Focal Muscle Vibration for Stroke Rehabilitation: A Review of Vibration Parameters and Protocols
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
3. Results
3.1. Search Results
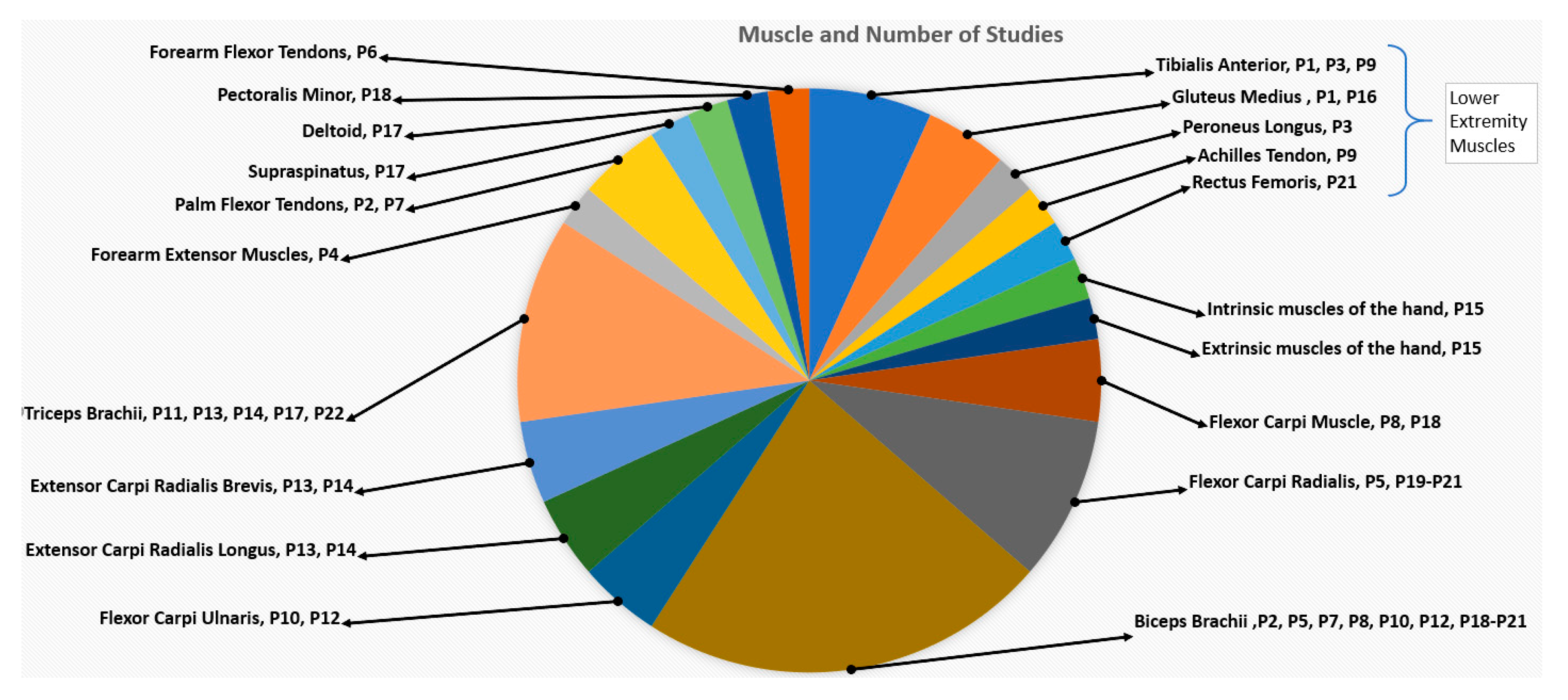
3.2. Participant Characteristics
3.3. Study Design
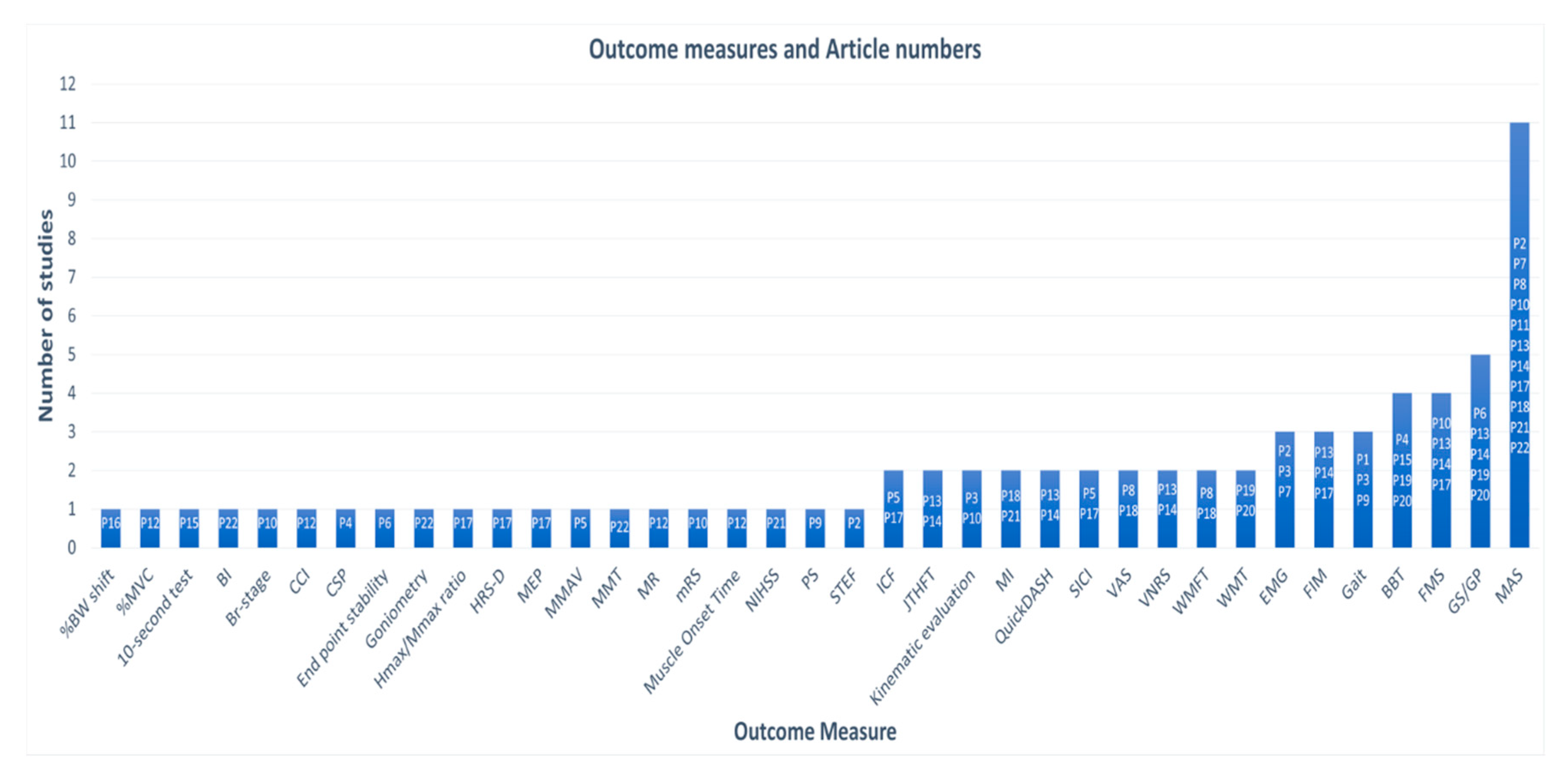
3.4. Vibration Parameteres and Protocols
3.4.1. Vibration Device
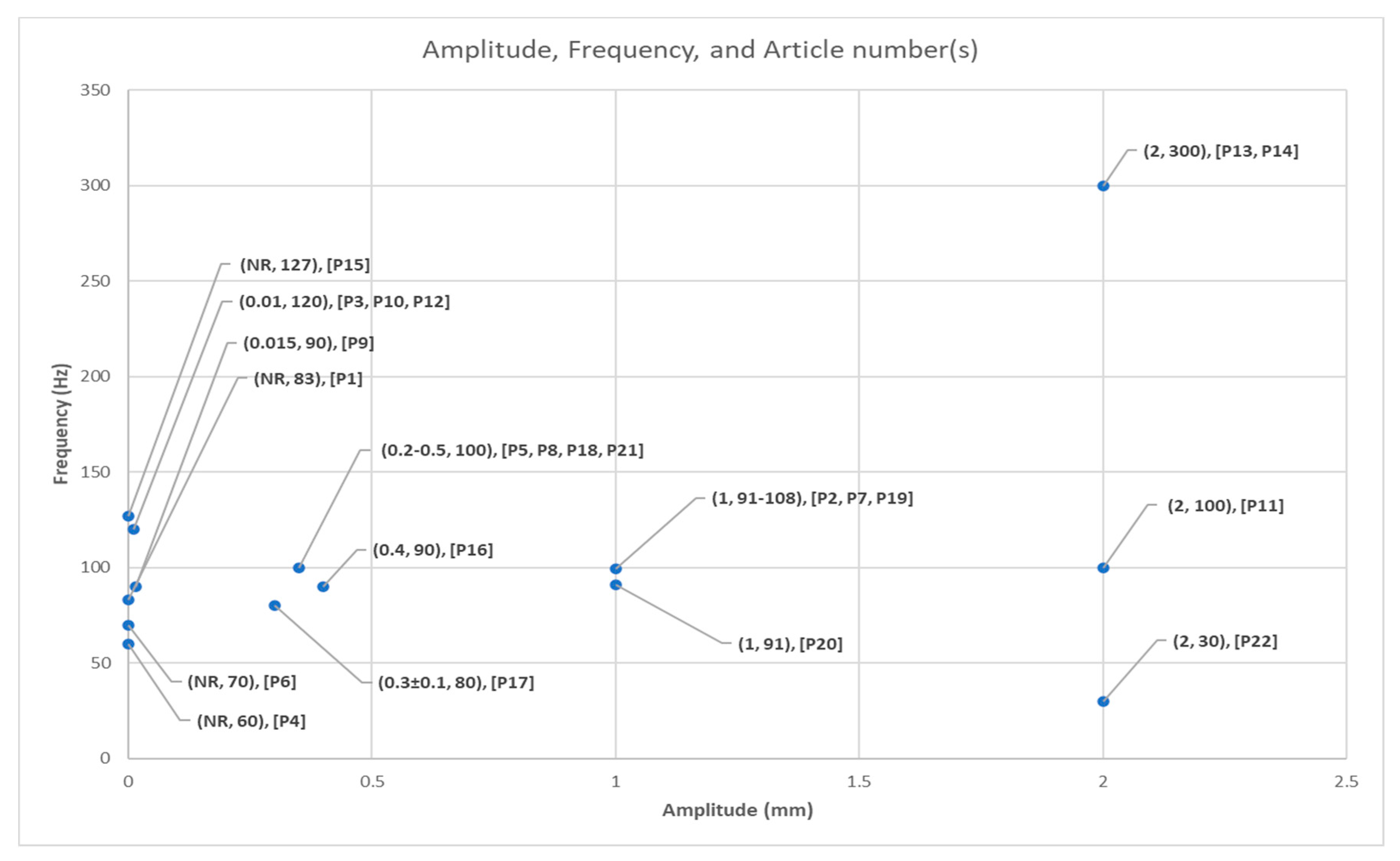
3.4.2. Vibration Frequency and Amplitude
3.4.3. Vibration Protocols
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD/PD | Anterior/Posterior Deltoid |
| AROM | Active Range of Motion |
| BB | Biceps Brachii |
| BBT | Box and Block Test |
| BI | Barthel Index |
| Br-stage | Brunnstorm stage |
| BW | Body Weight |
| CCI | Co-Contraction Index |
| CG | Control Group |
| CP | Conventional Physiotherapy |
| CSP | Cortical Silent Period |
| ECR | Extensor Carpi Radialis |
| EDC | Extensor Digitorum Communis |
| EG | Experimental Group |
| EMG | Electromyograph |
| EPS | End Point Stability |
| FAS | Functional Ability Scale |
| FCR | Flexor Carpi Radialis |
| FES | Functional Electrical Stimulation |
| FMV | Focal Muscle Vibration |
| FIM | Functional Independence Measure |
| FMS | Fugl-Meyer Scale |
| G1/G2/G3 | Group 1/2/3 |
| GP | Grip Pressure |
| GS | Grip Strength |
| HRS-D | Hamilton Rating Scale for Depression |
| HMR | Hmax/Mmax Ratio |
| ICF | Intracortical Fascilitation |
| JTHFT | Jebsen-Taylor Hand Function Test |
| KE | Kinematic Evaluation |
| MAS | Modified Ashworth Scale |
| MI | Motricity Index |
| MMAV | Motor Map Area and Volume |
| MR | Modulation Ratio |
| MOT | Muscle Onset Time |
| mRS | modified Rankin Scale |
| MVC | Maximal Voluntary Contraction |
| PS | Postural Sway |
| PT | Physical Therapy |
| RCT | Randomized Control Trial |
| RG | Rest Group |
| RMP | Progressive Modular Re-balancing |
| SICF | Short Interval Intracortical Facilitation |
| SICI | Short Interval Intracortical Inhibition |
| SSD | Single Subject Design |
| STEF | Simple Test for Evaluating hand Function |
| StG | Stretch Group |
| TB | Triceps Brachii |
| VAS | Visual Analog Scale |
| VNRS | Verbal Numerical Rating Scale |
| WMFT | Wolf Motor Function Test |
| WMT | Weinstein Monofilament Test |
References
- Katan, M.; Luft, A. Global burden of stroke. In Seminars in Neurology; Georg Thieme Verlag: Stuttgart, Germany, 2018. [Google Scholar]
- Bolognini, N.; Russo, C.; Edwards, D.J. The sensory side of post-stroke motor rehabilitation. Restor. Neurol. Neurosci. 2016, 34, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.L.; Tommasino, P.; Budhota, A.; Campolo, D. Upper extremity proprioception in healthy aging and stroke populations, and the effects of therapist-and robot-based rehabilitation therapies on proprioceptive function. Front. Hum. Neurosci. 2015, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, H.T.; van Limbeek, J.; Geurts, A.C.; Zwarts, M.J. Motor recovery after stroke: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2002, 83, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Nyström, K.V.; Yavagal, D.R.; Luciano, J.; Nogueira, R.G.; Zorowitz, R.D.; Khalessi, A.A.; Bushnell, C.; Barsan, W.G.; Panagos, P.; et al. Recommendations for the establishment of stroke systems of care: A 2019 update: A policy statement from the American Stroke Association. Stroke 2019, 50, e187–e210. [Google Scholar] [CrossRef] [PubMed]
- Westwater-Wood, S.; Adams, N.; Kerry, R. The use of proprioceptive neuromuscular facilitation in physiotherapy practice. Phys. Ther. Rev. 2010, 15, 23–28. [Google Scholar] [CrossRef]
- Oujamaa, L.; Relave, I.; Froger, J.; Mottet, D.; Pelissier, J.Y. Rehabilitation of arm function after stroke. Literature review. Ann. Phys. Rehabil. Med. 2009, 52, 269–293. [PubMed]
- Hong, Z.; Sui, M.; Zhuang, Z.; Liu, H.; Zheng, X.; Cai, C.; Jin, D. Effectiveness of neuromuscular electrical stimulation on lower limbs of patients with hemiplegia after chronic stroke: A systematic review. Arch. Phys. Med. Rehabil. 2018, 99, 1011–1022. [Google Scholar] [CrossRef]
- Quandt, F.; Hummel, F.C. The influence of functional electrical stimulation on hand motor recovery in stroke patients: A review. Exp. Transl. Stroke Med. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Laufer, Y.; Elboim-Gabyzon, M. Does sensory transcutaneous electrical stimulation enhance motor recovery following a stroke? A systematic review. Neurorehabilit. Neural Repair 2011, 25, 799–809. [Google Scholar] [CrossRef]
- Mahmood, A.; Veluswamy, S.K.; Hombali, A.; Mullick, A.; Manikandan, N.; Solomon, J.M. Effect of transcutaneous electrical nerve stimulation on spasticity in adults with stroke: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 751–768. [Google Scholar] [CrossRef]
- Stanton, R.; Ada, L.; Dean, C.M.; Preston, E. Biofeedback improves activities of the lower limb after stroke: A systematic review. J. Physiother. 2011, 57, 145–155. [Google Scholar] [CrossRef]
- van Duijnhoven, H.J.; Heeren, A.; Peters, M.A.; Veerbeek, J.M.; Kwakkel, G.; Geurts, A.C.; Weerdesteyn, V. Effects of exercise therapy on balance capacity in chronic stroke: Systematic review and meta-analysis. Stroke 2016, 47, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Ammann, B.C.; Knols, R.H.; Baschung, P.; De Bie, R.A.; Bruin, E.D. Application of principles of exercise training in sub-acute and chronic stroke survivors: A systematic review. BMC Neurol. 2014, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.P.; Keeling, J.; Lin, B.; Henderson, M.; Hale, L.A. The impact of bilateral therapy on upper limb function after chronic stroke: A systematic review. Disabil. Rehabil. 2010, 32, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruzado, D.; Merchán-Baeza, J.A.; González-Sánchez, M.; Cuesta-Vargas, A.I. Systematic review of mirror therapy compared with conventional rehabilitation in upper extremity function in stroke survivors. Aust. Occup. Ther. J. 2017, 64, 91–112. [Google Scholar] [CrossRef]
- Bertani, R.; Melegari, C.; Maria, C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Bruni, M.F.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J. Clin. Neurosci. 2018, 48, 11–17. [Google Scholar] [CrossRef]
- Iruthayarajah, J.; McIntyre, A.; Cotoi, A.; Macaluso, S.; Teasell, R. The use of virtual reality for balance among individuals with chronic stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2017, 24, 68–79. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, Y.J.; Park, S.W. The effects of virtual reality training on function in chronic stroke patients: A systematic review and meta-analysis. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Teasell, R.; Mehta, S.; Pereira, S.; McIntyre, A.; Janzen, S.; Allen, L.; Lobo, L.; Viana, R. Time to rethink long-term rehabilitation management of stroke patients. Top. Stroke Rehabil. 2012, 19, 457–462. [Google Scholar]
- Teasell, R.W.; Fernandez, M.M.; McIntyre, A.; Mehta, S. Rethinking the continuum of stroke rehabilitation. Arch. Phys. Med. Rehabil. 2014, 95, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Forster, A. Review of stroke rehabilitation. BMJ 2007, 334, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, F. Application of Vibration Training in People with Common Neurological Disorders. In Manual of Vibration Exercise and Vibration Therapy; Springer International: Cham, Switzerland, 2020; pp. 343–353. [Google Scholar]
- Schröder, J.; Truijen, S.; Van Criekinge, T.; Saeys, W. Peripheral somatosensory stimulation and postural recovery after stroke—A systematic review. Top. Stroke Rehabil. 2018, 25, 312–320. [Google Scholar]
- Celletti, C.; Suppa, A.; Bianchini, E.; Lakin, S.; Toscano, M.; La Torre, G.; Di Piero, V.; Camerota, F. Promoting post-stroke recovery through focal or whole body vibration: Criticisms and prospects from a narrative review. Neurol. Sci. 2020, 41, 11–24. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Romagnoli, C.; Annino, G. Effectiveness of focal muscle vibration on hemiplegic upper extremity spasticity in individuals with stroke: A systematic review. NeuroRehabilitation 2019, 45, 471–481. [Google Scholar] [CrossRef]
- Murillo, N.; Valls-Sole, J.; Vidal, J.; Opisso, E.; Medina, J.; Kumru, H. Focal vibration in neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2014, 50, 231–242. [Google Scholar]
- Huang, M.; Pang, M.Y.C. Muscle activity and vibration transmissibility during whole-body vibration in chronic stroke. Scand. J. Med. Sci. Sports 2019, 29, 816–825. [Google Scholar] [CrossRef]
- Steyvers, M.; Levin, O.; Van Baelen, M.; Swinnen, S.P. Corticospinal excitability changes following prolonged muscle tendon vibration. Neuroreport 2003, 14, 1901–1905. [Google Scholar] [CrossRef]
- Yang, F.; Butler, A.J. Efficacy of controlled whole-body vibration training on improving fall risk factors in stroke survivors: A meta-analysis. Neurorehabilit. Neural Repair 2020, 34, 275–288. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.W.; Lee, H.S. Comparison of the Effectiveness of Whole Body Vibration in Stroke Patients: A Meta-Analysis. BioMed Res. Int. 2018, 2018, 5083634. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, B.; Taiar, R.; Furness, T.; Bernardo-Filho, M. Clinical Approaches of Whole-Body Vibration Exercises in Individuals with Stroke: A Narrative Revision. Rehabil. Res. Pract. 2018, 2018. [Google Scholar] [CrossRef]
- Lee, A.; Kim, H.; Kim, J.; Choi, D.S.; Jung, J.H.; Lee, J.; Kim, Y.H. Modulating Effects of Whole-body Vibration on Cortical Activity and Gait Function in Chronic Stroke Patients. Brain Neurorehabilit. 2019, 13. [Google Scholar] [CrossRef]
- Yang, X.; Wang, P.; Liu, C.; He, C.; Reinhardt, J.D. The effect of whole body vibration on balance, gait performance and mobility in people with stroke: A systematic review and meta-analysis. Clin. Rehabil. 2015, 29, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, G.; Wang, Y. Effects of whole body vibration training on people with chronic stroke: A systematic review and meta-analysis. Top. Stroke Rehabil. 2015, 22, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-R.; Huang, M.; Lam, F.M.; Pang, M.Y. Effects of whole-body vibration therapy on body functions and structures, activity, and participation poststroke: A systematic review. Phys. Ther. 2014, 94, 1232–1251. [Google Scholar] [CrossRef]
- Pozo-Cruz, B.d.; Adsuar, J.C.; Parraca, J.A.; Pozo-Cruz, J.D.; Olivares, P.R.; Gusi, N. Using whole-body vibration training in patients affected with common neurological diseases: A systematic literature review. J. Altern. Complementary Med. 2012, 18, 29–41. [Google Scholar] [CrossRef]
- Huang, M.; Liao, L.-R.; Pang, M.Y. Effects of whole body vibration on muscle spasticity for people with central nervous system disorders: A systematic review. Clin. Rehabil. 2017, 31, 23–33. [Google Scholar] [CrossRef]
- Kawahira, K.; Higashihara, K.; Matsumoto, S.; Shimodozono, M.; Etoh, S.; Tanaka, N.; Sueyoshi, Y. New functional vibratory stimulation device for extremities in patients with stroke. Int. J. Rehabil. Res. 2004, 27, 335–337. [Google Scholar] [CrossRef]
- Paoloni, M.; Mangone, M.; Scettri, P.; Procaccianti, R.; Cometa, A.; Santilli, V. Segmental muscle vibration improves walking in chronic stroke patients with foot drop: A randomized controlled trial. Neurorehabil. Neural Repair 2010, 24, 254–262. [Google Scholar] [CrossRef]
- Lee, S.-W.; Cho, K.-H.; Lee, W.-H. Effect of a local vibration stimulus training programme on postural sway and gait in chronic stroke patients: A randomized controlled trial. Clin. Rehabil. 2013, 27, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Bonan, I.; Butet, S.; Jamal, K.; Yelnik, A.; Ponche, S.T.; Leplaideur, S. Difference between individuals with left and right hemiparesis in the effect of gluteus medius vibration on body weight shifting. Neurophysiol. Clin. 2017, 47, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Noma, T.; Matsumoto, S.; Etoh, S.; Shimodozono, M.; Kawahira, K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients. Brain Inj. 2009, 23, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Binder, C. Vibration-induced effects in stroke patients with spastic hemiparesis—A pilot study. Restor. Neurol. Neurosci. 2010, 28, 729–735. [Google Scholar] [CrossRef]
- Marconi, B.; Filippi, G.M.; Koch, G.; Giacobbe, V.; Pecchioli, C.; Versace, V.; Camerota, F.; Saraceni, V.M.; Caltagirone, C. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil. Neural Repair 2011, 25, 48–60. [Google Scholar] [CrossRef]
- Conrad, O.M.; Scheidt, R.A.; Schmit, B.D. Effects of wrist tendon vibration on targeted upper-arm movements in poststroke hemiparesis. Neurorehabil. Neural Repair 2011, 25, 61–70. [Google Scholar] [CrossRef]
- Noma, T.; Matsumoto, S.; Shimodozono, M.; Etoh, S.; Kawahira, K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients: A proof-of-principle study. J. Rehabil. Med. 2012, 44, 325–330. [Google Scholar] [CrossRef]
- Caliandro, P.; Celletti, C.; Padua, L.; Minciotti, I.; Russo, G.; Granata, G.; La Torre, G.; Granieri, E.; Camerota, F. Focal muscle vibration in the treatment of upper limb spasticity: A pilot randomized controlled trial in patients with chronic stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1656–1661. [Google Scholar] [CrossRef]
- Tavernese, E.; Paoloni, M.; Mangone, M.; Mandic, V.; Sale, P.; Franceschini, M.; Santilli, V. Segmental muscle vibration improves reaching movement in patients with chronic stroke. A randomized controlled trial. NeuroRehabilitation 2013, 32, 591–599. [Google Scholar] [CrossRef]
- Casale, R.; Damiani, C.; Maestri, R.; Fundarò, C.; Chimento, P.; Foti, C. Localized 100 Hz vibration improves function and reduces upper limb spasticity: A double-blind controlled study. Eur. J. Phys. Rehabil. Med. 2014, 50, 495–504. [Google Scholar]
- Paoloni, M.; Tavernese, E.; Fini, M.; Sale, P.; Franceschini, M.; Santilli, V.; Mangone, M. Segmental muscle vibration modifies muscle activation during reaching in chronic stroke: A pilot study. NeuroRehabilitation 2014, 35, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Constantino, C.; Galuppo, L.; Romiti, D. Efficacy of mechano-acoustic vibration on strength, pain, and function in poststroke rehabilitation: A pilot study. Top. Stroke Rehabil. 2014, 21, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.; Galuppo, L.; Romiti, D. Short-term effect of local muscle vibration treatment versus sham therapy on upper limb in chronic post-stroke patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 32–40. [Google Scholar] [PubMed]
- Go, J.-E.; Lee, S.-H. Effect of sensorimotor stimulation on chronic stroke patients’ upper extremity function: A preliminary study. J. Phys. Ther. Sci. 2016, 28, 3350–3353. [Google Scholar] [CrossRef]
- Calabro, R.S.; Naro, A.; Russo, M.; Milardi, D.; Leo, A.; Filoni, S.; Trinchera, A.; Bramanti, P. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS ONE 2017, 12, e0185936. [Google Scholar] [CrossRef]
- Celletti, C.; Sinibaldi, E.; Pierelli, F.; Monari, G.; Camerota, F. Focal muscle vibration and progressive modular rebalancing with neurokinetic facilitations in post-stroke recovery of upper limb. Clin. Ther. 2017, 168, e33–e36. [Google Scholar]
- Jung, S.-M. The effects of vibratory stimulation employed to forearm and arm flexor muscles on upper limb function in patients with chronic stroke. J. Phys. Ther. Sci. 2017, 29, 1620–1622. [Google Scholar] [CrossRef][Green Version]
- Choi, W.-H. Effects of repeated vibratory stimulation of wrist and elbow flexors on hand dexterity, strength, and sensory function in patients with chronic stroke: A pilot study. J. Phys. Ther. Sci. 2017, 29, 605–608. [Google Scholar] [CrossRef][Green Version]
- Annino, G.; Alashram, A.R.; Alghwiri, A.A.; Romagnoli, C.; Messina, G.; Tancredi, V.; Padua, E.; Mercuri, N.B. Effect of segmental muscle vibration on upper extremity functional ability poststroke: A randomized controlled trial. Medicine 2019, 98. [Google Scholar] [CrossRef]
- Toscano, M.; Celletti, C.; Viganò, A.; Altarocca, A.; Giuliani, G.; Jannini, T.B.; Mastria, G.; Ruggiero, M.; Maestrini, I.; Vicenzini, E.; et al. Short-Term Effects of Focal Muscle Vibration on Motor Recovery After Acute Stroke: A Pilot Randomized Sham-Controlled Study. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Volpe, D.; Giantin, M.G.; Fasano, A. A wearable proprioceptive stabilizer (Equistasi®) for rehabilitation of postural instability in Parkinson’s disease: A phase II randomized double-blind, double-dummy, controlled study. PLoS ONE 2014, 9, e112065. [Google Scholar] [CrossRef] [PubMed]
- Serio, F.; Minosa, C.; De Luca, M.; Conte, P.; Albani, G.; Peppe, A. Focal Vibration Training (Equistasi®) to Improve Posture Stability. A Retrospective Study in Parkinson’s Disease. Sensors 2019, 19, 2101. [Google Scholar] [CrossRef] [PubMed]
- Peppe, A.; Paravati, S.; Baldassarre, M.G.; Bakdounes, L.; Guiotto, A.; Pavadan, D.; Sawacha, Z.; Clerici, D.; Bottino, S.; Cau, N.; et al. Proprioceptive Focal Stimulation (Equistasi®) may improve the quality of gait in middle-moderate Parkinson’s disease patients. Double-blind, double-dummy, randomized, crossover, Italian Multicentric study. Front. Neurol. 2019, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; Carotenuto, A.; Aceto, M.G.; Cerillo, I.; Silvestre, F.; Arace, F.; Paone, P.; Orefice, G.; Iodice, R. The effects of mechanical focal vibration (Equistasi®) on walking impairment in multiple sclerosis patients: A randomized, double-blinded vs placebo study. Mult. Scler. J. 2016, 22. [Google Scholar] [CrossRef]
- Schirinzi, T.; Romano, A.; Favetta, M.; Sancesario, A.; Burattini, R.; Summa, S.; Della Bella, G.; Castelli, E.; Bertini, E.; Petrarca, M.; et al. Non-invasive focal mechanical vibrations delivered by wearable devices: An open-label pilot study in childhood Ataxia. Front. Neurol. 2018, 9, 849. [Google Scholar] [CrossRef]
- Leonardi, L.; Aceto, M.G.; Marcotulli, C.; Arcuria, G.; Serrao, M.; Pierelli, F.; Paone, P.; Filla, A.; Roca, A.; Casali, C. A wearable proprioceptive stabilizer for rehabilitation of limb and gait ataxia in hereditary cerebellar ataxias: A pilot open-labeled study. Neurol. Sci. 2017, 38, 459–463. [Google Scholar] [CrossRef]
- Cochrane, D. The acute effect of direct vibration on muscular power performance in master athletes. Int. J. Sports Med. 2016, 37, 144–148. [Google Scholar] [CrossRef]
- Cochrane, D.J. Effectiveness of using wearable vibration therapy to alleviate muscle soreness. Eur. J. Appl. Physiol. 2017, 117, 501–509. [Google Scholar] [CrossRef]
- Cochrane, D.J.; Cochrane, F.; Roake, J.A. An exploratory study of vibration therapy on muscle function in patients with peripheral artery disease. J. Vasc. Surg. 2020, 71, 1340–1345. [Google Scholar] [CrossRef]
- Souron, R.; Besson, T.; Millet, G.Y.; Lapole, T. Acute and chronic neuromuscular adaptations to local vibration training. Eur. J. Appl. Physiol. 2017, 117, 1939–1964. [Google Scholar] [CrossRef]
- Steyvers, M.; Levin, O.; Verschueren, S.M.; Swinnen, S.P. Frequency-dependent effects of muscle tendon vibration on corticospinal excitability: A TMS study. Exp. Brain Res. 2003, 151, 9–14. [Google Scholar] [CrossRef]
- Necking, L.E.; Lundström, R.; Dahlin, L.B.; Lundborg, G.; Thornell, L.E.; Friden, J. Tissue displacement is a causative factor in vibration-induced muscle injury. J. Hand Surg. Br. Eur. Vol. 1996, 21, 753–757. [Google Scholar] [CrossRef]
- Hagbarth, E.K.; Eklund, G. The effects of muscle vibration in spasticity, rigidity, and cerebellar disorders. J. Neurol. Neurosurg. Psychiatry 1968, 31, 207–213. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Mancinelli, R.; Toniolo, L.; Cancellara, L.; Paoli, A.; Puglielli, C.; Iodice, P.; Doria, C.; Bosco, G.; d’Amelio, L.; et al. Effects of local vibrations on skeletal muscle trophism in elderly people: Mechanical, cellular, and molecular events. Int. J. Mol. Med. 2009, 24, 503–512. [Google Scholar] [CrossRef]
- Nelson, M.C.; Murray, W.M.; Dewald, J.P.A. Motor Impairment-Related Alterations in Biceps and Triceps Brachii Fascicle Lengths in Chronic Hemiparetic Stroke. Neurorehabil. Neural Repair 2018, 32, 799–809. [Google Scholar] [CrossRef]
- de Gooijer-van de Groep, K.L.; de Groot, J.H.; van der Krogt, H.; de Vlugt, E.; Arendzen, J.H.; Meskers, C.G. Early Shortening of Wrist Flexor Muscles Coincides With Poor Recovery After Stroke. Neurorehabil. Neural Repair 2018, 32, 645–654. [Google Scholar] [CrossRef]
- Dutta, A.; Paulus, W.; Nitsche, M.A. Translational methods for non-invasive electrical stimulation to facilitate gait rehabilitation following stroke-the future directions. Neurosci. Biomed. Eng. 2013, 1, 22–33. [Google Scholar] [CrossRef]
- Chae, J.; Quinn, A.; El-Hayek, K.; Santing, J.; Berezovski, R.; Harley, M. Delay in initiation and termination of tibialis anterior contraction in lower-limb hemiparesis: Relationship to lower-limb motor impairment and mobility. Arch. Phys. Med. Rehabil. 2006, 87, 1230–1234. [Google Scholar] [CrossRef]
- Üstün, T.B.; Chatterji, S.; Bickenbach, J.; Kostanjsek, N.; Schneider, M. The International Classification of Functioning, Disability and Health: A new tool for understanding disability and health. Disabil. Rehabil. 2003, 25, 565–571. [Google Scholar]
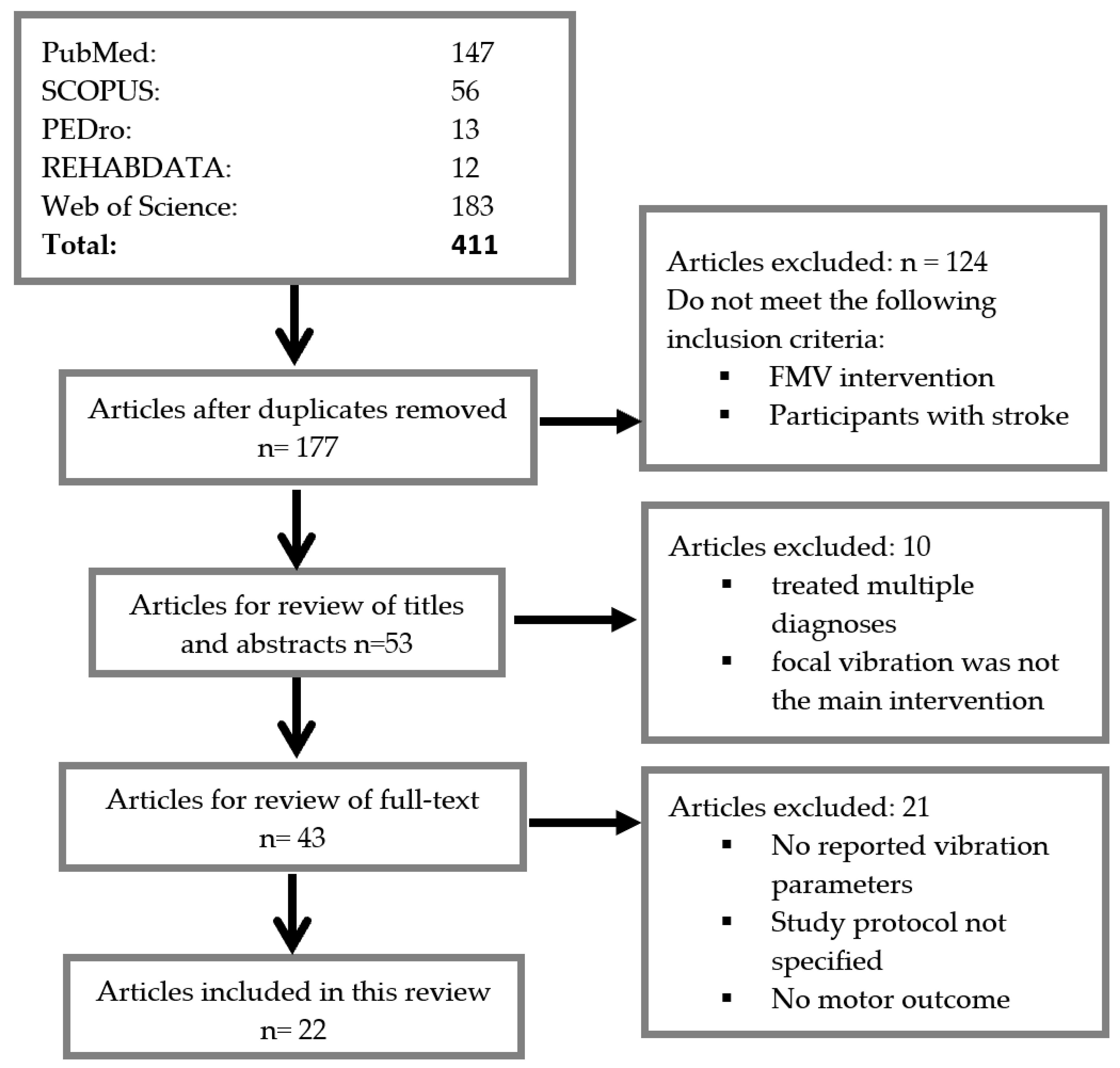
| Article and No. | Design and Participant Characteristics | Outcome Measure | Results |
|---|---|---|---|
| P1. Kawahira et al. 2004 | Design: Single group pre-post Subjects (n): 13 Age: 58.2 ± 9.7 Stroke type: Chronic Hemiplegia, R/L (n): 9/4 Intervention: FMV | Gait speed | Gait speed improved; Time to walk 10 m: Pre:13.7 ± 4.0 s Post: 12.8 ± 3.9 s |
| P2. Noma et al. 2009 | Design: Single group pre-post Subjects (n): 14 Age: 57.3 ± 19.1 Stroke type: Acute Hemiplegia, R/L (n): 8/6 Intervention: FMV | MAS (Modified Ashworth Scale) EMG (Electromyograph) | MAS pre, immediately after FMV, and 30 min post: Biceps brachii: 2.1 ± 1.0; 0.2 ± 0.4; 1 ± 1 Wrist flexor: 2.5 ± 0.8; 0.3 ± 0.6; 1.4 ± 0.9 Finger flexors: 2.5 ± 0.6; 0.2 ± 0.4; 1.1 ± 1 F-wave amplitude pre, immediately after FMV, and every 5 min post: 593 ± 255, 417 ± 282, 360 ± 234, 366 ± 260, 368 ± 249, 351 ± 238, 366 ± 205, 367 ± 202 mV F/M ratios pre, immediately after FMV, and every 5 min post: 4.9 ± 1.8, 3.6 ± 2.5, 3.1 ± 2.0, 3.1 ± 2.2, 3.1 ± 2.1, 3.0 ± 2.2, 3.1 ± 1.8, 3.1 ± 1.7% |
| P3. Paolini et al. 2010 | Design: RCT (Randomized Control Trial) Subjects (n): 44; EG (Experimental Group) 22, CG (Control Group) 22 Age: EG 59.5 ± 13.3 CG 62.5 ± 9.5 Stroke type: Chronic Hemiplegia, R/L (n): 23/21 Intervention: EG- FMV and PT, CG- PT (Physical Therapy) | Gait EMG (Electromyograph) KE (Kinematic Evaluation) | EG: Improvement in Gait Toe-off on paretic side (%): 62.6 ± 5.8, 59.6 ± 5.5 Stride length on normal side: 0.71 ± 0.20, 0.82 ± 0.18 Stride length on paretic side: 0.70 ± 0.19, 0.79 ± 0.17 Swing velocity normal side: 1.32 ± 0.34, 1.53 ± 0.39 Gait speed: 0.44 ± 0.13, 0.53 ± 0.13 |
| P4. Liepert et al. 2010 | Design: Single group pre-post Subjects (n): 10 Age: 57 ± 13 Stroke type: Chronic Hemiplegia, R/L (n): 8/2 Intervention: FMV | BBT (Box and Block Test) CSP (Cortical Silent Period) | BBT 20% less time to complete CSP Significant prolongation in the affected and the healthy flexor carpi radialis muscle. The duration of the CSP was not different between affected and non-affected muscles. |
| P5. Marconi et al. 2011 | Design: RCT Subjects (n): 30; EG15, CG 15 Age: EG 63.6 ± 7.6, CG 66.3 ± 11 Stroke type: Chronic Hemiplegia, R/L (n): 17/13 Intervention: EG- FMV and PT, CG- PT | MMAV (Motor Mao Area and Volume) SICI (Short Interval Intracortical Inhibition) SICF (Short Interval Intracortical Facilitation) | EG: Significant reduction in FCR vol. map and increase in EDC. SICI increased in FCR and reduced in EDC. Changes persisted up to 2 weeks after vibration. CG: No significant changes |
| P6. Conrad et al. 2011 | Design: Single group pre-post Subjects (n): 10 Age: 54 ± 9 Stroke type: Chronic Hemiplegia, R/L (n): 4/6 Intervention: FMV | EPS (End Point Stability) Muscle activity GP (Grip Pressure) | Improved EPS The mean absolute distance between hand position and target location at the end of a trial was 3.6 ± 1.2 cm. Stroke survivors had more success at making medial/lateral than proximal/distal. Muscle activity Decreased stability error: Se, Pre 0.133 ± 0.048, Se, Post 0.077 ± 0.025 GP: 39.1 ± 13.3, 33.5 ± 11.3, 32.6 ± 10.4 |
| P7. Noma et al. 2012 | Design: RCT Subjects (n): 36; RG – 12, StG – 12, EG – 12 Age: RG 61 (27–83), StG 61.5 (41–83), EG 57.5 (38–83) Stroke type: Acute Hemiplegia, R/L (n): 16/20 Intervention: RG (Rest Group) – no FMV, StG (Stretch Group))– FMV, EG – sham FMV | MAS EMG (F-wave) | RG: No significant changes observed StG: Decrease in F-wave amp. and F/M ratio immediately after vibration, but not 30 min later. EG: Significant improvements in F-wave & MAS scores immediately after vibration which also remained 30 min later. |
| P8. Caliandro et al. 2012 | Design: RCT Subjects (n): 49; EG 28, CG 21 Age: EG 57.42 ± 12.79, CG 61.85 ± 15.74 Stroke type: Chronic Hemiplegia, R/L (n): 23/26 Intervention: EG- PT and FMV, CG- PT and sham | WMFT FAS (Wolf Motor Function Test Functional Ability Scale) MAS (Modified Ashworth Scale) VAS (Visual Analog Scale) | EG: number of patients with more than 0.37 points increase in WMFT FAS post-intervention increased CG: No improvement in WMFT FAS No significant changes in MAS & VAS in CG or EG after 1 month of the intervention |
| P9. Lee et al. 2013 | Design: RCT Subjects (n): 31; EG 16, CG 15 Age: SG 53.31 ± 8.37, CG 55.73 ± 8.27 Stroke type: Chronic Hemiplegia, R/L (n): 16/15 Intervention: EG- PT, FES +FMV, CG- PT, FES+sham | PS (Postural Sway) Gait | PS: Greater improvements in distance with eyes-open (−11.91 vs. 0.80) and eyes-closed (−20.67 vs. −0.34) and velocity with eyes-open (−0.40 vs. 0.03) and eyes-closed (−0.69 vs. −0.01) in EG than CG. Gait: Greater improvement in gait speed (15.06 vs. 2.85), cadence (8.46 vs. 1.55), step length (7.90 vs. 3.64), and single limb support time (0.12 vs. 0.01) in EG than CG. |
| P10. Tavernese et al. 2013 | Design: RCT Subjects (n): 44; EG 24, CG 20 Age: EG 58.9 ± 14.7, CG 58.3 ± 12.4 Stroke type: Chronic Hemiplegia, R/L (n): 14/30 Intervention: EG- PT and FMV, CG- PT | Br-stage mRS (modified Rankin Scale) KE (Kinematic Evaluation) FMS (Fugl-Meyer Scale) MAS (Modified Ashworth Scale) | Normalized jerk significantly decreased in the EG, but not in the CG. Linear velocity significantly increased in the EG, but not in the CG. Angular velocity at shoulder significantly improved in EG, but not in CG. The movement duration significantly decreased in EG, but not in CG. The distance to target significantly decreased in the EG, but not in CG. |
| P11. Casale et al. 2014 | Design: RCT Subjects (n): 30; EG 15, CG 15 Age: EG 64.7 ± 5.4, CG 65.1 ± 5.8 Stroke type: NR Hemiplegia, R/L (n): 26/4 Intervention: EG- PT and FMV, CG- PT and sham | MAS Robot sided motor task changes | EG: MAS significantly improved at T1 and T2 with respect to T0 CG: the improvement reached statistical significance only at T2 Similar results were observed for time to complete the tasks. |
| P12. Paolini et al. 2014 | Design: RCT Subjects (n): 22; EG 12, CG 10 Age: EG 59.5 ± 13.3, CG 62.5 ± 9.5 Stroke type: Chronic Hemiplegia, R/L (n): 8/14 Intervention: EG- Exercise and FMV, CG- Exercise | MOT (Muscle Onset Time) CCI (Co-Contraction Index) MR (Modulation Ratio) %MVC (%Maximal Voluntary Contraction) | Significant differences between pre- and post- in the EG as regards the PD and ECR muscle onset times. No differences in CG. PD muscle onset time significantly closer to zero in the EG than in the CG. Patients in the EG had significantly lower CCI for the pairs BB/TB, PD/BB, and AD/BB. No differences in the CCI in the CG, except for Anterior and Posterior Deltoid. Post-CCI significantly lower in the EG for PD/BB and Anterior Deltoid/Biceps Brachii. Significantly better modulated AD and BB in EG. No differences in the CG. Post EG modulated the AD and BB significantly better than CG. BB %MCV value significantly lower in EG. |
| P13. Constantino et al. 2014 | Design: Single group pre-post Subjects (n): 16 Age: 61.6 ± 15.5 Stroke type: Chronic Hemiplegia, R/L (n): 15/1 Intervention: FMV | GS (Grip Strength) MAS (Modified Ashworth Scale) Quick-DASH (The Disabilities of the Arm, Shoulder and Hand Score) FIM (Functional Independence Measure) FMS (Fugl-Meyer Scale) VNRS (Verbal Numerical Rating Scale) JTHFT (Jebsen-Taylor Hand Function Test) | GS in the paretic hand improved SP2 14.75 ± 8.39, 18.31 ± 9.38; SP3 16.50 ± 9.86, 19.50 ± 11.00 MAS: shoulder (1.44 ± 1.21, 1.00 ± 0.97), elbow (1.88 ± 1.15, 1.38 ± 1.09), wrist (1.63 ± 1.31, 1.00 ± 1.03) QuickDASH: 39.90 ± 16.01, 26.98 ± 17.13 FIM: 80.50 ± 1533, 82.75 ± 14.69 FMS: 85.00 ± 18.50, 96.75 ± 16.93 VNRS: 2.88 ± 3.01, 1.31 ± 1.30 JTHFT: 190.60 ± 125.63, 159.67 ± 117.16 |
| P14. Costantino et al. 2016 | Design: RCT Subjects (n): 32; EG 17, CG 15 Age: EG 62.59 ± 15.39, CG 60.47 ± 16.0 Stroke type: Chronic Hemiplegia, R/L (n): 9/23 Intervention: EG- FMV, CG- sham FMV | GS (Grip Strength) MAS (Modified Ashworth Scale) Quick-DASH (The Disabilities of the Arm, Shoulder and Hand Score) FIM (Functional Independence Measure) FMS (Fugl-Meyer Scale) VNRS (Verbal Numerical Rating Scale) JTHFT (Jebsen-Taylor Hand Function Test) | GS in the paretic hand improved in EG (SP 2: 13.88 ± 8.88, 17.24 ± 10.11; SP3: 15.71 ± 10.09, 18.53 ± 11.37); in CG, a slight difference (SP2 unchanged at 17.33 ± 11.79; SP3 18.07 ± 11.25, 18.00 ± 11.26) MAS: shoulder (EG 1.59 ± 1.33, 1.12 ± 1.05; CG unchanged at 1.73 ± 1.28), elbow (EG: 2.00 ± 1.22, 1.47 ± 1.12; CG: 1.93 ± 1.22, 1.87 ± 1.19), wrist (EG: 1.76 ± 1.39, 1.18 ± 1.24; CG: 1.67 ± 1.35, 1.60 ± 1.30). QuickDASH: EG 41.17 ± 16.35, 29.01 ± 18.56, CG 40.55 ± 25.49, 39.74 ± 24.69 FIM: EG 79.24 ± 15.83, 81.35 ± 15.35, CG 83.27 ± 10.96, 83.53 ± 11.06 FMS: EG 82.82 ± 20.04, 94.24 ± 19.40 CG 83.33 ± 17.81, 84.27 ± 17.25 VNRS: EG 2.88 ± 2.91, 1.24 ± 1.30 CG 2.13 ± 2.72, 1.80 ± 2.60 JTHFT: EG 168.18 ± 133.48, 140.88 ± 121.74 CG 223.20 ± 163.89, 206.07 ± 153.53 |
| P15. Go Eun-Ji et al. 2016 | Design: SSD Subjects (n): 3 Age: 58.3 Stroke type: Chronic Hemiplegia, R/L (n): 2/1 Intervention: FMV | BBT (Box and Block Test) 10-s Test FMS | Significant improvement in BBT and 10-s test. BBT: Participant 1 non-affected: 62.0 to 67.7; affected: 39.0 to 47.3 Participant 2 non-affected: 55.8 to 64.4; affected: 26.3 to 34.1 Participant 3 non-affected 33.3 to 40.0; affected 18.3 to 28.6 10-s test: Participant 1 FIMT 3.0 to 4.2, HPST 11.3 to 14.3, FTT 25.0 to 32.2 Participant 2 FIMT 2.0 to 3.4, HPST 7.0 to 10.6, FTT 26.3 to 34.1 Participant 3 FIMT 2.0 to 2.2, HPST 9.3 to 10.4, FTT 20.3 to 27.5 |
| P16. Bonan et al. 2017 | Design: Two group pre-post Subjects (n): 80; EG 40, CG 40 Age: EG 54.7 ± 10.5, CG 54.7 ± 10.5 Stroke type: Acute Hemiplegia, R/L (n): 21/19 Intervention: EG- FMV, CG- FMV | %BW Shift (% Body Weight Shift) | The evaluation was repeated 4-to-6 weeks (session 2) after the first test (session 1). Session 1: %shift1 for left HP patients (1.5% (5.3)) significantly lower than healthy subjects (4.8% (4.1)) and the right HP patients (4.9% (3.6)). Session 2: No significant difference between the 3 groups for %shift2. |
| P17. Calabro et al. 2017 | Design: RCT Subjects (n): 20; EG 10, CG 10 Age: EG 66 ± 5, CG 67 ± 4 Stroke type: Chronic Hemiplegia, R/L (n): 20/0 Intervention: EG- FMV+Robot, CG- sham FMV+Robot | MAS (Modified Ashworth Scale) SICI (Short Interval Intracortical Inhibition) HMR (Hmax/Mmax Ratio) FMS (Fugl-Meyer Scale) FIM (Functional Independence Measure) HRS-D (Hamilton Rating Scale for Depression) MEP (Motor-Evoked Potential) ICF (Intracortical Fascilitation) | EG: greater reduction in the MAS and HMR and a more evident increase of SICI was observed up to 4 weeks after the end of the treatment, compared with CG. A significant correlation was found between the degree of MAS reduction and SICI increase in the agonist spastic muscles. |
| P18. Celletti et al. 2017 | Design: RCT Subjects (n): 18; G1-3 6 each Age: G1 43 (38–63), G2 43 (30–57), G3 62.5 (46–69) Stroke type: Chronic Hemiplegia, R/L (n): 9/9 Intervention: G1- FMV+RMP, G2- FMV+CP, G3- CP | WMFT (Wolf Motor Function Test) MAS (Modified Ashworth Scale) VAS (Visual Analog Scale) MI (Motricity Index) | Group 1: Increased WMFT (20, 48) and MI (39.5, 68.5), reduced VAS (5, 1.75) and MAS (2, 1.1). Group 2: Increased WMFT (24, 36) and MI (37,43), reduced VAS (5.75, 4) and MAS (2.6, 2.2). Group 3: Only reduced MAS. |
| P19. Jung Sang-Mi et al. 2017 | Design: Single group pre-post Subjects (n): 10 Age: 62.6 ± 8.6 Stroke type: Chronic Hemiplegia, R/L (n): 5/5 Intervention: FMV | GS (Grip Strength) BBT (Box and Block Test) WMT (Weinstein Monofilament Test) | Significant improvement in GS and BBT. All improvements retained after 2 weeks. GS: 11.4 ± 5.4, 13.4 ± 6.9, 12.6 ± 6.3 BBT: 13.3 ± 8.2, 17.1 ± 8.5, 15.1 ± 8.3 |
| P20. Choi et al. 2017 | Design: RCT Subjects (n): 10; EG 5, CG 5 Age: EG 62 ± 9, CG 59 ± 10.1 Stroke type: Chronic Hemiplegia, R/L (n): 5/5 Intervention: EG FMV, CG PT | BBT (Box and Block Test) GS (Grip Strength) WMT (Weinstein Monofilament Test) | BBT scores: EG: 18.6 ± 9.3, 22.2 ± 9.2; CG 20.4 ± 9.3, 21.8 ± 9.0 Significant changes in BBT in EG and CG, no significant differences between EG and CG. GS and WMT scores did not improve significantly. |
| P21. Toscano et al. 2019 | Design: RCT Subjects (n): 22; EG 10, CG 12 Age: EG 64.7 ± 17.2, CG 69.5 ± 7.3 Stroke type: Acute Hemiplegia, R/L (n): 10/12 Intervention: EG FMV, CG sham FMV | NIHSS (National Institutes of Health Stroke Scale) FMS (Fugl-Meyer Scale) MI (Motricity Index) MAS (Modified Ashworth Scale) | EG patients showed a better clinical improvement in terms of stroke severity assessed by NIHSS, FMS, and MI than did CG patients. |
| P22. Annino et al. 2019 | Design: RCT Subjects (n): 37; EG 19, CG 18 Age: EG 67.8 ± 8.3, CG 69.4 ± 10.4 Stroke type: Chronic Hemiplegia, R/L (n): 19/18 Intervention: EG- FMV and PT, CG- PT | BI (Barthel Index) Gonio-metry MAS (Modified Ashworth Scale) MMT ( Manual Muscle Testing) | EG: BI scores: 71.9 ± 22.9, 76.8 ± 21.7 Goniometry: 115 ± 9.5, 116.2 ± 9.5 MAS: 1.7 ± 0.7, 1.1 ± 0.8 MMT flexor/extensor: 4 ± 0.8/4 ± 0.6, 4.2 ± 0.7/4.2 ± 0.7 CG: BI scores: 78.6 ± 20.3, 81.0 ± 19.9 Goniometry: 116.9 ± 9.7, 118.6 ± 9.1 MAS: Not statistically improved MMT flexor/extensor: 3.7 ± 0.9/3.7 ± 0.8, 4 ± 0.8/3.8 ± 0.7 |
| Article | Device | FR (Hz) | A (mm) | Vibration Protocol |
|---|---|---|---|---|
| P1. Kawahira et al. 2004 | Custom Device | 83 | NR | Anterior tibial and gluteus medius 1 single session for 14 s |
| P2. Noma et al. 2009 | Thrive MD-01 | 91–108 | 1 | Palm flexor tendon and biceps brachii 1 single session for 5 min |
| P3. Paolini et al. 2010 | Horus | 120 | 0.01 | Peroneus longus and tibialis anterior 4 weeks (30 min/day, 3 day/week) |
| P4. Liepert et al. 2010 | Custom Device | 60 | NR | Forearm extensor muscles 1 single session for 5 min |
| P5. Marconi et al. 2011 | CROSYSTEM, NEMOCO | 100 | 0.2–0.5 | Flexor carpi radialis, biceps brachii 3 consecutive days (30 min/day) |
| P6. Conrad et al. 2011 | Custom Device | 70 | NR | Forearm flexor tendons 1 single session with 40 trials (5 s/trial) |
| P7. Noma et al. 2012 | Thrive MD-01 | 91–108 | 1 | Palmar flexor tendon, biceps brachii 1 single session for 5 min |
| P8. Caliandro et al. 2012 | CROSYSTEM, NEMOCO | 100 | 0.2–0.5 | Pectoralis minor, biceps brachii and flexor carpi 3 consecutive days (30 min/day) |
| P9. Lee et al. 2013 | NR | 90 | 0.015 | Achilles tendon, tibialis anterior 6 weeks (30 min/day, 3 day/week) |
| P10. Tavernese et al. 2013 | Horus | 120 | 0.01 | Biceps brachii and flexor carpi ulnaris 2 weeks (30 min/day, 5 day/week) |
| P11. Casale et al. 2014 | VIBRA | 100 | 2 | Triceps brachii 2 weeks (30 min/day, 5 day/week) |
| P12. Paolini et al. 2014 | Horus | 120 | 0.01 | Biceps brachii, flexor carpi ulnaris 2 weeks (30 min/day, 5 day/week) |
| P13. Constantino et al. 2014 | ViSS Device | 300 | 2 | Extensor carpi radialis longus, carpi radialis brevis, triceps brachii 4 weeks (30 min/day, 3 day/week) |
| P14. Costantino et al. 2016 | ViSS Device | 300 | 2 | Carpi radialis longus, carpi radialis brevis, triceps brachii 4 weeks (30 min/day, 3 day/week) |
| P15. Go Eun-Ji et al. 2016 | Toothbrush | 127 | NR | Hand intrinsic/extrinsic muscles 6 weeks (30 min/day, 2 day/week) |
| P16. Bonan et al. 2017 | TechnoConcept VB115 | 90 | 0.4 | Non-paretic gluteus medius 1 session 35 s FMV after 15 s rest, repeatedly |
| P17. Calabro et al. 2017 | VIBRA | 80 | 0.3 ± 0.1 | Triceps brachii, supraspinatus, deltoid 8 weeks (1 h/day, 5 day/week) |
| P18. Celletti 2017 | NR | 100 | 0.2–0.5 | Pectoralis minor, biceps brachii, and flexor carpi 6 weeks (30 min/day, 3 consecutive day/week) |
| P19. Jung Sang-Mi 2017 | THRIVE MD-01 | 91–108 | 1 | Biceps brachii and flexor carpi radialis 2 weeks (30 min/day, 3 day/week) |
| P20. Choi et al. 2017 | THRIVE MD-01 | 91 | 1 | Biceps brachii and flexor carpi radialis 4 weeks (30 min/day, 3 day/week) |
| P21. Toscano et al. 2019 | CROSYSTEM, NEMOCO | 100 | 0.2–0.5 | Rectus femoris, biceps brachii, and flexor carpi radialis 3 consecutive days (30 min/day) |
| P22. Annino et al. 2019 | NR | 30 | 2 | Triceps brachii 8 weeks (30 min/day, 3 day/week) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chandrashekhar, R.; Rippetoe, J.; Ghazi, M. Focal Muscle Vibration for Stroke Rehabilitation: A Review of Vibration Parameters and Protocols. Appl. Sci. 2020, 10, 8270. https://doi.org/10.3390/app10228270
Wang H, Chandrashekhar R, Rippetoe J, Ghazi M. Focal Muscle Vibration for Stroke Rehabilitation: A Review of Vibration Parameters and Protocols. Applied Sciences. 2020; 10(22):8270. https://doi.org/10.3390/app10228270
Chicago/Turabian StyleWang, Hongwu, Raghuveer Chandrashekhar, Josiah Rippetoe, and Mustafa Ghazi. 2020. "Focal Muscle Vibration for Stroke Rehabilitation: A Review of Vibration Parameters and Protocols" Applied Sciences 10, no. 22: 8270. https://doi.org/10.3390/app10228270
APA StyleWang, H., Chandrashekhar, R., Rippetoe, J., & Ghazi, M. (2020). Focal Muscle Vibration for Stroke Rehabilitation: A Review of Vibration Parameters and Protocols. Applied Sciences, 10(22), 8270. https://doi.org/10.3390/app10228270






