Anaerobic Digestion of Steam-Exploded Wheat Straw and Co-Digestion Strategies for Enhanced Biogas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Analytical Techniques
2.3. Biochemical Methane Potential Test
2.4. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Characterisation of the Feedstock
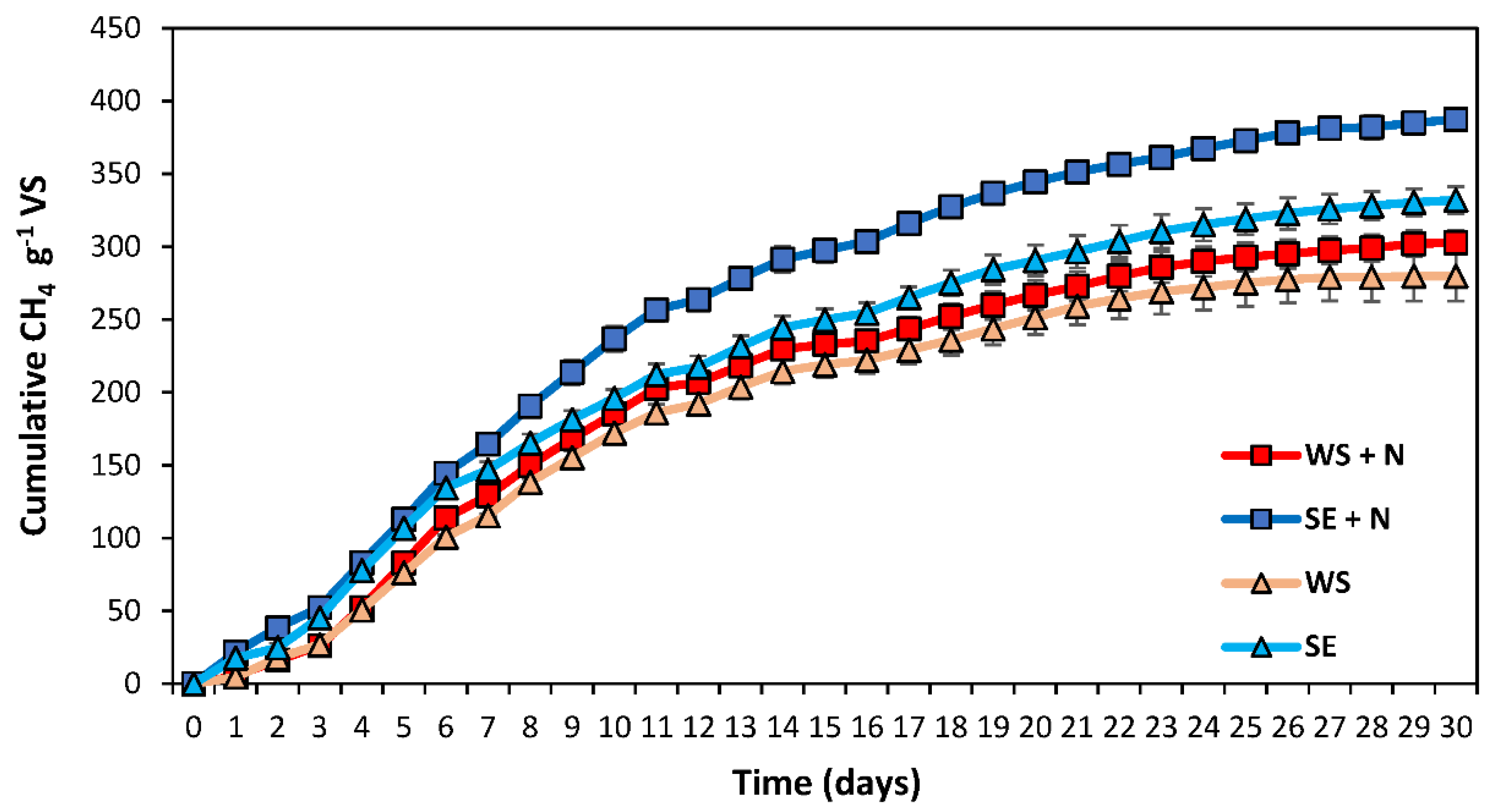
3.2. BMP—Inorganic Nitrogen Addition
3.3. BMP—Comparison of WS, RM and DDGS as AD Feedstock
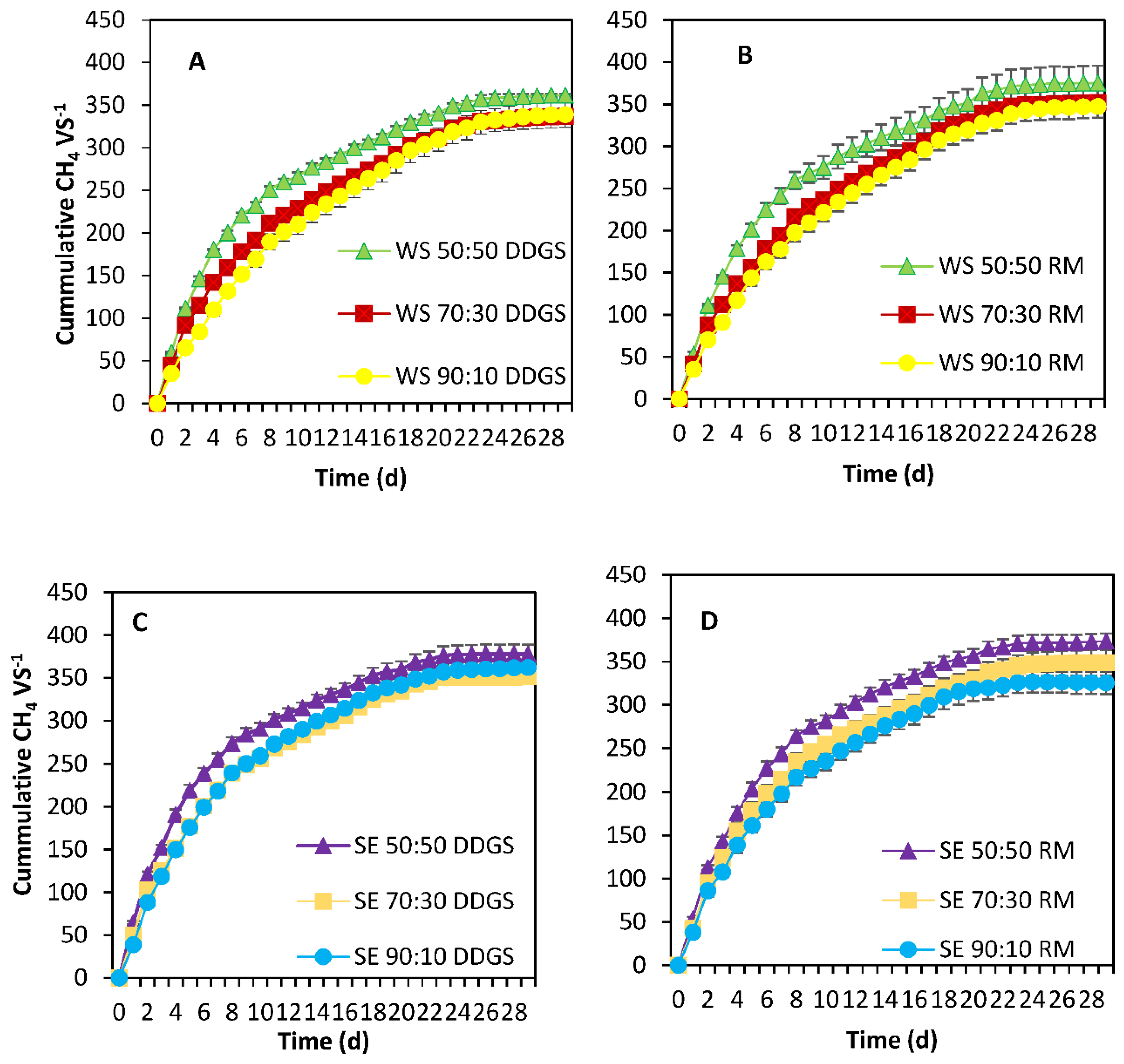
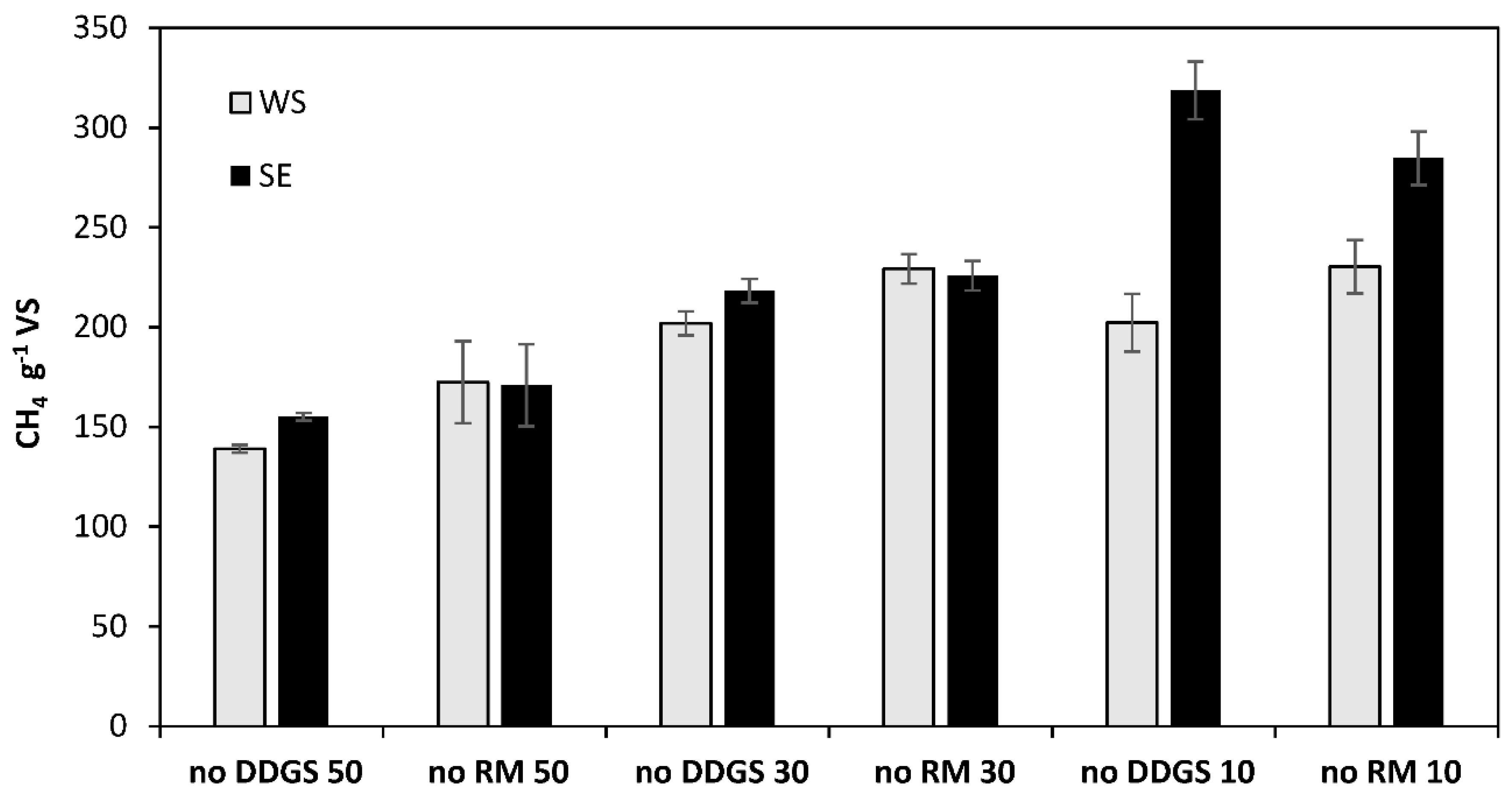
3.4. BMP—Co-Digestion Scenarios
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, H.; Chen, C.; Cai, D.; Fu, C.; Li, P.; Qin, P.; Tan, T. Simultaneous saccharification and juice co-fermentation for high-titer ethanol production using sweet sorghum stalk. Renew. Energy 2019, 134, 44–53. [Google Scholar] [CrossRef]
- Barcelos, C.A.; Maeda, R.N.; Santa Anna, L.M.M.; Pereira, N. Sweet sorghum as a whole-crop feedstock for ethanol production. Biomass Bioenergy 2016, 94, 46–56. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Nguyen, Q.D.; Westereng, B.; Nilsen, P.J.; Eijsink, V.G.H. Screening of steam explosion conditions for glucose production from non-impregnated wheat straw. Biomass Bioenergy 2011, 35, 4879–4886. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, B.H.; Wang, Y.; Yong, X.Y.; Yang, Z.H.; Jia, H.H.; Jiang, M.; Wei, P. Effect of steam explosion pretreatment on the anaerobic digestion of rice straw. R. Soc. Chem. 2016, 6, 88417–88425. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T.; Kumar, R. Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 2012, 43, 273–282. [Google Scholar] [CrossRef]
- Fjørtoft, K.; Morken, J.; Hanssen, J.F.; Briseid, T. Pre-treatment methods for straw for farm-scale biogas plants. Biomass Bioenergy 2019, 124, 88–94. [Google Scholar] [CrossRef]
- Agriculture and Horticulture Development Board (AHDB). Available online: https://ahdb.org.uk/farm-costs-at-a-glance (accessed on 10 October 2020).
- Fernandes, T.V.; Klaasse Bos, G.J.; Zeeman, G.; Sanders, J.P.M.; van Lier, J.B. Effects of thermo-chemical pre-treatment on anaerobic biodegradability and hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 2575–2579. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Wan, H.; Chen, Y.; Wang, X.; Yang, G.; Ren, G. Anaerobic co-digestion of animal manure and wheat straw for optimized biogas production by the addition of magnetite and zeolite. Energy Convers. Manag. 2015, 97, 132–139. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Donoso-Bravo, A.; Nilsen, P.J.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Influence of thermal pretreatment on the biochemical methane potential of wheat straw. Bioresour. Technol. 2013, 143, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Heeg, K.; Mumme, J. Anaerobic digestion of wheat straw—Performance of continuous solid-state digestion. Bioresour. Technol. 2013, 146, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.M.; Linjordet, R.; Morken, J. Effects of steam explosion and co-digestion in the methane production from Salix by mesophilic batch assays. Bioresour. Technol. 2012, 104, 749–756. [Google Scholar] [CrossRef]
- Yan, Z.; Song, Z.; Li, D.; Yuan, Y.; Liu, X.; Zheng, T. The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour. Technol. 2015, 177. [Google Scholar] [CrossRef]
- Shi, X.; Guo, X.; Zuo, J.; Wang, Y.; Zhang, M. A comparative study of thermophilic and mesophilic anaerobic co-digestion of food waste and wheat straw: Process stability and microbial community structure shifts. Waste Manag. 2018, 75, 261–269. [Google Scholar] [CrossRef]
- Hassan, M.; Ding, W.; Shi, Z.; Zhao, S. Methane enhancement through co-digestion of chicken manure and thermo-oxidative cleaved wheat straw with waste activated sludge: A C/N optimization case. Bioresour. Technol. 2016, 211, 534–541. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Prabhakumari, P.C.; Kosik, O.; Lovegrove, A.; Shewry, P.R.; Charalampopoulos, D. Extractability and characteristics of proteins deriving from wheat DDGS. Food Chem. 2016, 198, 12–19. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Kosik, O.; Prabhakumari, P.C.; Lovegrove, A.; Frazier, R.A.; Shewry, P.R.; Charalampopoulos, D. Biorefinery strategies for upgrading Distillers’ Dried Grains with Solubles (DDGS). Process Biochem. 2015, 50, 2194–2207. [Google Scholar] [CrossRef]
- Kolesárová, N.; Hutňan, M.; Špalková, V.; Lazor, M. Anaerobic treatment of rapeseed meal. Chem. Pap. 2013, 67, 1569–1576. [Google Scholar] [CrossRef]
- European Commission. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2018, L 328, 82–289. [Google Scholar]
- Ziganshin, A.M.; Schmidt, T.; Scholwin, F.; Il’Inskaya, O.N.; Harms, H.; Kleinsteuber, S. Bacteria and archaea involved in anaerobic digestion of distillers grains with solubles. Appl. Microbiol. Biotechnol. 2011, 89, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hu, Y. Comparison of reactor configurations for biogas production from rapeseed straw. Bioresorces 2016, 11, 9970–9985. [Google Scholar] [CrossRef]
- Cesaro, A.; Velten, S.; Belgiorno, V.; Kuchta, K. Ultrasonic Pretreatment Of Ddgs For Anaerobic Digestion. Semant. Sch. 2013, 5–7. Available online: https://www.semanticscholar.org/paper/ULTRASONIC-PRETREATMENT-OF-DDGS-FOR-ANAEROBIC-Ces%C3%A1ro-Velten/f03c05d4c637570bf81f11fe1809dd72718b1848 (accessed on 10 October 2020).
- Antonopoulou, G.; Stamatelou, K.; Lyberatos, G. Exploitation of Rapeseed and Sunflower Residues for Methane Generation Through Anaerobic Digestion: The Effect of Pretreatment. Chem. Eng. Trans. 2010, 20, 253–258. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington DC, USA, 2012; p. 1360. ISBN 978-087553-013-0. [Google Scholar]
- AOAC International. Official Methods of Analysis, 15th ed.; AOAC International: Gaithersburg, MD, USA, 1990; ISBN 0849328993. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 analytical procedure—Determination of structural carbohydrates and lignin in Biomass. Lab. Anal. Proced. 2012, 17. Technical Report: NREL/TP-510-42618. [Google Scholar]
- Strömberg, S.; Nistor, M.; Liu, J. Towards eliminating systematic errors caused by the experimental conditions in Biochemical Methane Potential (BMP) tests. Waste Manag. 2014, 34, 1939–1948. [Google Scholar] [CrossRef]
- Flores, G.A.E.; Fotidis, I.A.; Karakashev, D.B.; Kjellberg, K.; Angelidaki, I. Effects of Benzalkonium Chloride, Proxel LV, P3 Hypochloran, Triton X-100 and DOWFAX 63N10 on anaerobic digestion processes. Bioresour. Technol. 2015, 193, 393–400. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Menardo, S.; Bauer, A.; Theuretzbacher, F.; Piringer, G.; Nilsen, P.J.; Balsari, P.; Pavliska, O.; Amon, T. Biogas Production from Steam-Exploded Miscanthus and Utilization of Biogas Energy and CO2 in Greenhouses. Bioenergy Res. 2013, 6, 620–630. [Google Scholar] [CrossRef]
- Amel, B.D.; Nawel, B.; Khelifa, B.; Mohammed, G.; Manon, J.; Salima, K.G.; Farida, N.; Hocine, H.; Bernard, O.; Jean-Luc, C.; et al. Characterization of a purified thermostable xylanase from Caldicoprobacter algeriensis sp. nov. strain TH7C1T. Carbohydr. Res. 2016, 419, 60–68. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, B.; Xiao, Q.; Wu, S. A kinetics modeling study on the inhibition of glucose on cellulosome of Clostridium thermocellum. Bioresour. Technol. 2015, 190, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gyenge, L.; Raduly, B.; Barrera, R.; Font, X.; Lanyi, S.; Abraham, B. Efficiency of biogas production from corn bioe-thanol by-products using different inocula. In Proceedings of the 2013 4th International Youth Conference on Energy (IYCE), Siofok, Hungary, 6–8 June 2013. [Google Scholar] [CrossRef]
- Harith, Z.T.; Charalampopoulos, D.; Chatzifragkou, A. Rapeseed meal hydrolysate as substrate for microbial astaxanthin production. Biochem. Eng. J. 2019, 151, 107330. [Google Scholar] [CrossRef]
- Tomás-pejó, E.; Fermoso, J.; Herrador, E.; Hernando, H.; Jiménez-sánchez, S.; Ballesteros, M. Valorization of steam-exploded wheat straw through a biorefinery approach: Bioethanol and bio-oil co-production. Fuel 2017, 199, 403–412. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, P.; Zhang, G.; Wang, Y.; Yang, A. Degradation properties of protein and carbohydrate during sludge anaerobic digestion. Bioresour. Technol. 2015, 192, 126–130. [Google Scholar] [CrossRef] [PubMed]

| %, w/w (db) | Wheat Straw (WS) | Steam-Exploded Straw (SE) | DDGS | RM | Inoculum |
|---|---|---|---|---|---|
| Total solids (TS) | 36.06 ± 0.52 | 21.04 ± 0.57 | 91.46 ± 0.29 | 92.42 ± 0.65 | 3.37 ± 0.13 |
| Volatile solids (VS) | 33.38 ± 0.63 | 19.37 ± 0.53 | 86.1 ± 0.34 | 86.39 ± 0.68 | 2.53 ± 0.11 |
| Crude protein | 0.29 ± 0.01 | 0.47 ± 0.02 | 28.3 ± 0.5 | 25.28 ± 0.15 | n.d. |
| Cellulose (glucose) | 37.41 ± 0.77 | 47.92 ± 0.50 | 11.1 ± 0.4 | 20.17 ± 2.32 | n.d. |
| Hemicellulose | 27.56 ± 0.75 | 28.23 ± 0.12 | 20.3 ± 1.7 | 14.03 ± 2.03 | n.d. |
| Acid insoluble lignin | 28.09 ± 2.6 | 25.28 ± 1.68 | n.d. | 16.08 ± 0.15 | n.d. |
| Acid soluble lignin | 2.38 ± 0.02 | 2.05 ± 0.11 | 2.9 ± 0.1 | 1.9 ± 0.1 | n.d. |
| WS (% w/w) | SE (% w/w) | DDGS (% w/w) | RM (% w/w) | C/N (w/w) | |
|---|---|---|---|---|---|
| WS-DDGS | 50 | - | 50 | - | 21 |
| WS-DDGS | 70 | - | 30 | - | 26 |
| WS-DDGS | 90 | - | 10 | - | 30 |
| WS-RM | 50 | - | - | 50 | 19 |
| WS-RM | 70 | - | - | 30 | 24 |
| WS-RM | 90 | - | - | 10 | 30 |
| SE-DDGS | - | 50 | 50 | - | 15 |
| SE-DDGS | - | 70 | 30 | - | 24 |
| SE-DDGS | - | 90 | 10 | - | 35 |
| SE-RM | - | 50 | - | 50 | 15 |
| SE-RM | - | 70 | - | 30 | 25 |
| SE-RM | - | 90 | - | 10 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaldis, F.; Cysneiros, D.; Day, J.; G. Karatzas, K.-A.; Chatzifragkou, A. Anaerobic Digestion of Steam-Exploded Wheat Straw and Co-Digestion Strategies for Enhanced Biogas Production. Appl. Sci. 2020, 10, 8284. https://doi.org/10.3390/app10228284
Kaldis F, Cysneiros D, Day J, G. Karatzas K-A, Chatzifragkou A. Anaerobic Digestion of Steam-Exploded Wheat Straw and Co-Digestion Strategies for Enhanced Biogas Production. Applied Sciences. 2020; 10(22):8284. https://doi.org/10.3390/app10228284
Chicago/Turabian StyleKaldis, Fokion, Denise Cysneiros, James Day, Kimon-Andreas G. Karatzas, and Afroditi Chatzifragkou. 2020. "Anaerobic Digestion of Steam-Exploded Wheat Straw and Co-Digestion Strategies for Enhanced Biogas Production" Applied Sciences 10, no. 22: 8284. https://doi.org/10.3390/app10228284
APA StyleKaldis, F., Cysneiros, D., Day, J., G. Karatzas, K.-A., & Chatzifragkou, A. (2020). Anaerobic Digestion of Steam-Exploded Wheat Straw and Co-Digestion Strategies for Enhanced Biogas Production. Applied Sciences, 10(22), 8284. https://doi.org/10.3390/app10228284






