307, 123270. [Google Scholar] [CrossRef]
- Jongrungruangchok, S.; Kittakoop, P.; Yongsmith, B.; Bavovada, R.; Tanasupawat, S.; Lartpornmatulee, N.; Thebtaranonth, Y. Azaphilone pigments from a yellow mutant of the fungus Monascus kaoliang. Phytochemistry 2004, 65, 2569–2575. [Google Scholar] [CrossRef]
- Patakova, P. Monascus secondary metabolites: Production and biological activity. J. Ind. Microbiol. Biotechnol. 2013, 40, 169–181. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, W.; Chen, F.A. Monascus pilosus MS-1 strain with high-yield monacolin K but no citrinin. Food Sci. Biotechnol. 2016, 25, 1115–1122. [Google Scholar] [CrossRef]
- Mostafa, M.E.; Abbady, M.S. Secondary metabolites and bioactivity of the Monascus pigments review article. Glob. J. Biotechnol. Biochem. 2014, 9, 1–13. [Google Scholar]
- Yu, X.; Wu, H.; Zhang, J. Effect of Monascus as a nitrite substitute on color, lipid oxidation, and proteolysis of fermented meat mince. Food Sci. Biotechnol. 2015, 24, 575–581. [Google Scholar] [CrossRef]
- Rosales, E.; Pazos, M.; Sanromán, M.Á. Solid-state fermentation for food applications. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2018, pp. 319–355. [Google Scholar]
- Mondal, S.; Pandit, S.G.; Puttananjaiah, M.H.; Harohally, N.V.; Dhale, M.A. Structural and functional characterization of new pigment molecule Monashin from Monascus purpureus CFR410–11. Process Biochem. 2019, 82, 173–178. [Google Scholar] [CrossRef]
- Wu, W.T.; Wang, P.M.; Chang, Y.Y.; Huang, T.K.; Chien, Y.H. Suspended rice particles for cultivation of Monascus purpureus in a tower-type bioreactor. Appl. Microbiol. Biotechnol. 2000, 53, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Espinosa, R.M.; Webb, C. Submerged fermentation in wheat substrates for production of Monascus pigments. World J. Microbiol. Biotechnol. 2003, 19, 329–336. [Google Scholar] [CrossRef]
- Darwesh, O.M.; El-Maraghy, S.H.; Abdel-Rahman, H.M.; Zaghloul, R.A. Improvement of paper wastes conversion to bioethanol using novel cellulose degrading fungal isolate. Fuel 2020, 262, 116518. [Google Scholar] [CrossRef]
- Hsu, F.L.; Wang, P.M.; Lu, S.Y.; Wu, W.T. A combined solid-state and submerged cultivation integrated with adsorptive product extraction for production of Monascus red pigments. Bioprocess. Biosyst. Eng. 2002, 25, 165–168. [Google Scholar]
- Chen, Y.P.; Tseng, C.P.; Chien, I.L.; Wang, W.Y.; Liaw, L.L.; Yuan, G.F. Exploring the distribution of citrinin biosynthesis related genes among Monascus species. J. Agric. Food. Chem. 2008, 56, 11767–11772. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, T.H.; Lee, M.H.; Su, N.W. Biologically active components and nutraceuticals in the Monascus-fermented rice: A review. Appl. Microbiol. Biotechnol. 2008, 77, 965–973. [Google Scholar] [CrossRef]
- Iriart, X.; Fior, A.; Blanchet, D.; Berry, A.; Neron, P.; Aznar, C. Monascus ruber: Invasive gastric infection caused by dried and salted fish consumption. J. Clin. Microbiol. 2010, 48, 3800–3802. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Eida, M.F.; Matter, I.A. Isolation, screening and optimization of L-asparaginase producing bacterial strains inhabiting agricultural soils. Biosci. Res. 2018, 15, 2802–2812. [Google Scholar]
- Elshahawy, I.; Abouelnasr, H.M.; Lashin, S.M.; Darwesh, O.M. First report of Pythium aphanidermatum infecting tomato in Egypt and its control using biogenic silver nanoparticles. J. Plant Prot. Res. 2018, 58, 137–151. [Google Scholar]
- Hasanin, M.S.; Darwesh, O.M.; Matter, I.A.; El-Saied, H. Isolation and characterization of non-cellulolytic Aspergillus flavus EGYPTA5 exhibiting selective ligninolytic potential. Biocatal. Agric. Biotechnol. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Darwesh, O.M.; El-Hawary, A.S.; El Kelany, U.S.; El-Sherbiny, G.M. Nematicidal activity of thermostable alkaline protease produced by Saccharomonospora viridis strain Hw G550. Biotechnol. Rep. 2019, 24, e00386. [Google Scholar] [CrossRef] [PubMed]
- Orozco, S.F.B.; Kilikian, B.V. Effect of pH on citrinin and red pigments production by Monascus purpureus CCT3802. World J. Microbiol. Biotechnol. 2008, 24, 263–268. [Google Scholar] [CrossRef]
- Moharram, A.M.; Mostafa, M.E.; Ismail, M.A. Chemical profile of Monascus ruber strains. Food Technol. Biotechnol. 2012, 50, 490–499. [Google Scholar]
- Carvalho, J.C.; Oishi, B.O.; Pandey, A.; Soccol, C.R. Biopigments from Monascus: Strains selection, citrinin production and color stability. Braz. Arch. Biol. Technol. 2005, 48, 885–894. [Google Scholar] [CrossRef]
- Elsayed, M.E.; Salem, M.A.; Selem, S.B. Effect of feeding at different levels of chromium picolinate and magnesium sulfate on diabetic rats. J. Food Dairy Sci. 2015, 6, 379–392. [Google Scholar] [CrossRef]
- Kumari, H.M.; Naidu, K.A.; Vishwanatha, S.; Narasimhamurthy, K.; Vijayalakshmi, G. Safety evaluation of Monascus purpureus red mould rice in albino rats. Food Chem. Toxicol. 2009, 47, 1739–1746. [Google Scholar] [CrossRef]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef]
- Bouksir, K.; Kazzaz, M.; Fehri, H.F.; Bouziane, H.; Bouksir, H.; El Haskouri, F. Monascus ruber: A new of onychomycosis in the north of Morocco (Tetouan). J. Mycol. Med. 2018, 28, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Eida, M.F.; Darwesh, O.M.; Matter, I.A. Cultivation of oleaginous microalgae Scenedesmus obliquus on secondary treated municipal wastewater as growth medium for biodiesel production. J. Ecol. Eng. 2018, 19, 38–51. [Google Scholar] [CrossRef]
- Sadek, Z.I.; Abdel-Rahman, M.A.; Azab, M.S.; Darwesh, O.M.; Hassan, M.S. Microbiological evaluation of infant foods quality and molecular detection of Bacillus cereus toxins relating genes. Toxicol. Rep. 2018, 5, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Kheiralla, Z.H.; Hewedy, M.A.H.; Mohammed, H.R.; Darwesh, O.M. Isolation of pigment producing actinomycetes from rhizosphere soil and application it in textiles dyeing. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2128–2136. [Google Scholar]
- Chatterjee, S.; Maity, S.; Chattopadhyay, P.; Sarkar, A.; Laskar, S.; Sen, S.K. Characterization of red pigment from Monascus in submerged culture red pigment from Monascus purpureus. J. Appl. Sci. Res. 2008, 5, 2102–2108. [Google Scholar]
- Long, C.; Cui, J.; Xie, S.; Zhang, D.; Liu, M.; Zhang, Z.; Huang, Z.; Zeng, B. The alpha-amylase MrAMY1 is better than MrAMY2 in rice starch degradation, which promotes Monascus pigments production in Monascus ruber. 3 Biotech 2020, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hilares, R.T.; de Souza, R.A.; Marcelino, P.F.; da Silva, S.S.; Dragone, G.; Mussatto, S.I.; Santos, J.C. Sugarcane bagasse hydrolysate as a potential feedstock for red pigment production by Monascus ruber. Food Chem. 2018, 245, 786–791. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F.; Moawad, H.; Oh, Y.K. Influence of nitrogen source and growth phase on extracellular biosynthesis of silver nanoparticles using cultural filtrates of Scenedesmus obliquus. Appl. Sci. 2019, 9, 1465. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Poorniammal, R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr. J. Biotechnol. 2008, 7, 1894–1898. [Google Scholar] [CrossRef]
- Shuler, M.L.; Kargi, F. Bioprocess considerations in using plant cell cultures. In Bioprocess Engineering (Basic Concepts), 2nd ed.; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 2002; pp. 405–419. [Google Scholar]
- Jeon, C.P.; Lee, J.B.; Choi, S.Y.; Shin, J.W.; Lee, O.S.; Choi, C.S.; Rhee, C.H.; Kwon, G.S. Optimal culture condition for production of water-soluble red pigments by Monascus purpureus. J. Korean Soc. Food Sci. Nutr. 2006, 35, 493–498. [Google Scholar]
- Padmavathi, T.; Prabhudessai, T. A solid liquid state culture method to stimulate Monascus pigments by intervention of different substrates. Int. Res. J. Biol. Sci. 2013, 2, 22–29. [Google Scholar]
- Babitha, S.; Soccol, C.R.; Pandey, A. Solid-state fermentation for the production of Monascus pigments from jackfruit seed. Bioresour. Technol. 2007, 98, 1554–1560. [Google Scholar] [CrossRef]
- Ouyang, W.; Liu, X.; Wang, Y.; Huang, Z.; Li, X. Addition of genistein to the fermentation process reduces citrinin production by Monascus via changes at the transcription level. Food Chem. 2020, 128410. [Google Scholar] [CrossRef]
- Joshi, V.K.; Attri, D.; Bala, A.; Bhushan, S. Microbial pigments. Indian J. Biotechnol. 2003, 2, 362–369. [Google Scholar]
- Babitha, S.; Soccol, C.R.; Pandey, A. Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. J. Basic Microbiol. 2007, 47, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lockard, V.G.; Phillips, R.D.; Hayes, A.W.; Berndt, W.O.; O’Neal, R.M. Citrinin nephrotoxicity in rats: A light and electron microscopic study. Exp. Mol. Pathol. 1980, 32, 226–240. [Google Scholar] [CrossRef]
- Kogika, M.M.; Hagiwara, M.K.; Mirandola, R.M. Experimental citrinin nephrotoxicosis in dogs: Renal function evaluation. Vet. Hum. Toxicol. 1993, 35, 136–140. [Google Scholar]
- Da Lozzo, E.J.; Oliveira, M.B.M.; Carnieri, E.G.S. Citrinin-induced mitochondrial permeability transition. J. Biochem. Mol. Toxicol. 1998, 12, 291–297. [Google Scholar] [CrossRef]
- Lin, T.F.; Demain, A.L. Effect of nutrition of Monascus sp. on formation of red pigments. Appl. Microbiol. Biotechnol. 1991, 36, 70–75. [Google Scholar] [CrossRef]
- Blanc, P.; Laussac, J.P.; Le Bars, J.; Le Bars, P.; Loret, M.O.; Pareilleux, A.; Prome, D.; Prome, J.C.; Santerre, A.L.; Goma, G. Characterization of monascidin A from Monascus as citrinin. Int. J. Food Microbiol. 1995, 27, 201–213. [Google Scholar] [CrossRef]
- Blanc, P.J.; Loret, M.O.; Goma, G. Production of citrinin by various species of Monascus. Biotechnol. Lett. 1995, 17, 291–294. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.B.; Chen, D.; Zhang, A.M.; Niu, S.Q. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Poorniammal, R.; Gunasekaran, S.; Ariharasivakumar, G. Toxicity evaluation of fungal food colourant from Thermomyces sp. in albino mice. J. Sci. Ind. Res. 2011, 70, 773–777. [Google Scholar]
- Masango, M.; Myburgh, J.; Botha, C.; Labuschagne, L.; Naicker, D. A comparison of in vivo and in vitro assays to assess the toxicity of algal blooms. Water Res. 2008, 42, 3241–3248. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Mahmood, H.Z.A.; Shahid, Z.; Jain, R.; Chen, G. Etanercept-associated Nephropathy. Cureus 2019, 11, e5419. [Google Scholar] [CrossRef] [PubMed]
- Serfilippi, L.M.; Stackhouse Pallman, D.R.; Gruebbel, M.M.; Kern, T.J.; Spainhour, C.B. Assessment of retinal degeneration in outbred albino mice. Comp. Med. 2004, 54, 69–76. [Google Scholar] [PubMed]
- Baranova, M.Á.R.I.A.; Mala, P.; Burdova, O.Ľ.G.A.; Hadbavny, M.; Sabolová, G. Effect of natural pigment of Monascus purpureus on the organoleptic characters of processed cheeses. Bull. Vet. Inst. Pulawy 2004, 48, 59–62. [Google Scholar]
- Vidyalakshmi, R.; Paranthaman, R.; Murugesh, S.; Singaravadivel, K. Microbial bioconversion of rice broken to food grade pigments. Glob. J. Biotechnol. Biochem. 2009, 4, 84–87. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).

