Pure Rotational Spectrum of Benzophenone Detected by Broadband Microwave Spectrometer in the 2–8 GHz Range
Abstract
1. Introduction
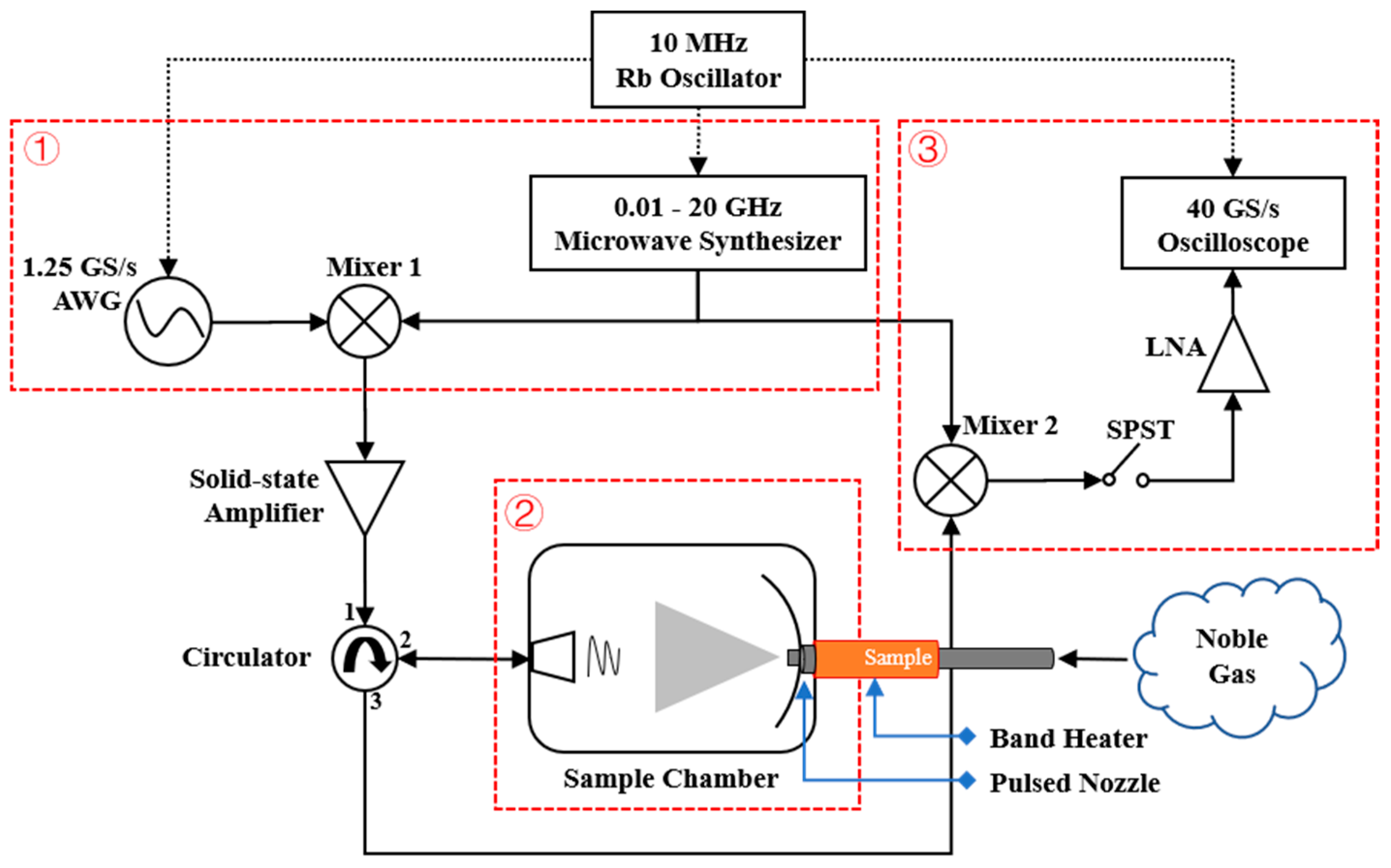
2. Experiment
2.1. Experimental Instrument and Methods
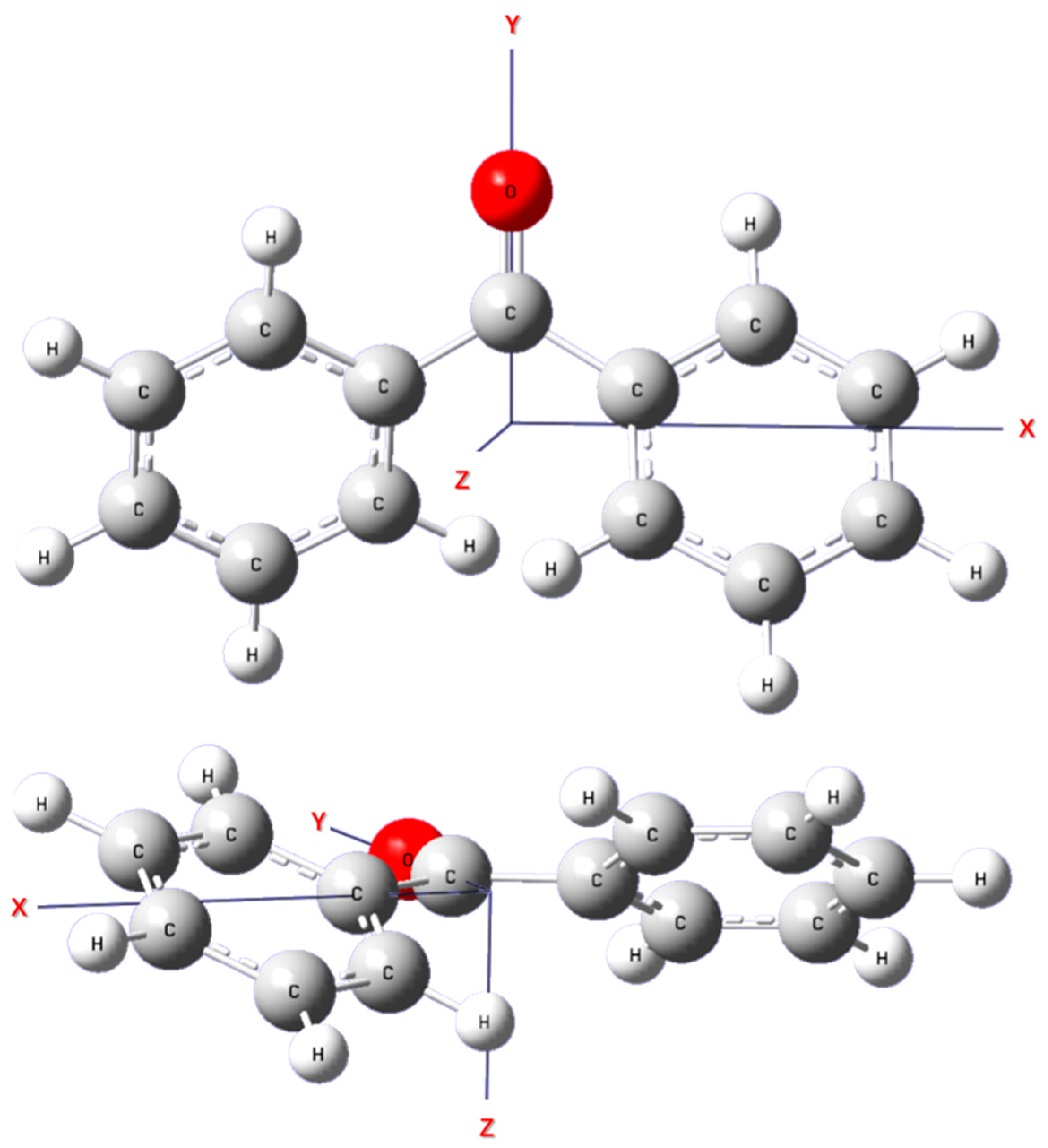
2.2. Theoretical Calculation
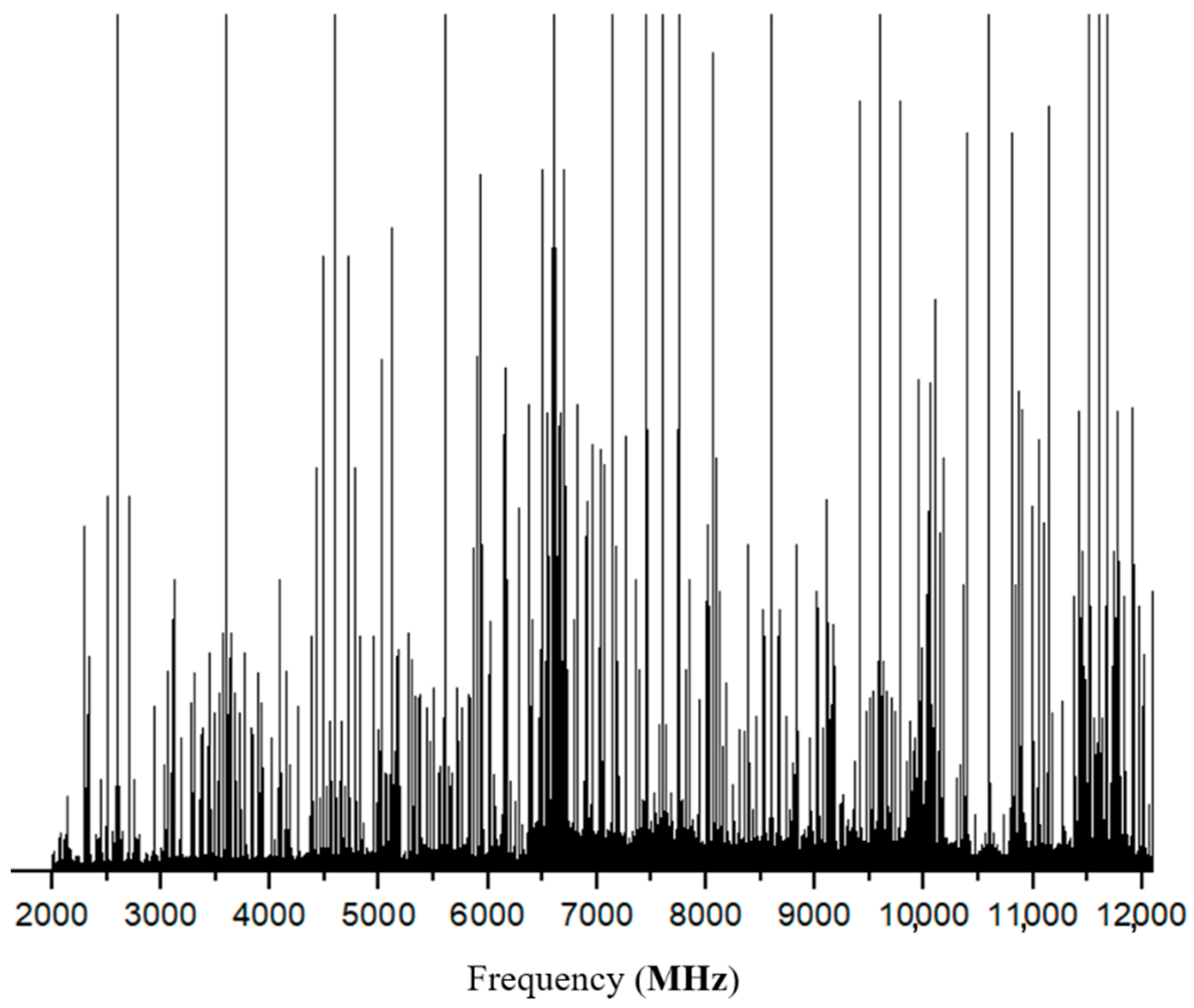
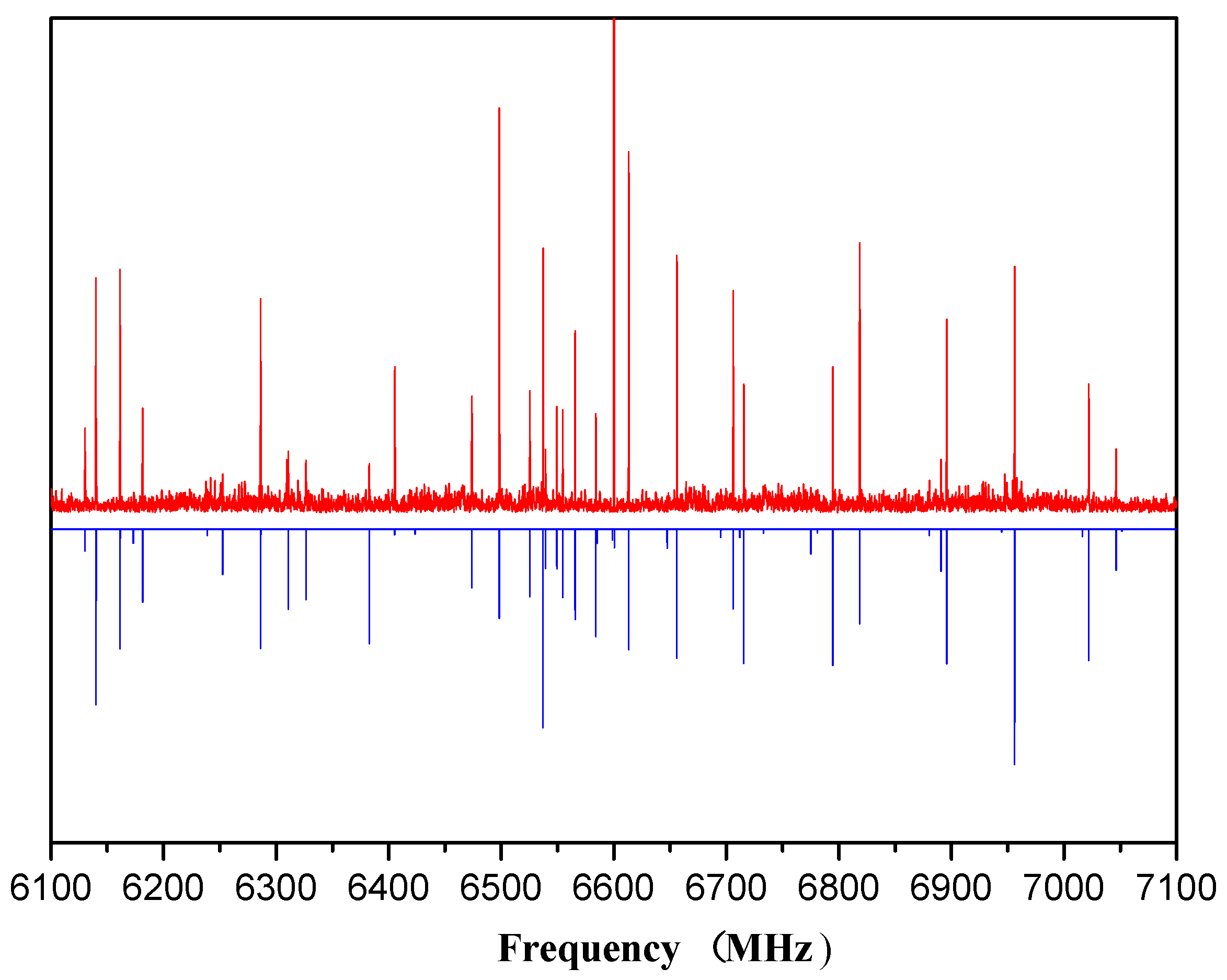
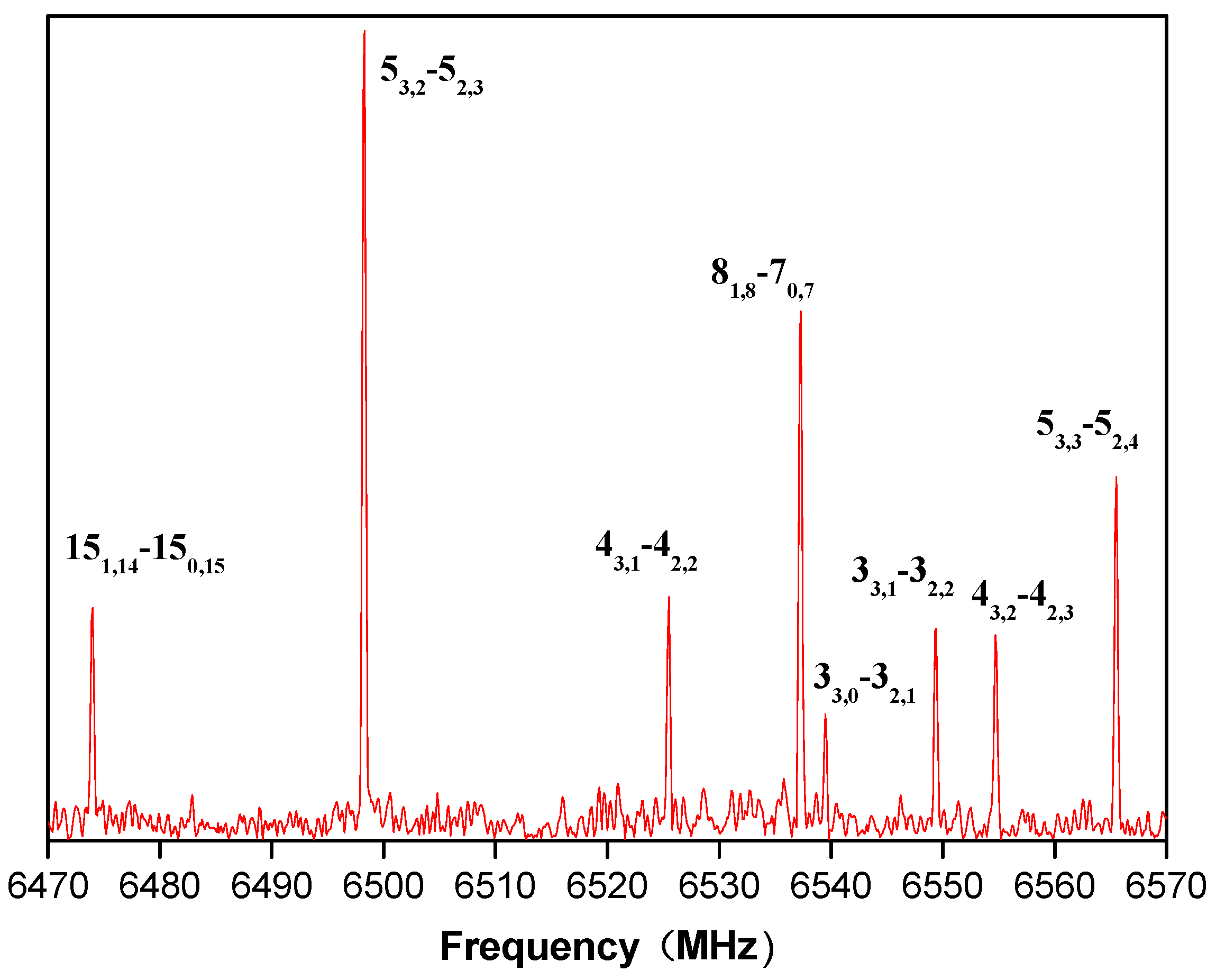
3. Assignment and Results
4. Discussion and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furia, T.E.; Bellanca, N. Fenarolis Handbook of Flavor Ingredients. CRC Press Clevel. 1975, 1, 43. [Google Scholar]
- Chang, F.; Li, W.Y.; Xia, F.; Yan, Z.Y.; Xiong, J.; Wang, J.Q. Highly Selective Oxidation of Diphenylmethane to Benzophenone over Co/MCM-41. Chem. Lett. 2005, 34, 1540. [Google Scholar] [CrossRef]
- Chhabra, R.S. NTP Technical Report on the Toxicity Studies of Benzophenone. Natl. Toxicol. Program Toxic. Rep. Ser. Number 2000, 61, 1–53. [Google Scholar]
- Allamandola, L.J.; Tielens, A.G.G.M.; Barker, J.R. Interstellar Polycyclic Aromatic Hydrocarbons: The Infrared Emission Bands, the Excitation/Emission Mechanism, and the Astrophysical Implications. Astrophlys. J. Suppl. S 1989, 71, 733. [Google Scholar] [CrossRef] [PubMed]
- Léger, J.L.A. A New Component of the Interstellar Matter: Small Grains and Large Aromatic Molecules. Puget Annu. Rev. Astron. Astr. 1989, 27, 161–198. [Google Scholar]
- Finlayson-Pitts, J.B.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Helmig, D.; Müller, J.; Klein, W. Volatile Organic Substances in a Forest Atmosphere. Chemosphere 1989, 19, 1399–1412. [Google Scholar] [CrossRef]
- West, C.; Sedo, G.; Wijngaarden, J.V. Newly assigned microwave transitions and a global analysis of the combined microwave/millimeter wave rotational spectra of 9-fluorenone and benzophenone. J. Mol. Spectrosc. 2017, 335, 43. [Google Scholar] [CrossRef]
- Maris, A.; Melandri, S.; Caminati, W.; Favero, P.G. Free jet absorption millimeter wave spectrum of benzophenone. Chem. Phys. Lett. 1996, 256, 509. [Google Scholar] [CrossRef]
- Jiao, C.; Duan, S.W.; Wu, Y.; Sun, M.; Chen, Q.; Fang, P.Y.; Wang, D.P. Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy. Appl. Sci. 2020, 10, 7650. [Google Scholar] [CrossRef]
- Ding, M.Y.; Li, Y.J.; Xu, Z.R.; Jiao, C.; Duan, S.W.; Sun, M.; Li, L.; Zhang, Y.Z.; Chen, Q.; Li, Y.M.; et al. Application of Broadband Fourier Transform Microwave Spectroscopy to Study Complexes and Chemical Reactions. Chin. J. Anal. Chem. 2019, 47, 779–784. [Google Scholar]
- Brown, G.G.; Dian, B.C.; Douglass, K.O.; Geyer, S.M.; Shipman, S.T.; Pate, B.H. A broadband Fourier transform microwave spectrometer based on chirped pulse excitation. Rev. Sci. Instrum. 2008, 79, 053103. [Google Scholar] [CrossRef] [PubMed]
- Park, G.B.; Field, R.W. Perspective: The first ten years of broadband chirped pulse Fourier transform microwave spectroscopy. J. Chem. Phys. 2016, 144, 200901. [Google Scholar] [CrossRef] [PubMed]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Thompson, H.B. Calculation of Cartesian Coordinates and Their Derivatives from Internal Molecular Coordinates. J. Chem. Phys. 1967, 47, 3407–3410. [Google Scholar] [CrossRef]
- Watson, J.K.G.; Durig, J. Vibrational Spectra and Structure; Elsevier: Amsterdam, The Netherlands, 1977; Volume 6, pp. 1–89. [Google Scholar]
- Pickett, H.M. The fitting and prediction of vibration-rotation spectra with spin interactions. J. Mol. Spectrosc. 1991, 148, 371. [Google Scholar] [CrossRef]
- Available online: http://www.ifpan.edu.pl/~kisiel/asym/pickett/crib.htm#errors (accessed on 18 November 2020).
- Schaefer, T.; Penner, G.H. The STO 3G MO structure and internal rotational potential of benzophenone. J. Phys. Chem. 1986, 85, 6249. [Google Scholar] [CrossRef]
- Gough, K.M.; Wildman, T.A. Hindered internal rotation in benzophenone. J. Am. Chem. Soc. 1990, 112, 9141. [Google Scholar] [CrossRef]
- Bauder, A. Fundamentals of Rotational Spectroscopy. In Handbook of High-Resolution Spectroscopy; Quack, M., Merkt, F., Eds.; Wiley, John Wiley, Sons, Ltd.: New York, NY, USA, 2011; pp. 57–116. [Google Scholar]
| Parameters | MP2/cc-pVDZ | B3LYP/cc-pVDZ |
|---|---|---|
| A(MHz) | 1666.94 | 1716.36 |
| B(MHz) | 410.30 | 405.16 |
| C(MHz) | 349.82 | 345.92 |
| I. a(amu·Å2) | 303.18 | 294.45 |
| I. b(amu·Å2) | 1231.72 | 1247.36 |
| I. c(amu·Å2) | 1444.68 | 1460.98 |
| P. a(amu·Å2) | 1186.61 | 1206.95 |
| P. b(amu·Å2) | 258.07 | 254.03 |
| P. c(amu·Å2) | 45.11 | 40.42 |
| (Debye) | 3.20 | 2.82 |
| Δ(amu·Å2) a | −90.22 | −80.83 |
| kappa | −0.91 | −0.91 |
| Parameters | Combined | Previous Work a | |
|---|---|---|---|
| Frequency range (GHz) | 2.0–14.0 | 8.0–14.0 | 60.0–73.0 |
| A (MHz) | 1692.889219(31) | 1692.88929(23) | 1692.916(35) |
| B (MHz) | 412.644660(11) | 412.644594(92) | 412.620(17) |
| C (MHz) | 353.874564(11) | 353.874501(88) | 353.884(17) |
| DJ (kHz) | 0.010635(19) | 0.01053(14) | 0.0122(17) |
| DJK (kHz) | −0.043444(83) | −0.04372(54) | −0.0517(60) |
| DK (kHz) | 0.41417(59) | 0.4195(43) | 0.462(62) |
| d1 (kHz) | −0.0014406(23) | −0.001442(17) | |
| d2 (kHz) | −0.00099(15) | −0.0003385(68) | |
| σ(kHz) | 2.6 | 8 | 110 |
| N | 304 | 166 | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Yang, M.; Huang, C.; Duan, S.; Sun, M.; Chen, Q.; Jiao, C.; Wu, Y. Pure Rotational Spectrum of Benzophenone Detected by Broadband Microwave Spectrometer in the 2–8 GHz Range. Appl. Sci. 2020, 10, 8471. https://doi.org/10.3390/app10238471
Tan H, Yang M, Huang C, Duan S, Sun M, Chen Q, Jiao C, Wu Y. Pure Rotational Spectrum of Benzophenone Detected by Broadband Microwave Spectrometer in the 2–8 GHz Range. Applied Sciences. 2020; 10(23):8471. https://doi.org/10.3390/app10238471
Chicago/Turabian StyleTan, Haoyang, Miaoling Yang, Chenbo Huang, Shengwen Duan, Ming Sun, Qian Chen, Chao Jiao, and Yi Wu. 2020. "Pure Rotational Spectrum of Benzophenone Detected by Broadband Microwave Spectrometer in the 2–8 GHz Range" Applied Sciences 10, no. 23: 8471. https://doi.org/10.3390/app10238471
APA StyleTan, H., Yang, M., Huang, C., Duan, S., Sun, M., Chen, Q., Jiao, C., & Wu, Y. (2020). Pure Rotational Spectrum of Benzophenone Detected by Broadband Microwave Spectrometer in the 2–8 GHz Range. Applied Sciences, 10(23), 8471. https://doi.org/10.3390/app10238471




