Abstract
Several plant extracts are being investigated to produce edible coatings, mainly due to their antioxidant and antimicrobial activities. In this study, licorice root extracts were produced by ultrasound-assisted extraction and were combined with chitosan to elaborate edible coatings. Different solvents and temperatures were used in the extraction process, and the antioxidant and antimicrobial activity of the extracts were assessed. The most bioactive extracts were selected for the development of the edible coatings. The rheological properties of the coatings were studied, and they were applied on strawberry to evaluate their physicochemical and microbiological properties. The addition of licorice extract to chitosan resulted in positive effects on the rheological properties of the coatings: the incorporation of phytochemicals to chitosan decreased the shear stress and improved the restructuring ability of the coating solutions. The films presented a reduction of the Burger model parameter, indicating a reduction of rigidity. Furthermore, the strawberry coated with chitosan and licorice extract maintained good quality parameters during storage and showed the best microbiological preservation in comparison with controls. Hence, the use of chitosan with licorice extract is a potential strategy to produce edible coating for improving the postharvest quality of fruits.
1. Introduction
An increase in consumer requirements for safe food has led to the development of new improved packaging systems, including active, intelligent and edible materials. The use of edible biopolymers in food-packaging applications has become an alternative due to their film-forming properties and environmentally friendly behavior [1]. Edible coatings and films are different: edible films are used as wrapping packaging materials, while edible coatings can be directly applied on the surface of food products [2]. Edible coatings are natural, safe and ecologically friendly substitutes, suitable to be applied to reduce water transfer, gaseous exchange and oxidation of fresh products [3]. The edible coatings should preserve the quality, nutritional value and texture of food products by reducing moisture loss and oxygen effects, while maintaining adherence, without altering the original taste and odor [4]. Furthermore, the combination of natural food grade substances in the coating should improve the physical properties of the formed films [5]. In fact, the rheological properties of the film-forming solution, such as thickness, spreadability and uniformity of the liquid coating layer, and the film performance, can be significantly affected by the type and composition of the coating constituents, such as polysaccharides, protein, hydrocolloid or composite materials. In general, a reduction in the solution’s viscosity provides a processing advantage during high-shear processing operations, whereas high apparent viscosity at low-shear rates provides a better application by dipping [4].
Chitosan is a cationic biopolymer that can be mainly obtained from the deacetylation process of chitin, a renewable natural resource [2] which is widely used in the coating of fresh products. Biological properties have been attributed to chitosan, such as antibacterial and antifungal properties [6]. Then, the application of this substance has been applied in several areas such as agriculture, cosmetic, pharmacy and also food industry [7].
Chitosan has been studied and used for coating fish, meat, fruits and vegetables, to improve the quality and extend the food shelf life [8]. In addition, chitosan can be combined with other functional compounds with antioxidant, antimicrobial or other activities, to enhance the quality and efficacy of the coating [9]. Antioxidants, antimicrobial agents, coloring agents, flavors, nutrients, prebiotics and probiotics are examples of functional compounds that can be incorporated into edible chitosan-based coatings [10]. Particularly, for the case of strawberry (Fragaria × ananassa), some works studied the application of chitosan-based coatings [11,12] but a relatively high moisture permeability was reported [2]. Then, the incorporation of natural bioactive compounds such as nisin, natamycin, pomegranate, grape seed extracts [13], aloe vera gel [14], Thymus capitatus [15] and Mentha spicata essential oils [16] and green tea extract [17], were studied, with the aim of enhancing the performance of the edible coating and the preservation of strawberry. This fruit has been intensively studied, because it is highly consumed [18] and is a relevant source of bioactive compounds such as vitamin C, vitamin E, β-carotene and anthocyanins [19]. Nevertheless, several factors can reduce the fruit quality and limit its commercialization, such as intrinsic physiology, physiological and mechanical lesion, fungal infections and postharvest decay [20,21,22].
On the other hand, licorice is a traditional therapeutic herb, which grows in various parts of the world, and is well recognized due to its bioactive properties, including antioxidant, anti-fungal, anti-ulcer, anti-inflammatory, as well as anticancer and antiviral. Licorice was commonly used in traditional medicine recipes for the treatment of respiratory complaints, inflammatory disorder and liver problems [23,24]. Accordingly, licorice extracts, particular those obtained from the root, were studied as natural food preservatives. Jiang et al. [25] investigated the efficacy of the addition of licorice extract in the preparation of meat patty to inhibit lipid oxidation during refrigerated and frozen storage. Qui et al. [26] combined chitosan citric acid and aqueous licorice extract to preserve the quality of sea bass (Lateolabrax japonicas) fillets by preventing lipid oxidation and microbial growth and thus extending the fish shelf life. Mandanipour et al. [27] evaluated the influence of an ethanolic licorice extract combined with chitosan for controlling blue mold and extending the shelf life in the postharvest storage of apples.
Different methods have been employed for the production of extracts. Among these, the ultrasound-assisted extraction (UAE) is a versatile, flexible and efficient technique employed for the extraction of bioactive compounds [28] due to its high reproducibility, and it is very appreciated for the reduction of solvent consumption [29]. Application of ultrasounds causes the implosion of the solvent cavitation bubbles, leading to the disruption of the vegetal cell membranes. This action facilitates the penetration of solvent into the cells, thereby improving mass transfer and increasing the releasing bioactive compounds.
In this study, ultrasound-assisted extraction of licorice root was studied, using solvents of different polarities, to examine the bioactive composition and the antioxidant and antimicrobial activity of the extracts. Selected extracts were tested for the elaboration of chitosan-based edible coatings. The rheological properties of the coating solutions were studied to assess their ability to form films. Furthermore, the rheological properties of the films were analyzed in order to determine the influence of the incorporation of licorice extract. Finally, a study to evaluate the effect of the application of the edible coatings on strawberry was accomplished, considering the physicochemical and microbiological characteristics of the berries during storage.
2. Materials and Methods
2.1. Chemicals and Fruit
Sodium carbonate anhydrous (99.5% purity) and ethanol (99.5% purity) were purchased from Panreac (Barcelona, Spain). Folin–Ciocalteu reagent, Gallic acid standard (>98% purity), (±)-6-hydroxy-2.5.7.8-tetramethyll-chromane-2-carboxylic acid (Trolox, 97% purity), 2.2-diphenyl-1-pycrilhydrazyl (DPPH, 95% purity), chloramphenicol (≥98% purity), low molecular weight chitosan deacetylated chitin, tween-20, tween-80, acetic acid (≥99.5% FCC, Food Grade), glycerol (>99%, FCC, FG), isoliquiritigenin, liquiritin, glycyrrhizic acid ammonium salt, liquiritigenin and glabridin, were purchased from Sigma–Aldrich (St. Louis, MO, USA). BBL Mueller Hinton II Broth and Difco Wilkins-Chalgren Agar were purchased from Becton, Dickinson and Company (France). Sodium hydroxide (ACS, Reag. Ph Eur, ISO) was purchased from EMSURE®, Merck (Germany).
Strawberries were purchased from a local market. They were brought to the lab for the experimental studies and used immediately. Strawberries were selected for uniformity of color, shape and size and with the absence of physical defects or decay.
2.2. Preparation of Licorice Extracts
Roots of licorice were obtained from Murciana herbalist’s (Murcia, Spain) and ground using a Premill 250 hammer mill (Lleal S.A., Granollers, Spain). Then, UAE using an ultrasonic probe (Branson Digital Sonifier 550 model, Danbury, CT, USA) with an electric power of 550 W and frequency of 60 kHz was accomplished. The extractions were carried out for 15 min with a plant/solvent ratio of 1:10, at temperatures of 25 and 50 °C. Four different solvents were used: ethanol, methanol, ethanol:water (50:50) and methanol:water (50:50) (Table 1). The extracts obtained were stored at −20 °C until their use.

Table 1.
Extraction yields (% mass extracted related to the mass of licorice root) of licorice root ultrasound-assisted extraction (UAE).
2.3. Content of Total Phenolic Compounds (TPC) and Antioxidant Activity
The content of TPC of licorice extract was determined following the method of Folin–Ciocalteu [30]. Initially, 10 μL of extract was mixed with 600 μL of milliQ water and 50 μL of Folin–Ciocalteu reagent. The content was thoroughly mixed and after 3 min, 150 μL of sodium carbonate solution (20% mass) and 190 μL of milliQ water were added to the mixture. After 2 h at room temperature in darkness, the absorbance was measured at 760 nm using a Genesys 10S UV-Vis spectrophotometer (Thermo Fischer Scientific Inc., MA, Waltham, USA). The results were expressed as GAE (mg of gallic acid equivalents/g of extract).
The ability of licorice extracts to scavenge DPPH free radicals was determined according to the method described by Brand-Williams et al. [31]. For the reaction, 25 µL of samples were added to 975 µL of DPPH radical in ethanol (6.1 × 10−5), which was prepared daily. The reaction took place at room temperature, in the dark, until it reached a plateau. Then, the absorbance was measured at 515 nm in a Genesys 10S UV-Vis spectrophotometer (Thermo Fischer scientific, Waltham, MA, USA). The DPPH concentration in the reaction medium was calculated from a calibration curve (linear regression). A control sample, containing the same volume of solvent instead of the extract, was used to measure the maximum DPPH absorbance. Trolox was used as a reference standard, so results were expressed as TEAC values (µmol Trolox/g extract). All analyses were done in triplicate.
2.4. Determination of Antimicrobial Activity of Licorice Extracts
The licorice extracts were tested against a Gram-positive bacterium, Staphylococcus aureus ATCC 25923, and a Gram-negative bacteria, Escherichia coli ATCC 25922, following the methods described by Quintana et al. [32]. These tests were carried out to assess a general comparison of the antimicrobial capacity of the extracts and decide which of them could be the most effective for the edible coating. A broth microdilution method was used for the determination of IC50 values (i.e., the concentration required to obtain 50% inhibition of growth after 24 h of incubation at 37 °C). All tests were performed in Mueller–Hinton broth supplemented with 0.5% tween-20. The inoculum of bacterial strains was prepared from overnight Mueller–Hinton broth cultures at 37 °C. Test strains were suspended in Muller–Hinton (bacteria) broth to give a final density 107 CFU(Colony forming units)/mL. The extract and fractions were diluted in ethanol ranging from 1 to 50 mg/mL. The 96-microwell plates were prepared by dispensing into each well 185 μL of culture broth, 10 µl of the different extract’s dilutions, antibiotic solution (chloramphenicol as positive control) or solvent (ethanol as negative control), and 5 µL of the inoculums. In addition, blanks were prepared by adding 190 µL of broth medium to the solvent or licorice extracts wells. The final volume of each well was 200 µl. After dispensing the inoculum, the plates were read in an Infinite 200 PRO plate reader (TECAN, Trading AG, Männedorf, Switzerland) spectrophotometer at 620 nm for T0 (Zero Time). Then, the plates were incubated at 37 °C for 24 h and the absorbance was read for TF (Final Time). Each test was performed in triplicate and repeated twice.
2.5. HPLC Analysis
High Performance Liquid Chromatography (HPLC) analysis was performed as previously described by the authors [33] to identify and quantify main bioactive compounds of licorice, i.e., isoliquiritigenin, liquiritin, glycyrrhizic acid, liquiritigenin and glabridin. A Prominence-i LC-2030C 3D Plus (Shimadzu) unit equipped with a quaternary solvent delivery system, an autosampler, diode-array detection (DAD) detector and a RP-C18 (250 × 4.6 mm; 3 μm) column was used. The column temperature was set at 25 °C. The mobile phase was 0.026% aqueous H3PO4 (v/v) (A) and acetonitrile, applying the following gradient elution: 80–75% (0–20 min), 75–66% (20–30 min), 66–50% (30–50 min), 50–40 (50–60min) and 40% (60–80min) of A. After 5 min, the initial conditions were achieved. The flow rate was 0.7 mL/min and was kept constant during analysis. Injection volume was 20 μL and detection was accomplished at 254, 280 and 370 nm. Calibration curves with the standards were used to determine the content of these bioactive compounds in the different extracts.
2.6. Preparation of Coating Forming Solutions
Edible coatings were prepared with chitosan and the addition of different amounts (1% and 5%) of the licorice extract (LE). Chitosan solution was prepared following the procedures described by Ali et al. [34] with some modifications. Briefly, 4.0 g of chitosan was dissolved in 200 mL of distilled water containing 1.0 mL of glacial acetic acid. The solution was heated with constant stirring for 12 h. The pH of the solution was adjusted to 5.5 with 1 N NaOH, and 0.2 mL of tween-80 was added as emulsifier. Then, 1% or 5% of licorice extract was added and homogenized using an Ultra Turrax homogenizer (T18 basic IKA, Staufen, Germany) at 7500 rpm for 3 min.
2.6.1. Rheological Properties of Coating Solutions and Films
Rheological assay was done in order to evaluate the steady and viscoelastic properties of edible coating solutions employing a modular advanced rheometer system Mars 60, Haake (Thermo-Scientific, Karlsruhe Germany), equipped with a coaxial cylinder (inner radius 12.54 mm, outer radius 11.60 mm, cylinder length 37.6 mm).
Steady-State Shear Test
The continuous shear test was performed at a temperature of 25 °C, over a shear rate in the range of 10−3 to 103 s–1, to study the influence of the natural extract on the rheological behavior. Experimental data were fitted to the rheological Power Law model, according to Equation (1):
where is the shear stress (Pa), is the shear rate (s−1), is the consistency coefficient ( and is the flux index (dimensionless).
Oscillatory Test
The stress amplitude sweep test was carried out within the range of 0.01 to 1000 Pa and with angular frequency of 0.1 Hz on all samples, in order to determine the linear viscoelastic regime (LVR). Based on the results of these experiments, the frequency sweep was done at 0.1 Pa, to keep the stress in the LVR, within the range of 0.01 to 100 rad·s−1. Thermo-viscoelasticity properties were investigated in a ramp temperature from 20 to 80 °C, under constant frequency (0.1 Hz) in the LVR and at a heating rate of 5 °C/min. Dynamic data were obtained in oscillatory shear experiments. The data recorded include the storage modulus which provides the elastic component, the loss modulus () which is related to the viscous component of the material and the loss tangent (Tan δ) which is the ratio G″/G′ and provides the ratio of elastic to viscous response of the material under consideration.
Preparation of the Film
In order to evaluate the influence of the addition of licorice extracts to chitosan on the ability of the coating solutions to form films, the casting method [35] was used to prepare the films. The coating solutions were casted in sample holder followed by drying at 47 °C for 12 h. The dried films were peeled and stored in a desiccator containing silica gel to prevent moisture absorption. Films of 0.5 mm of thickness were obtained.
Creep and Recovery
The rheological measurement of films was conducted using a parallel plate geometry (diameter 35 min, gap 0.5 mm). The storage modulus () and loss modulus () were measured from 0.1 to 20 rad·s−1 at 25 °C in the LVR. Creep and recovery tests were performed at 25 °C for each sample (with the same size). The tests were recorded at constant stress amplitude at 25 °C for 180 s, followed by release of the stress and recovery for 180 s. Creep curves were analyzed according to the Burgers model with one Kelvin-Voigt element [36] (Equation (2)):
where is compliance, is the time, is the instantaneous compliance (Pa−1), is the retarded compliance (Pa−1), is the retardation time of Kelvin-Voight element (s) and is Newtonian viscosity (Pa·s).
2.7. Application of Coatings on Strawberry and Quality Parameters
The strawberries were dipped into three different edible coating samples (chitosan, chitosan + 1% LE, and chitosan + 5% LE) for 1 min. Then, the fruits were air-dried, packed in commercial corrugated boxes and stored at 4.0 ± 1 °C. Uncoated strawberries were used as control. Twenty-five berries for each coating treatment were used and the experiments were performed in duplicate. Quality characteristics of control and coated fruits were determined during storing at 4.0 °C.
2.7.1. Fungal Decay Percentage
Strawberries were visually evaluated for the presence of mold growth during the storage time (10 days). Any strawberry with visible spoilage was considered to be decayed. Fungal decay percentage was calculated by using the following equation:
2.7.2. Determination of Weight Loss
Strawberries were weighed just after coating and air drying. Then, berries’ weight was monitored during 10 days after coating. Weight loss was calculated as the percentage of loss related to initial weight (Equation (4)):
2.7.3. Determination of pH and Titratable Acidity (TA)
Following the procedures describe by the Association of Official Agricultural Chemist -AOAC method [37], the pH was measured using a pH-meter. TA was determined by titrating the diluted juice (5 g fruit diluted in 95 mL distilled water) up to pH 8.2 using 0.1N NaOH. The results were expressed as the percent of citric acid (Equation (5)):
where V(NaOH) is the volume (mL) of NaOH consumed for titration, 0.1 is the molarity of the NaOH solution, 0.064 is a conversion factor for citric acid and m is the mass of the aliquot sample taken for analysis. Measurements were done in triplicate.
2.7.4. Content of Total Phenolic Compounds (TPC)
Strawberries’ pulp was finely chopped and 5 g of it was extracted and homogenized with 10 mL of methanol. After a cleaning-up step via centrifugation (5 min at 4500 rpm and 25 °C) and filtration, the supernatants were recovered and allowed to stand at room temperature for evaporation of solvent. The experiments were done in triplicate. Total phenolic content of the extracted pulp was determined using the Folin–Ciocalteu method [30].
2.7.5. Microbiological Analysis of Strawberries
The microbiological analysis of strawberries during storage was measured according to Haiji et al. [38] with slight modifications. The mesophilic and psychrophilic bacteria and yeast count were considered the most comprehensive method to evaluate the microbiological quality of the strawberries. On day 1 and until day 10 of storage, 10 g of fruit was aseptically transferred into stomacher bags, and 100 mL of sterile saline solution was added to each sample. Serial decimal dilutions of the homogenized sample were prepared. Determination of total aerobic bacteria was carried out on Plate Count Agar (37 °C for 48 h). The enumeration results were signified as log CFU (colony forming units)/g.
2.8. Statistical Analysis
Two replicates were prepared for each coating treatment and for each day. Each sample was measured in triplicate. All data were expressed as mean ± standard error, and the statistical analysis of data was performed using R software version 3.6.2. The significant difference between the treatments (three coating treatments) and days were determined using one-way analysis of variance (ANOVA) with Tukey’s HSD (honestly-significant difference) test, grouping at the 95% confidence level.
3. Results and Discussion
3.1. Extraction Yield of Licorice Extracts and Quantification of Bioactive Compounds
Extraction yields are reported in Table 1, together with the deviations obtained in duplicate experiments. Taking into account the solvents used, extraction yield increased in the following order: ethanol, methanol, methanol:water (50:50) and ethanol:water (50:50). Based on the dielectric constants of the solvents (ethanol 24.3, methanol 32.7 and water 78.4, at 25 °C), it can be established that the addition of 50% water to the organic solvents and thus, the increase of solvent polarity, resulted in a significant increase of extraction yield (p < 0.05). Nevertheless, extraction temperature did not affect yield significantly (p > 0.05), at least in the range of temperatures explored (25 and 50 °C).
While the addition of 50% water to methanol produced nearly a 2-fold increase of extraction yield, the addition of 50% water to ethanol resulted in a 6.5-fold yield increase. Then, the combination of a medium polar solvent (ethanol) with a high polar solvent (water) was the most suitable alternative to obtain high yields in the extraction of licorice root. These results are in accordance to other reported extraction studies, such as the work of Nieto et al. [39] concerning the extraction of grape stems, Arranz et al. [40] in the extraction of marjoram with different ethanol:water mixtures, or Kaderides et al. [41] in the UAE extraction of pomegranate peels, reporting an increase in the extraction yield with an increase in solvent polarity. Moreover, it was found that the combination of solvents is more efficient for extraction of phenolic compounds than a single solvent [42].
The main well-known bioactive compounds of licorice (glycyrrhizin, glabridin, liquiritigenin, isoliquiritigenin and liquiritin) were identified and quantified by HPLC analysis and the results obtained are reported in Table 2. Values in the range of 79.0 to 157.9 mg of these bioactive compounds were determined per gram of the different extracts obtained. Except in the case of ethanolic extracts, the higher concentrations were determined for glycyrrhizin, followed by glabridin, and in considerably lower concentrations, liquiritigenin, isoliquiritigenin and liquiritin.

Table 2.
Concentration (mg/g extract) of bioactive compounds identified in licorice UAE extracts (High Performance Liquid Chromatography—HPLC analysis).
In the case of ethanolic extracts, the concentration of glabridin was higher than glycyrrhizin, while significantly lower concentrations were observed for the rest of the components. Glabridin, an isoflavan, and glycyrrhizin, a triterpene glycoside, are the most abundant and distinctive compounds of licorice roots with several recognized pharmacological properties [43,44]. While glabridin is nearly insoluble in water, the glycosylated sugars present in glycyrrhizin provides some polarity to this compound and thus is better extracted using water. Figure 1 shows the concentration of these compounds in the extracts (mg/g) obtained at 25 °C as a function of the dielectric constant of the solvent used. In the case of water:organic solvent mixtures, the dielectric constant was estimated as the weighted average of the pure solvent dielectric constants. As it can be observed in Figure 1, the extraction of glycyrrhizin increased considerably as the solvent dielectric constant increased, while the opposite effect is observed for the non-polar compound glabridin. Similar results were observed in the case of licorice root UAE extracts obtained at 50 °C.
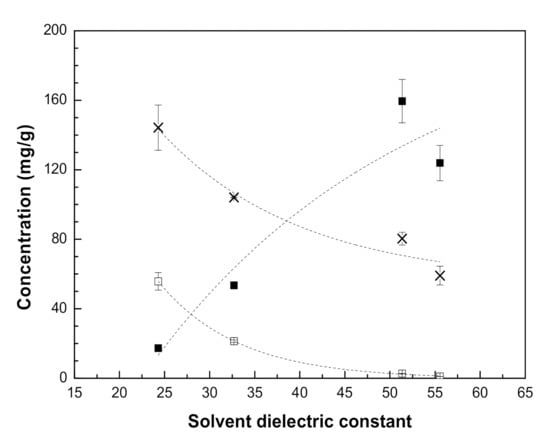
Figure 1.
Concentration of glabridin (☐), glycyrrhizin (■) and (✕) total phenolic compounds (TPC) in UAE licorice extracts as a function of the estimated dielectric constant of the solvent (extraction temperature 25 °C); (-----) trend line.
3.2. TPC, Antioxidant and Antimicrobial Activity of Licorice Extracts
3.2.1. TPC and Antioxidant Activity
The TPC of licorice extracts (mg GAE/g), together with their antioxidant (DPPH test) and antimicrobial activities (IC50 values), are reported in Table 3. The extracts with the higher content of TPC were obtained with ethanol (ELe25 and ELe50), followed by methanol (MLe25 and MLe50), ethanol:water (EWLe25 and EWLe50) and methanol:water (MWLe25 and MWLe50), with values in the range 60–160 mg GAE/g extract. Figure 1 shows that the TPC values of the extracts obtained at 25 °C decreased when the dielectric constant of the solvent increased. Similar results were obtained at the extraction temperature of 50 °C. Then, it can be established that the addition of water to the organic solvents (methanol or ethanol) enhanced the extraction yields, while the TPC concentration decreased, maybe due to the extraction of other polar compounds, as suggested by Spingo et al. [45]. For example, the glycyrrhizic acid (GA) molecule has several hydroxyl groups and thus, it is easily soluble when extracted with polar solvents [46]. Then, higher concentrations of GA were obtained in the extracts produced using ethanol:water (EWLe25 and EWLe50) and methanol:water (MWLe25 and MWLe50).

Table 3.
Total phenolic compounds (TPC), antioxidant activity (DPPH free radicals scavenging assay expressed as TEAC value) and antimicrobial activity (IC50 mg/mL) of licorice UAE extracts.
Concerning the effect of temperature, an increase of the extraction temperature (25 to 50 °C) increases the TPC content, showing significant differences (p < 0.05). A similar positive effect of temperature on total polyphenols recovery has been previously reported, for example in the extraction of olive leaves [47]. However, it should be noted that extremely high extraction temperature may promote possible degradation of phenolic compounds, or may enhance solvent loss through vaporization [48].
As reported elsewhere, phenolic compounds significantly contribute to the antioxidant activity of plant extracts [49,50]. It is generally stated that the higher the TPC value, the higher the antioxidant activity. In this work, a positive relationship between the antioxidant activity (evaluated by the DPPH assay) and the TPC content was obtained, considering all licorice UAE extracts produced. Figure 2 shows the linear correlation (R2 = 0.974) obtained between TPC (mg GAE/g) and TEAC (µmol Trolox/g) values. Regarding the effect of temperature and following the observed tendency in the case of TPC content, for all solvents used, the increase of extraction temperature increased the antioxidant activity of the extract. As mentioned before, the extracts obtained using 50% water were those with the lower TPC values and thus, these extracts are those with the lower antioxidant activity (Figure 2). This result strengthens the hypothesis that the addition of water to the organic solvent enhanced the extraction of polar but non-antioxidant compounds.
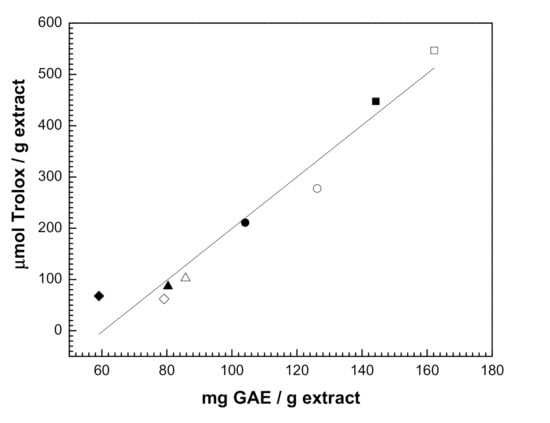
Figure 2.
Correlation between antioxidant activity (μmol Trolox/g) and total phenolic compounds (mg GAE/g) of licorice UAE extracts (Table 3). T = 25 °C: (■) ethanol, (●) methanol, (▲) ethanol:water and (◆) methanol:water. T = 50 °C: (□) ethanol, (○) methanol, (Δ) ethanol:water and (◊) methanol:water.
Among the identified and quantified licorice bioactive compounds, only in the case of glabridin, the less polar identified polyphenol, did an increase of its concentration show a positive effect on the antioxidant activity of the extract.
3.2.2. Antimicrobial Activity
Table 3 reports the IC50 values (concentration of the extract necessary to attain 50% inhibition) of the licorice UAE extracts tested against a Gram-positive bacteria, Staphylococcus aureus ATCC 25923, and a Gram-negative bacteria, Escherichia coli ATCC 25922. The IC50 values corresponding to Chloramphenicol, an antibiotic useful for the treatment of a number of bacterial infections, are 0.09 mg/mL for S. aureus bacteria and 0.08 mg/mL for E. coli. Only extracts obtained using mixtures of water and organic solvent (methanol or ethanol) exhibit antimicrobial activity. In comparison with Chloramphenicol, the IC50 values of these extracts are around one order of magnitude larger for both types of bacteria. The observed antimicrobial activity may be related to the high glycyrrhizin concentration observed in these extracts (>130 mg/g) due to the recognized antimicrobial activity of this compound [51] and licorice extracts [32].
3.2.3. Selection of Licorice Extract for Preparing Edible Coatings
Considering the Food and Drug Administration recommendation for the use of non-toxic and environmentally safe solvents, such as ethanol [52], and the antimicrobial and antioxidant activity of samples, the extract produced with ethanol:water (50:50) at 50 °C (EWLe50) was selected and produced in sufficient amounts in order to prepare the edible coatings and carry out the study of strawberries’ preservation. This licorice extract (LE) can be produced with high yield (c.a. 25%), and contains 85.71 ± 3.07 mg GAE/g, moderate antioxidant activity (102.52 ± 0.49 µmol Trolox/g) and good antimicrobial activity (IC50 values of 0.84 ± 0.04 and 1.43 ± 0.06 mg/mL for E. coli and S. aureus, respectively).
3.3. Rheology of the Edible Coating Solution
The rheological properties of coating solutions are of great importance because they affect the structure and apparent viscosity of the film matrix. The uniformity, the spreadability and the thickness of the coating could be greatly influenced by the flow characteristics of the coating solution [53]. Different chitosan-licorice coating solutions were prepared employing 1% (Ch + LE-1) and 5% (Ch + LE-5) of the licorice extract EWLe50 and pure chitosan (Ch) was used as control. In order to obtain a good knowledge of fluid behavior, the viscosity and viscoelastic properties of coating solutions were measured to assess the processability of the coating.
3.3.1. Steady Rheological Properties
Figure 3a shows the flow curve of different samples: the changes observed in shear stress are a consequence of the addition of licorice root extract to chitosan control, due to the interaction between chitosan and licorice phytochemicals and complexation. All coating solutions exhibited the behavior of non-Newtonian fluid and can be adjusted to the Power Law model (Equation (1)). The model parameters (K and ) are given in Table 4, and high fitting degree (R2 > 0.994) was achieved. The K (consistency coefficient) values decrease significantly and (flux index) values increase with the addition of LE. Chitosan solution presents a consistency coefficient of 0.469 ± 0.015 and flux index of 0.795 ± 0.005. Moreover, the samples Ch + LE-1 and Ch + LE-5 present K values of 3.72·10−4 ± 0.001 and 1.76·10−4 ± 0.001 and values of 1.436 ± 0.018 and 1.503 ± 0.012, respectively. values equal to 1 indicate the Newtonian fluids, lower than 1 indicate shear thinning fluids, while values higher than 1 indicate a shear thickening behavior [54]. Then, the chitosan coating solution presents a shear thinning behavior due to alignment of molecules in the direction of flow, and a decrease of viscosity with the increase of shear rate. Similar results were obtain by Zhang [55] in a coating solution of chitosan/zein with the addition of alpha-tocopherol.
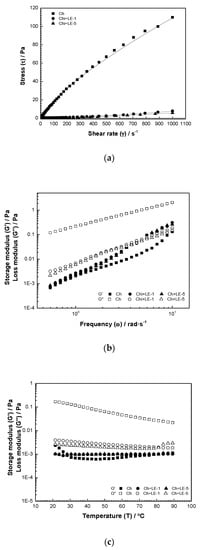
Figure 3.
Rheological properties of edible coating solutions produced with chitosan and chitosan-licorice: (a) flow curve, (b) frequency sweep and (c) temperature sweep.

Table 4.
Power Law parameters obtained for the edible coating solutions. Ch = Chitosan; Ch + LE-1 = Chitosan + 1% of licorice extract; Ch + LE-5 = Chitosan + 5% of licorice extract.
On the other hand, the addition of LE caused a decrease in the shear stresses and a non-Newtonian behavior. The shear thickening behavior (n > 1) observed in samples Ch + LE-1 and Ch + LE-5 are probably a consequence of particle hydro-clustering and an order-to-disorder transition. At large shear rates and stresses, convective and hydrodynamic force dominate over inter-particle force, and cause hydro-clustering of particles [56,57]. The rheological properties of coating solutions are consistently used among the literature to provide data on films’ microstructure, thickness, coating spreadability and uniformity on substrate. In general, highly viscous solutions retained air bubbles in the casting process, and low viscous solutions facilitated its spreading on the plate where the films were formed [58].
3.3.2. Dynamic Rheological Properties
The study of dynamic viscoelastic properties of coating solutions led to obtain information about the molecular entanglement and molecular network formation during drying [59]. The LVR used in this study was the maximum stress value in the flat region of storage modulus () and stress curve. Stress value applied for Ch, Ch + LE-1 and Ch + LE-5 was of 0.1 Pa.
Figure 3b shows the frequency dependence of the storage () and loss modules () of the different edible coating solutions studied, from 0.01 to 100 rad·s−1. According to mechanical spectra (Figure 3b), increased and values were observed with the increase of angular frequency (ω), with exhibiting a larger increase for all the samples in comparison with . Higher values were obtained for chitosan-licorice solutions, indicating that these solutions will behave as liquids during the mixer process for lower processing times. Then, to deform the material, the supplied energy would be lower in the case of the chitosan-licorice coatings [60].
Furthermore, > for the pure chitosan coating solution in the entire frequency range applied, without any crossover point observed, exhibited the typical behavior of liquid-like solutions [61]. But, in the case of chitosan-licorice solutions, crossover points were reached, indicating a longest time required for chain disengagement of the polymer structures in the solution [62,63]. This observed frequency dependence of and indicates that chitosan-licorice solutions are a useful class of materials to be applied as films in food coating, since they can enhance adhesiveness and the hardness of the coating solution.
Figure 3c shows the effect of temperature on and values of the edible chitosan and chitosan-licorice coating solutions for a heating rate of 5 °C/min in the LVR and 0.1 Hz frequency. The effect of temperature on the variation of and of the coating solutions exposes the phase transitions and elasticity and allows the selection of appropriate temperature ranges for the formulation and the application of the coating solution on the product.
For all coatings, was higher than in the whole range of temperatures studied, denoting the liquid-like behavior. Chitosan coating solution presents higher values than samples with 1% and 5% of LE, the ones which present similar values. Gelatinization does not occur because the high temperatures accelerate the mobility of chitosan molecules in the solution and reduce the intermolecular hydrogen bonding interactions, removing energized water molecules surrounding the chitosan chains [64].
3.3.3. Rheological Properties of Edible Film
The physicochemical and functional properties of edible films, such as mechanical, rheological and optical properties, are directly related to their microstructure and the interaction between film components and the drying conditions [65]. The rheological properties of edible films were evaluated in order to analyze the influence of combining chitosan with licorice extracts in the coating solutions, and to assess the particular characteristics and modifications in comparison with using only chitosan.
All the films were flexible and easily detachable from the sample holder, without evident defects in the form of cracks or pores. Figure 4a shows the frequency dependence of storage modulus () and loss modulus () of films of Ch, Ch + LE-1 and Ch + LE-5. For Ch films, >> over the entire frequency range studied, indicating that chitosan film exhibits dominant elastic behavior. > in the case of Ch + LE-1, and the film presents a more robust network and exhibits a gel-like behavior. Finally, for Ch + LE-5 film, > exhibiting a viscous behavior. Then, the addition of licorice phytochemicals to chitosan increases the viscous properties of film, displaying a variation in . Tan δ (= /) increase with the addition of licorice extract to chitosan and values become Tan δ ≈ 1, corroborating that the viscous quality of the film is greatly affected by the addition of LE and suggesting that the films containing LE have higher flexibility compared to the chitosan film and thus, they are more suitable than pure chitosan for application as edible food coatings.
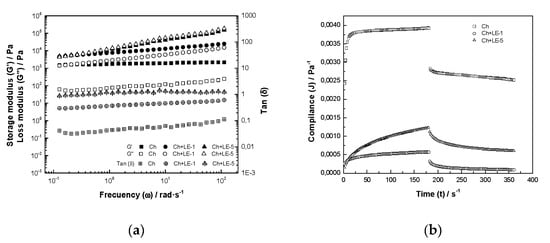
Figure 4.
Rheological properties of chitosan and chitosan-licorice edible films: (a) frequency sweep and (b) creep and recovery.
The creep compliance data are shown in Figure 4b. The extensional creep curves of all films showed a typical behavior of viscoelastic material, with varying degrees of viscoelasticity. The compliance of films decreases with the addition of licorice extracts and with the percentage amount of extract added. It can be inferred that films became more flexible with the addition of LE to chitosan, indicating an interaction between LE phytochemicals with possible hydrogen bonds formation.
The creep results were simulated by applying the Burger model (Equation (2)) and a satisfactory description of the essential features of the film viscoelasticity was attained, with high correlation coefficients (R2 > 0.999) (Table 5). The parameters of the Burger model reflect the structure of any system, and the decreasing/increasing of these parameters shows the weakening/strengthening of the structure. The instantaneous compliance () of chitosan was 3.793, and decreased to 0.482 and 0.801 for Ch + LE-1 and Ch + LE-5 respectively, indicating a reduction of rigidity or strength [66,67]. The retarded compliance () of chitosan was 1.408 mPa−1, decreasing to 0.481 and 0.626 respectively, with the addition of 1% and 5% of LE, indicating a reduction of the gel cohesive force and also a reduction of the resistance to deformation caused by the three-dimensional network structure [66,67]. Newtonian viscosity () increased significantly with the addition of LE to chitosan, revealing that mechanical strength and mobility of polymer chains increased with the addition of LE to chitosan in the coating formulation.

Table 5.
Burger model parameters obtained for the films. Ch = Chitosan; Ch + LE-1 = Chitosan + 1% of licorice extract; Ch + LE-5 = Chitosan + 5% of licorice extract.
Recovery percentages are an indirect measure of gel elasticity and the calculated values for the different coating films are given in Table 5. The recovery percentages of the films with LE extracts (83.78% and 51.09% for Ch + LE-1 and Ch + LE-5, respectively) were considerably higher than that of chitosan (35.82%). Then, the addition of licorice extract changed the viscous behavior and improved the elasticity of the films, with the Ch+LE-1 coating being the one which presents the highest recovery percentage (best gel elasticity).
3.4. Effect of Chitosan-Licorice Edible Coatings on Strawberries’ Quality
Four sets of strawberries were prepared to carry out the study of the effect of chitosan-licorice edible coatings on the fruit preservation: uncoated (control) fruit (C), coated with chitosan (Ch) and coated with Ch + LE-1 and Ch + LE-5. The samples were stored at 4 °C for 10 days.
3.4.1. Physicochemical Effects
In order to determine the effect of licorice extracts on the coated fruits, photographs were picked up during storage, which are shown in Figure 5. Pictures corresponding to 14 days of storage were also included in the figure, because the effect of coatings became very obvious. The coated strawberries presented a brilliant appearance, which was better than the uncoated sample.
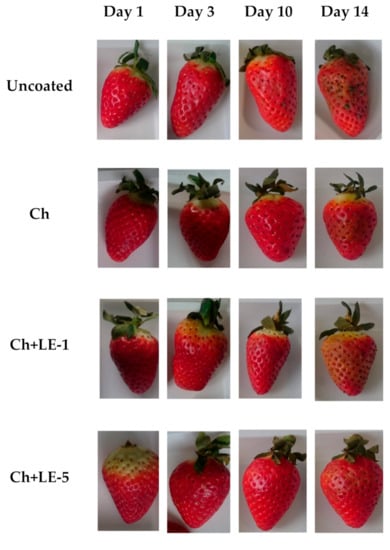
Figure 5.
Visual appearance of uncoated, chitosan- and chitosan-licorice-coated strawberries corresponding to 1, 3, 10 and 14 days of storage at 4 °C.
The coated forming on berries depends on the rheological parameters of the coating solutions, which present different shear stress and viscoelastic properties. The amount of coating solution adhered to strawberry during application strongly depends on the viscosity and the surface tension of the coating solutions. The roughness on the surface of strawberries require formulations with ingredients that can reduce the coating solution surface tension, in order to ensure coating uniformity and absence of void holes [4]. The total sugars present in fruit can act as a plasticizer, interacting with the polymer chains and generating “free” volumes within the chains, weakening the intermolecular forces and, consequently, reducing the resistance of the films [68]. Some authors determined that solutions with high viscosity and low surface tension promote a better film-forming surface [69]. In this study, the chitosan coating solution presented a viscosity of 170 mPa·s, while lower values were observed in the case of chitosan-licorice solutions (4.40 and 2.10 mPa·s for Ch + LE-1 and Ch + LE-5, respectively). Nevertheless, despite the observed viscosity reduction, the addition of LE improved the viscoelastic properties and allowed a better surface extensibility on berries, forming thinning coatings with higher uniformity and adherence, which can be related with the better physical appearance observed (Figure 5). The application of coatings did not affect the color of berries, maintaining good visual quality and no water migration on the surface was observed.
The presence of mold was visually observed in the control sample on day 10 (Figure 6), while no mold was observed in the coated berries. Ch + LE-5 coating exhibits the best preservation of strawberry appearance. The fungal decay of samples is depicted in Figure 6. The percentage of fungal decay was higher in control samples, followed by chitosan samples, while chitosan-licorice coatings did not present fungal decay during 10 days of storage. The licorice extract increased the antimicrobial properties of chitosan, according to the antibacterial activity assessed in this study and in other studies available in the literature [32].

Figure 6.
Fungal decay of uncoated and licorice-chitosan-coated strawberries stored for 10 days at 4 °C: (○) Ch (Chitosan); (■) C (uncoated).
Figure 7 shows the weight loss of uncoated and coated samples with the different films throughout the cold storage time. Postharvest mass loss of fruits and vegetables is mostly due to moisture loss through transpiration, and is recognized to accelerate the susceptibility of fruit and vegetables to physiological disorders [70,71]. After only three days of storing, the uncoated sample presented less weight loss than the samples treated with any of the coatings. Nevertheless, on day 10, the barrier effect exerted by the coatings could be observed, since all coatings reduced the loss of fruit weight in comparison with uncoated samples, with the coatings containing Ch + LE-1 and Ch + LE-5 being more efficient than the one containing solely chitosan. For these coatings, weight losses of samples are lower than 2% after 10 days of storing. Similar weight loss values were obtained by Martínez et al. [15] using chitosan coatings combined with Thymus capitatus essential oil. Furthermore, these loss weight values were lower than those observed by Petriccione et al. [72] when studying different species of strawberry coated with 2% chitosan (weight losses of 7–9%) or Ventura-Aguilar [73] using an edible film of chitosan combined with a Roselle calyces extract (weight loss of 6%).
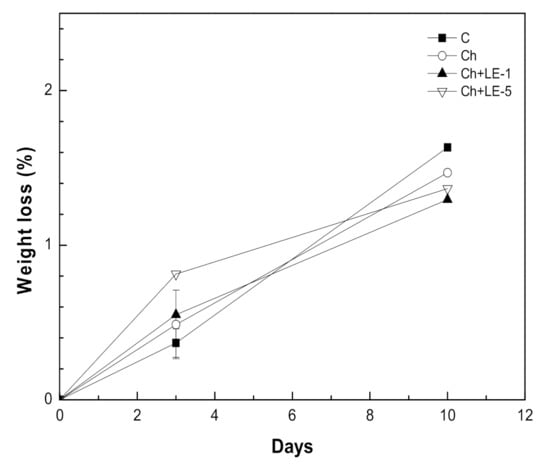
Figure 7.
Effect of different licorice-chitosan coatings on the weight loss of strawberries stored for 10 days at 4 °C: (○) Ch (chitosan) (▲) Ch + LE-1 (Chitosan + 1% of licorice extract); (▽) Ch + LE- 5 (Chitosan + 5% of licorice extract; (■) C = uncoated.
The effect of the different coatings in comparison with uncoated samples on the pH and titratable acidity (TA) of strawberries were also examined, since these properties are a measure of ripening effect. Fruit maturation produces the enzymatic reaction of sugars and the amounts of acids tend to decrease and the pH increases. The initial pH values of coated strawberries were slightly higher than uncoated samples (Figure 8a). Although there were some differences on day 1, pH values of coated samples did not change significantly during storage and no significant differences were observed between coated samples on day 10 (mean pH value is 4.05 ± 0.10), while a significantly higher pH value was obtained for the uncoated fruit (4.5 ± 0.20).
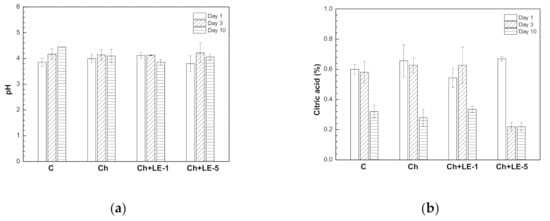
Figure 8.
Effect of different coatings based on chitosan and licorice extracts on (a) pH and (b) titratable acidity (TA) expressed as citric acid percentage of strawberries stored for 10 days at 4 °C. C = uncoated; Ch = chitosan; Ch + LE-1 = Chitosan + 1% of licorice extract; Ch + LE-5 = Chitosan + 5% of licorice extract. Means values and intervals of Tukey’s test at 95% according to the analysis of variance (ANOVA) test.
Furthermore, the TA values were significantly reduced (p < 0.05) both in coated and uncoated strawberries, but due to the nature of fruit organic acids, these usual decreases in fruit acidity during maturity did not induce a notable change in the pH value. As can be observed in Figure 8b, a significant decrease of TA values (p < 0.05) with storing time was observed in all cases, but differences between control and the different coatings were not significant. These results are in agreement with similar research conducted by Bhimrao et al. [74], who studied the extension of shelf life of strawberries using a coating formed with chitosan and whey protein isolate.
3.4.2. Total Phenolic Compounds Content and Microbiology Analysis
As stated before, the phenol content of a vegetal sample can be related with its antioxidant activity. Thus, the TPC content of the uncoated and coated strawberries was evaluated after 10 storing days, as an indicator of the coating antioxidant functional capacity (Table 6). The TPC values of uncoated strawberry on day 1 are in accordance with the values reported in the literature [75], which indicated that TPC values of 27 strawberry cultivars varied from 0.57 to 1.33 GAE mg/g of fresh weight.

Table 6.
Total phenolic compounds (TPC) of strawberries treated with different coatings. Ch = Chitosan; Ch + LE-1 = Chitosan + 1% of licorice extract; Ch + LE-5 = Chitosan + 5% of licorice extract.
The results given in Table 6 indicate that, on day 10 and for all cases (uncoated and coated fruit), the TPC values were lower after the cold storage. But, the type of coating had a significant effect on the observed TPC decrease, as is depicted in Figure 9, and the control samples (uncoated fruit) presented the highest TPC loss percentage (30.93 mg GAE/g), followed by chitosan coating (22.65 mg GAE/g), chitosan + 1% LE (13.1 mg GAE/g) and chitosan + 5% LE (13.3 mg GAE/g). Then, LE extract contributed significantly to preserve the phenolic compounds of samples.
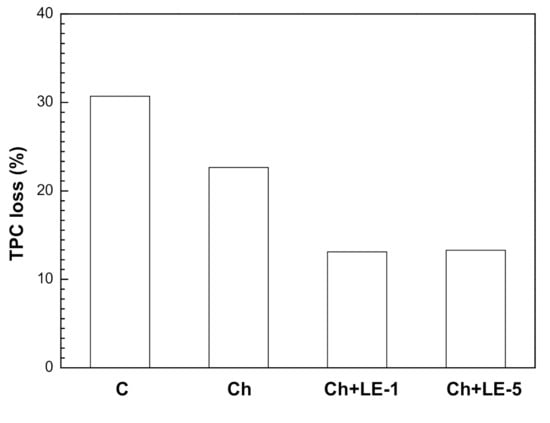
Figure 9.
Effect of chitosan-licorice coatings on total phenolic compounds (TPC) of strawberries stored for 10 days at 4 °C: C = uncoated; Ch = chitosan; LE1 = Chitosan + 1% of licorice extract; LE5 = Chitosan + 5% of licorice extract.
Concerning the microbiological analysis, Table 7 shows the aerobic bacteria and yeast determined in coated and uncoated strawberries after 6 days of cold storage. No initial growth of bacteria or yeasts was observed in the uncoated and coated strawberries. But after 6 days of storage, viable count of bacteria and yeasts were determined in uncoated samples and in berries coated with chitosan, while in the samples coated with chitosan-licorice mixtures, no growth was observed. Then, the addition of phytochemicals of licorice in order to prepared edible coating improved the antimicrobial properties of chitosan.

Table 7.
Effects of edible coatings prepared with chitosan and chitosan + licorice extract on the growth of mesophilic and psychrophilic bacteria (total aerobic psychrophilic bacteria) and yeasts during storage at 4 °C. Ch = Chitosan; Ch + LE-1 = Chitosan + 1% of licorice extract; Ch + LE-5 = Chitosan + 5% of licorice extract.
4. Conclusions
In this study, the ultrasound-assisted extraction using solvents with different polarities and two temperatures (25 and 50 °C) was carried out, in order to obtain bioactive licorice extracts (LE) for the elaboration of edible coatings. The polarity of the solvent contributed to the extraction of specific compounds, and while temperature did not significantly affect the extraction yield, it influenced the TPC content of samples. A higher concentration of glabridin was obtained with ethanol solvent, showing a positive effect on the antioxidant activity of the extract. Nevertheless, ethanolic licorice root extracts did not present antimicrobial activity. On the other hand, the extraction with mixtures of ethanol or methanol with water (50:50 v/v) enhanced the extraction of polar biomolecules (such as glycyrrhizin), and the extracts presented good antimicrobial activity but less antioxidant capacity in comparison with the ethanolic extract.
The edible coating solutions elaborated with pure chitosan and with chitosan and licorice ethanol:water extract, presented a non-Newtonian flow behavior. Solutions of pure chitosan presented shear thinning behavior, and the addition of LE decreased the shear stress value and presented a shear thickening behavior, probably due to particle hydro-clustering and an order-to-disorder transition. The viscoelastic properties of the coating solution show that the restructuring ability of Ch + LE physical gels improve the properties of film forming. Then, the addition of licorice phytochemical to chitosan increased the viscous properties of film, displayed a variation in the dissipation energy () and films became more flexible with the addition of LE. Furthermore, the addition of LE to chitosan decreased the shear stress values of samples, allowing better physical properties of the films applied on strawberries.
Concerning the preservation capacity, the addition of LE increased the antimicrobial properties of chitosan, according to the antibacterial activities studied in vitro. The values of weight loss of these coatings were significantly lower than those corresponding to pure chitosan. Additionally, the chitosan + LE edible coatings better preserved the content of phenolic compounds in the berries.
Then, the ultrasound-assisted extraction of bioactive compounds of licorice root using ethanol:water (50:50 v/v) is an alternative for producing bioactive licorice extracts with potential application in the elaboration of edible coatings with good rheological properties and preservative capacity.
Author Contributions
Conceptualization, M.R.G.-R.; L.A.G.-Z., and T.F.; methodology, S.E.Q.; M.R.G.-R.; L.A.G.-Z., and T.F.; software, O.L. and S.E.Q.; validation, T.F., formal analysis, S.E.Q., M.R.G.-R., L.A.G.-Z., and T.F.; investigation, S.E.Q., O.L., resources, S.E.Q., O.L., M.R.G.-R.; L.A.G.-Z. and T.F., writing—original draft preparation, S.E.Q. and T.F.; writing—review and editing, S.E.Q., O.L., L.A.G.-Z., M.R.G.-R. and T.F., supervision, T.F., M.R.G.-R. and L.A.G.-Z. and project administration, M.R.G.-R. and T.F.; funding acquisition, S.E.Q.; O.L., M.R.G.-R. and T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Comunidad Autónoma de Madrid, grant number P2013/ABI27, project Bolívar Gana con Ciencia, MinCiencias Contract 368-2019 and Programa Nacional de Innovación Agraria—PNIA of Perú, Contract: No. 152-2018-INIA-PNIA-PASANTIA.
Acknowledgments
The authors gratefully acknowledge the financial support from Comunidad Autónoma de Madrid (ALIBIRD, project: P2013/ABI2728). Somaris E. Quintana is grateful for the funding provided by Gobernación de Bolivar and Fundación Ceiba, Colombia, in the project “Bolívar Gana con Ciencia” and MinCiencias Contract 368-2019. Olimpia Ollalla thanks the Programa Nacional de Innovación Agraria—PNIA of Perú (Contract: No. 152-2018-INIA-PNIA-PASANTIA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Elsabee, M.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Pinotti, A.; Martino, M.N.; Zaritzky, N.E. Characterization of Starch and Composite Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 169–209. [Google Scholar]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Guillén, M.C.G. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; De Leon-Zapata, M.A.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura-Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Ramos-Aguiñaga, M.E.; et al. Basic and Applied Concepts of Edible Packaging for Foods. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–61. [Google Scholar]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Duan, J.; Cherian, G.; Zhao, Y. Quality enhancement in fresh and frozen lingcod (Ophiodon elongates) fillets by employment of fish oil incorporated chitosan coatings. Food Chem. 2010, 119, 524–532. [Google Scholar] [CrossRef]
- Odila Pereira, J.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT Food Sci. Technol. 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Ghaouth, A.; Arul, J.; Ponnampalam, R.; Boulet, M. Chitosan Coating Effect on Storability and Quality of Fresh Strawberries. J. Food Sci. 1991, 56, 1618–1620. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C. Antifungal effects of chitosan coating on fresh strawberries and raspberries during storage. J. Hortic. Sci. Biotechnol. 1998, 73, 763–767. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int. J. Food Sci. Technol. 2019, 55, 92–98. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Castañeda, C.G.G.; Valencia, M.E.; Tovar, C.D.G. The Effect of Edible Chitosan Coatings Incorporated with Thymus capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria x ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Apriyanti, D.; Rokhati, N.; Mawarni, N.; Khoiriyah, Z.; Istirokhatun, T. Edible Coating from Green Tea Extract and Chitosan to Preserve Strawberry (Fragaria vesca L.). In Proceedings of the The 24th Regional Symposium on Chemical Engineering (RSCE 2017), Semarang, Indonesia, 15–16 November 2017. [Google Scholar]
- Almenar, E.; Del-Valle, V.; Hernández-Muñoz, P.; Lagarón, J.M.; Catalá, R.; Gavara, R. Equilibrium modified atmosphere packaging of wild strawberries. J. Sci. Food Agric. 2007, 87, 1931–1939. [Google Scholar] [CrossRef]
- Van De Velde, F.; Tarola, A.M.; Güemes, D.; Pirovani, M.E. Bioactive Compounds and Antioxidant Capacity of Camarosa and Selva Strawberries (Fragaria x ananassa Duch.). Foods 2013, 2, 120–131. [Google Scholar] [CrossRef]
- Vu, K.; Hollingsworth, R.G.; Leroux, E.; Salmieri, S.; Lacroix, M. Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries. Food Res. Int. 2011, 44, 198–203. [Google Scholar] [CrossRef]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. LWT 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Oliveira, J.; Da Glória, E.M.; Da Silva, P.P.M.; Baggio, J.S.; Da Silva, P.P.M.; Ambrosio, C.M.; Spoto, M.H.F. Antifungal activity of essential oils associated with carboxymethylcellulose against Colletotrichum acutatum in strawberries. Sci. Hortic. 2019, 243, 261–267. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza spp. and Its Bioactive Constituents: Update and Review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef]
- Al-Ani, B.M.; Owaid, M.N.; Al-Saeedi, S.S.S. Fungal interaction between Trichoderma spp. and Pleurotus ostreatus on the enriched solid media with licorice Glycyrrhiza glabra root extract. Acta Ecol. Sin. 2018, 38, 268–273. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; True, A.D.; Zhou, L.; Xiong, Y.L. Inhibition of Lipid Oxidation and Rancidity in Precooked Pork Patties by Radical-Scavenging Licorice (Glycyrrhiza glabra) Extract. J. Food Sci. 2013, 78, C1686–C1694. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Chen, S.; Liu, G.; Yang, Q. Quality enhancement in the Japanese sea bass (Lateolabrax japonicas) fillets stored at 4 °C by chitosan coating incorporated with citric acid or licorice extract. Food Chem. 2014, 162, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Madanipour, S.; Alimohammadi, M.; Rezaie, S.; Nabizadeh, R.; Khaniki, G.J.; Hadi, M.; Yousefi, M.; Bidgoli, S.M.; Yousefzadeh, S. Influence of postharvest application of chitosan combined with ethanolic extract of liquorice on shelflife of apple fruit. J. Environ. Health Sci. Eng. 2019, 17, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravent6s, R.M. Analysis of total phenols and other oxidations substrates and antioxidants by means of Folin–Ciocalteu reagent. Polyphen. Flavonoids 1974, 25, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Quintana, S.E.; Cueva, C.; Villanueva-Bermejo, D.; Moreno-Arribas, M.V.; Fornari, T.; García-Risco, M.R. Antioxidant and antimicrobial assessment of licorice supercritical extracts. Ind. Crop. Prod. 2019, 139, 111496. [Google Scholar] [CrossRef]
- Wei, S.-S.; Yang, M.; Chen, X.; Wang, Q.-R.; Cui, Y.-J. Simultaneous determination and assignment of 13 major flavonoids and glycyrrhizic acid in licorices by HPLC-DAD and Orbirap mass spectrometry analyses. Chin. J. Nat. Med. 2015, 13, 232–240. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Torres-León, C.; Vicente, A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible films and coatings based on mango (var. Ataulfo) by-products to improve gas transfer rate of peach. LWT 2018, 97, 624–631. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- AOAC (Association of Official Analytical Chemist). Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries postharvest life. Food Hydrocoll. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of Grape Stems as a Source of Phenolic Antioxidants by Using a Sustainable Extraction Methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Villalva, M.; Guri, A.; Ortego-Hernández, E.; Jaime, L.; Reglero, G.; Santoyo, S.; Corredig, M. Protein matrices ensure safe and functional delivery of rosmarinic acid from marjoram (Origanum majorana) extracts. J. Sci. Food Agric. 2019, 99, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 2012, 11, 293–302. [Google Scholar]
- Yehuda, I.; Madar, Z.; Leikin-Frenkel, A.; Tamir, S. Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation. Mol. Nutr. Food Res. 2015, 59, 1041–1052. [Google Scholar] [CrossRef]
- Nassiri, A.M.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phyther. Res. 2008, 22, 709–724. [Google Scholar]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Tian, M.; Yan, H.; Row, K.H. Simultaneous extraction and separation of liquiritin, glycyrrhizic acid, and glabridin from licorice root with analytical and preparative chromatography. Biotechnol. Bioprocess. Eng. 2009, 13, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.-H.; You, F.; Zhang, Y.-L. Reduced pressure extraction of oleuropein from olive leaves (Olea europaea L.) with ultrasound assistance. Food Bioprod. Process. 2015, 93, 29–38. [Google Scholar] [CrossRef]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; García-Pérez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason. Sonochem. 2017, 34, 466–473. [Google Scholar] [CrossRef]
- Amado, I.R.; Franco, D.; Sánchez, M.; Zapata, C.; Vázquez, J. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Bermejo, D.; Zahran, F.; Troconis, D.; Villalva, M.; Reglero, G.; Fornari, T. Selective precipitation of phenolic compounds from Achillea millefolium L. extracts by supercritical anti-solvent technique. J. Supercrit. Fluids 2017, 120, 52–58. [Google Scholar] [CrossRef]
- Thakur, D.; Jain, A.; Ghoshal, G. Evaluation of phytochemical, antioxidant and antimicrobial properties of glycyrrhizin extracted from roots of Glycyrrhiza Glabra. J. Sci. Ind. Res. 2016, 75, 487–494. [Google Scholar]
- Bartnick, D.D.; Mohler, C.M.; Houlihan, M. Methods for the Production of Food Grade Extracts. WO 2006/047404 A3, 4 May 2006. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2006047404 (accessed on 31 October 2020).
- Chen, C.-H.; Kuo, W.-S.; Lai, L.-S. Rheological and physical characterization of film-forming solutions and edible films from tapioca starch/decolorized hsian-tsao leaf gum. Food Hydrocoll. 2009, 23, 2132–2140. [Google Scholar] [CrossRef]
- Haddarah, A.; Bassal, A.; Ismail, A.; Gaiani, C.; Ioannou, I.; Charbonnel, C.; Hamieh, T.; Ghoul, M.; Gaiani, C. The structural characteristics and rheological properties of Lebanese locust bean gum. J. Food Eng. 2014, 120, 204–214. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Effect of α-tocopherol antioxidant on rheological and physicochemical properties of chitosan/zein edible films. LWT 2020, 118, 108799. [Google Scholar] [CrossRef]
- Maranzano, B.J.; Wagner, N.J. The effects of particle size on reversible shear thickening of concentrated colloidal dispersions. J. Chem. Phys. 2001, 114, 10514–10527. [Google Scholar] [CrossRef]
- Maranzano, B.J.; Wagner, N.J. The effects of interparticle interactions and particle size on reversible shear thickening: Hard-sphere colloidal dispersions. J. Rheol. 2001, 45, 1205–1222. [Google Scholar] [CrossRef]
- Peressini, D.; Bravin, B.; Lapasin, R.; Rizzotti, C.; Sensidoni, A. Starch–methylcellulose based edible films: Rheological properties of film-forming dispersions. J. Food Eng. 2003, 59, 25–32. [Google Scholar] [CrossRef]
- Li, C.; Xiang, F.; Wu, K.; Jiang, F.; Ni, X. Changes in microstructure and rheological properties of konjac glucomannan/zein blend film-forming solution during drying. Carbohydr. Polym. 2020, 250, 116840. [Google Scholar] [CrossRef] [PubMed]
- Powles, J.G.; Rickayzen, G.; Heyes, D.M. Purely viscous fluids. Proc. R. Soc. A Math. Phys. Eng. Sci. 1999, 455, 3725–3742. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Ramírez, P.; Guerrero, A. Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll. 2010, 24, 659–666. [Google Scholar] [CrossRef]
- Lauten, R.A.; Nyström, B. Linear and nonlinear viscoelastic properties of aqueous solutions of cationic polyacrylamides. Macromol. Chem. Phys. 2000, 201, 677–684. [Google Scholar] [CrossRef]
- Rweiab, S.-P.; Chen, Y.-M.; Lin, W.-Y.; Chiang, W.-Y. Synthesis and Rheological Characterization of Water-Soluble Glycidyltrimethylammonium-Chitosan. Mar. Drugs 2014, 12, 5547–5562. [Google Scholar] [CrossRef]
- Tang, Y.-F.; Du, Y.; Hu, X.-W.; Shi, X.-W.; Kennedy, J.F. Rheological characterisation of a novel thermosensitive chitosan/poly(vinyl alcohol) blend hydrogel. Carbohydr. Polym. 2007, 67, 491–499. [Google Scholar] [CrossRef]
- Fabra, M.J.; Jiménez, A.; Atarés, L.; Talens, P.; Chiralt, A. Effect of Fatty Acids and Beeswax Addition on Properties of Sodium Caseinate Dispersions and Films. Biomacromolecules 2009, 10, 1500–1507. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, S.; Xiong, S.; Huang, Q. Steady, dynamic, and creep-recovery rheological properties of myofibrillar protein from grass carp muscle. Food Hydrocoll. 2016, 61, 48–56. [Google Scholar] [CrossRef]
- Toker, O.S.; Karaman, S.; Yuksel, F.; Dogan, M.; Kayacier, A.; Yilmaz, M.T. Temperature Dependency of Steady, Dynamic, and Creep-Recovery Rheological Properties of Ice Cream Mix. Food Bioprocess. Technol. 2013, 6, 2974–2985. [Google Scholar] [CrossRef]
- Martelli, M.R.; Barros, T.T.; De Moura, M.R.; Mattoso, L.H.C.; Assis, O.B.G. Effect of Chitosan Nanoparticles and Pectin Content on Mechanical Properties and Water Vapor Permeability of Banana Puree Films. J. Food Sci. 2013, 78, N98–N104. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Chiralt, A.; Albors, A.; González-Martínez, C. Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot. Postharvest Biol. Technol. 2009, 51, 263–271. [Google Scholar] [CrossRef]
- Alquezar, B.; Mesejo, C.; Alferez, F.; Agustí, M.; Zacarias, L. Morphological and ultrastructural changes in peel of ‘Navelate’ oranges in relation to variations in relative humidity during postharvest storage and development of peel pitting. Postharvest Biol. Technol. 2010, 56, 163–170. [Google Scholar] [CrossRef]
- Alferez, F.; Alquezar, B.; Burns, J.K.; Zacarias, L. Variation in water, osmotic and turgor potential in peel of ‘Marsh’ grapefruit during development of postharvest peel pitting. Postharvest Biol. Technol. 2010, 56, 44–49. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Bautista-Baños, S.; Flores-García, G.; Zavaleta-Avejar, L. Impact of chitosan based edible coatings functionalized with natural compounds on Colletotrichum fragariae development and the quality of strawberries. Food Chem. 2018, 262, 142–149. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef]
- Gomes, M.D.S.; Cardoso, M.D.G.; Guimarães, A.C.G.; Guerreiro, A.C.; Gago, C.M.L.; Boas, E.V.D.B.V.; Dias, C.M.B.; Manhita, A.C.C.; Faleiro, M.L.; Miguel, M.G.C.; et al. Effect of edible coatings with essential oils on the quality of red raspberries over shelf-life. J. Sci. Food Agric. 2017, 97, 929–938. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).