Impact of Osteotomy in Surgically Assisted Rapid Maxillary Expansion Using Tooth-Borne Appliance on the Formation of Stresses and Displacement Patterns in the Facial Skeleton—A Study Using Finite Element Analysis (FEA)
Abstract
1. Introduction
2. Materials and Methods
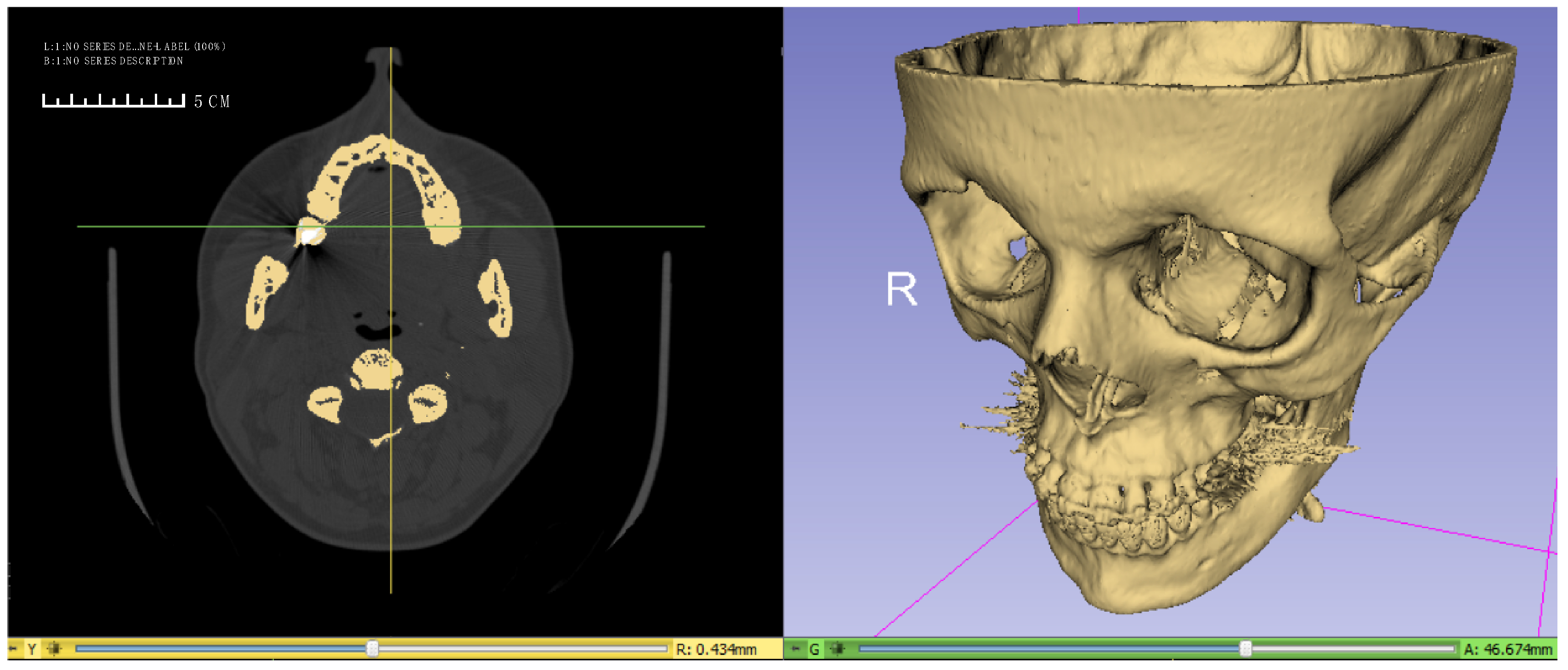
2.1. Construction of the Facial Skeleton Model for the Finite Element Analysis
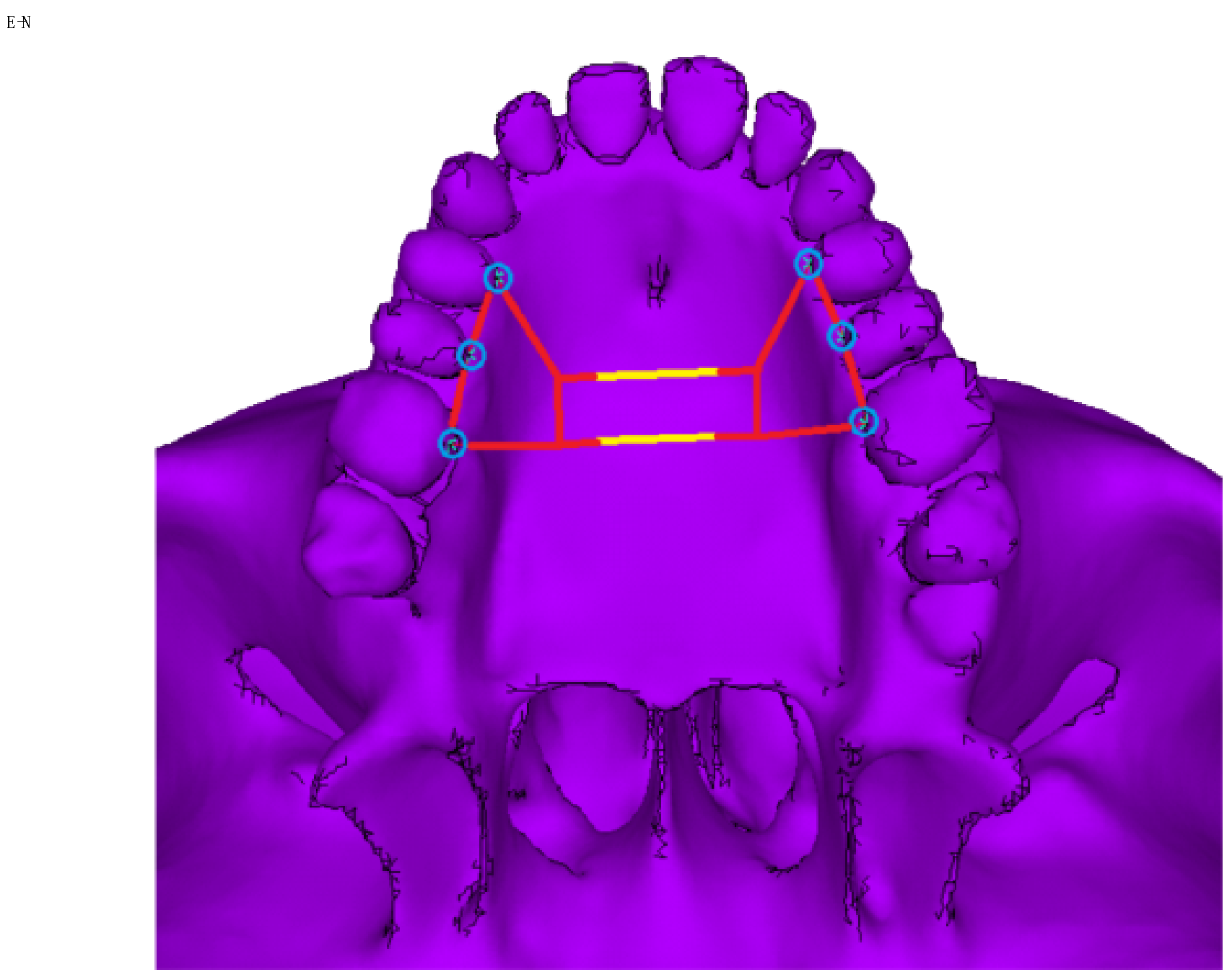
2.2. Construction of the Orthodontic Appliance
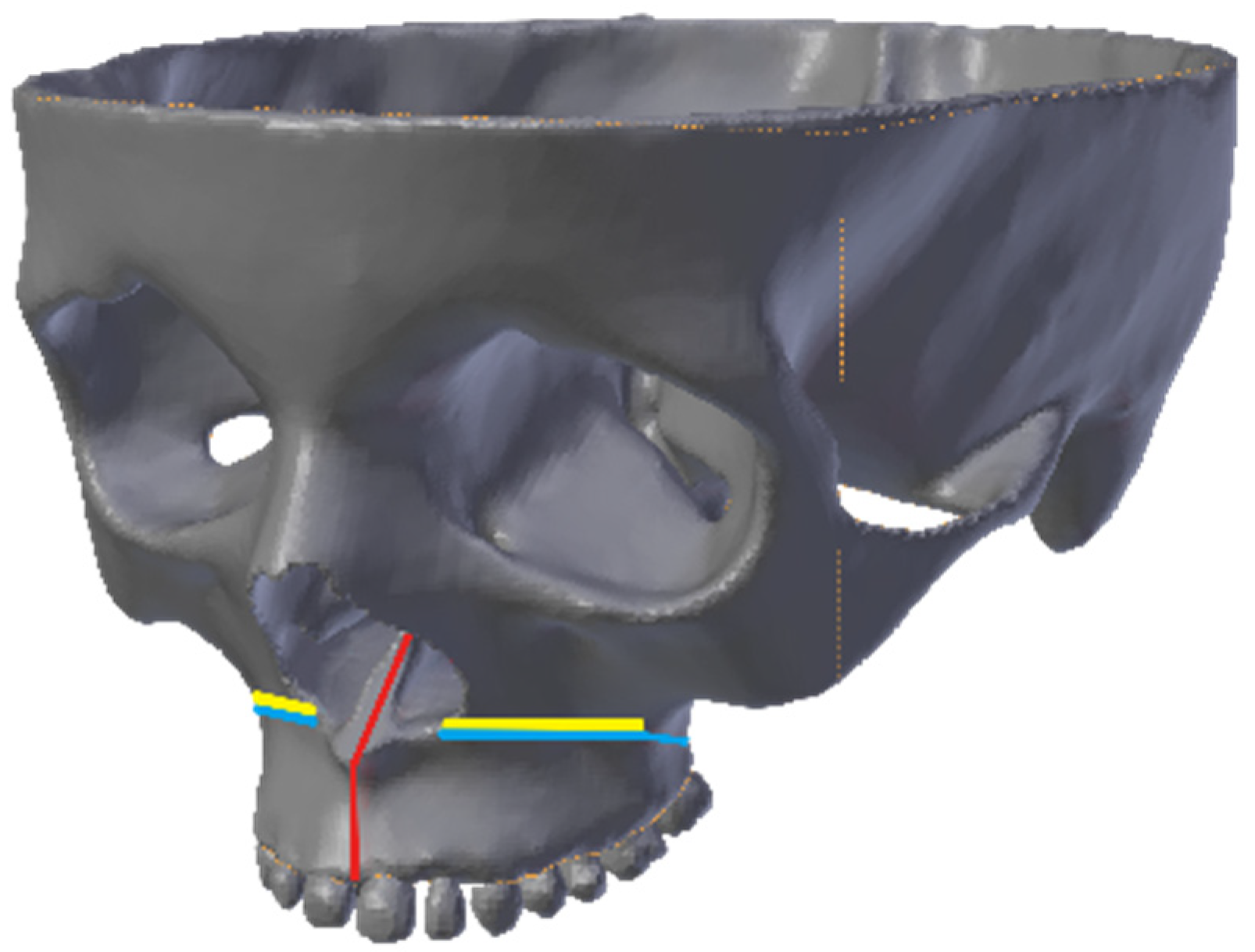
2.3. Types of Osteotomy on the Model of the Facial Skeleton
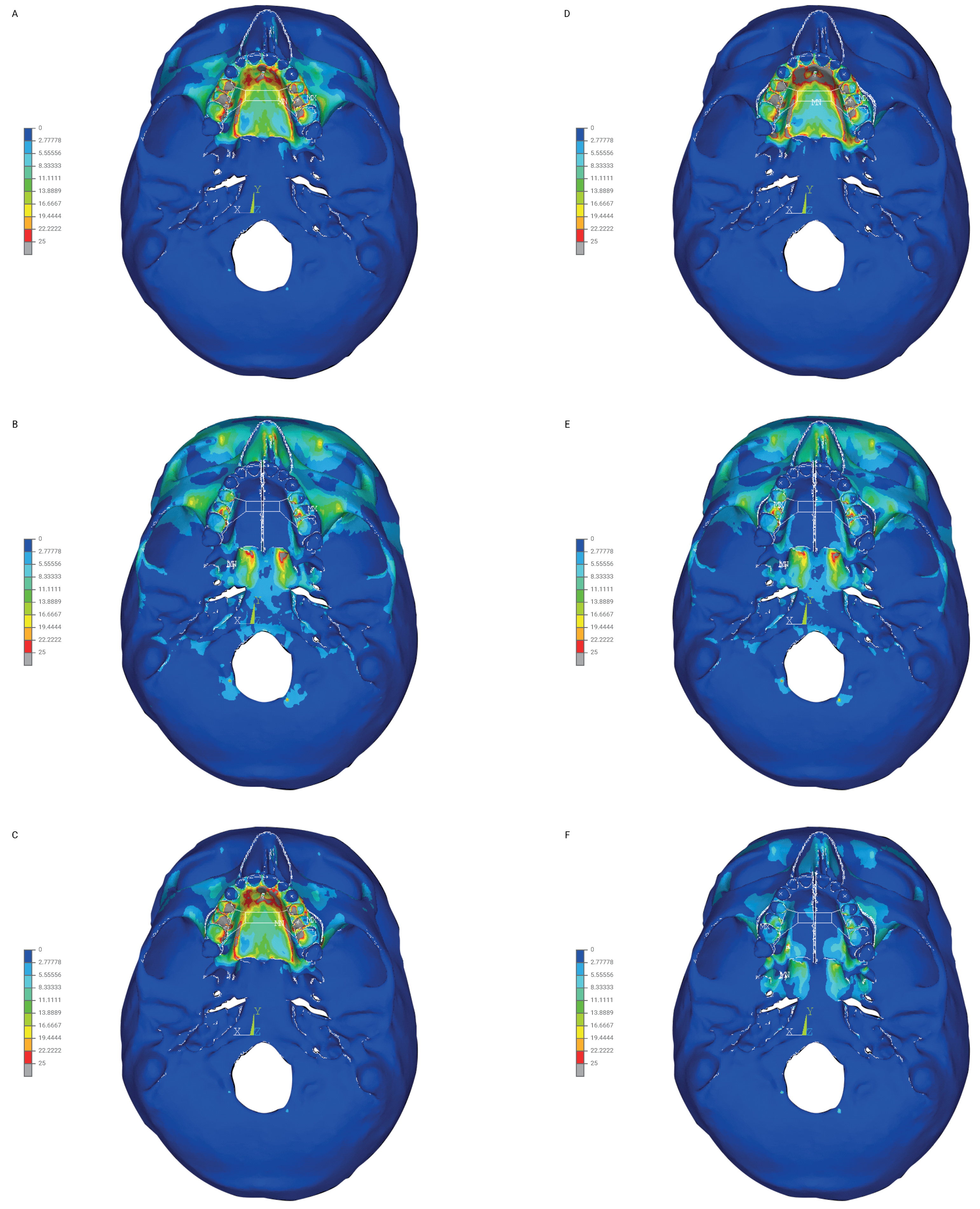
3. Results
3.1. Stress Reduced According to Huber (MPa)
3.2. Displacements of Selected Facial Skeleton Structures along the X, Y, Z Axes (mm)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verstraaten, J.; Kuijpers-Jagtman, A.M.; Mommaerts, M.Y.; Bergé, S.J.; Nada, R.M.; Schols, J.G. A systematic review of the effects of bone-borne surgical assisted rapid maxillary expansion. J. Cranio Maxillofac. Surg. 2010, 38, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.J.; White, R.P.; Proffit, W.R.; Turvey, T.A. Segmantal LeFort I osteotomy for management of transverse maxillary deficiency. J. Oral Maxillofac. Surg. 1997, 55, 728–731. [Google Scholar] [CrossRef]
- Erdinç, A.E.; Ugur, T.; Erbay, E. A comparison of different treatment techniques for posterior crossbite in the mixed dentition. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 287–300. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, Y.-C.; Park, J.-Y.; Hwang, W.-S. Miniscrew-assisted nonsurgical palatal expansion before orthognathic surgery for a patient with severe mandibular prognathism. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.D.; Shroff, B. Long-term Skeletal Changes with Rapid Maxillary Expansion: A Review of the Literature. Semin. Orthod. 2012, 18, 128–133. [Google Scholar] [CrossRef]
- Chamberland, S.; Proffit, W.R. Short-term and long-term stability of surgically assisted rapid palatal expansion revisited. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 815–822.e1. [Google Scholar] [CrossRef]
- Laptook, T. Conductive hearing loss and rapid maxillary expansion. Am. J. Orthod. 1981, 80, 325–331. [Google Scholar] [CrossRef]
- Haas, A.J. Palatal expansion: Just the beginning of dentofacial orthopedics. Am. J. Orthod. 1970, 57, 219–255. [Google Scholar] [CrossRef]
- Bishara, S.; Staley, R.N. Maxillary expansion: Clinical implications. Am. J. Orthod. Dentofac. Orthop. 1987, 91, 3–14. [Google Scholar] [CrossRef]
- Proffit, W.R.; Turvey, T.A.; Phillips, C. Orthognathic surgery: A hierarchy of stability. Int. J. Adult Orthod. Orthognath. Surg. 1996, 11, 191–204. [Google Scholar]
- Northway, W.; Meade, J.B. Surgically assisted rapid maxillary expansion: A comparison of technique, response, and stability. Angle Orthod. 1997, 67, 20. [Google Scholar]
- Koo, Y.-J.; Choi, S.-H.; Keum, B.-T.; Yu, H.-S.; Hwang, C.-J.; Melsen, B.; Lee, K.-J. Maxillomandibular arch width differences at estimated centers of resistance: Comparison between normal occlusion and skeletal Class III malocclusion. Korean, J. Orthod. 2017, 47, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Cavagnetto, D.; Fama, A.; Matarese, M.; Lucarelli, D.; Assandri, F. Short term effects of rapid maxillary expansion on breathing function assessed with spirometry: A case-control study. Saudi Dent. J. 2020. [Google Scholar] [CrossRef]
- Lanteri, V.; Farronato, M.; Ugolini, A.; Cossellu, G.; Gaffuri, F.; Parisi, F.M.R.; Cavagnetto, D.; Abate, A.; Maspero, C. Volumetric Changes in the Upper Airways after Rapid and Slow Maxillary Expansion in Growing Patients: A Case-Control Study. Materials 2020, 13, 2239. [Google Scholar] [CrossRef] [PubMed]
- Maspero, C.; Cavagnetto, D.; Abate, A.; Cressoni, P.; Farronato, M. Effects on the Facial Growth of Rapid Palatal Expansion in Growing Patients Affected by Juvenile Idiopathic Arthritis with Monolateral Involvement of the Temporomandibular Joints: A Case-Control Study on Posteroanterior and Lateral Cephalograms. J. Clin. Med. 2020, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.; Baumrind, S. Transverse Development of the Human Jaws Between the Ages of 8.5 and 15.5 Years, Studied Longitudinally With Use of Implants. J. Dent. Res. 1990, 69, 1298–1306. [Google Scholar] [CrossRef]
- Koudstaal, M.J.; Poort, L.; Van Der Wal, K.; Wolvius, E.; Prahl-Andersen, B.; Schulten, A. Surgically assisted rapid maxillary expansion (SARME): A review of the literature. Int. J. Oral Maxillofac. Surg. 2005, 34, 709–714. [Google Scholar] [CrossRef]
- M’Quillen, J.H. Review of Dental Literature and Art. Dental Cosmos 1860, 1, 535–540. [Google Scholar]
- Betts, N.J.; Vanarsdall, R.L.; Barber, H.D.; Higgins-Barber, K.; Fonseca, R.J. Diagnosis and treatment of transverse maxillary deficiency. Int. J. Adult Orthod. Orthognath. Surg. 1995, 10, 75–96. [Google Scholar]
- Chrcanovic, B.R.; Custódio, A.L.N. Orthodontic or surgically assisted rapid maxillary expansion. Oral Maxillofac. Surg. 2009, 13, 123–137. [Google Scholar] [CrossRef]
- Isaacson, R.J.; Ingram, A.H. Forces produced by rapid maxillary expansion, II. Forces present during treatment. Angle Orthod. 1964, 34, 261–270. [Google Scholar] [CrossRef]
- Pekhale, N.; Maheshwari, A.; Kumar, M.; Kerudi, V.V.; Patil, H.; Patil, B. Evaluation of stress patterns on maxillary posterior segment when intruded with mini implant anchorage: A three-dimensional finite element study. APOS Trends Orthod. 2016, 6, 18–23. [Google Scholar] [CrossRef]
- Marchetti, C.; Pironi, M.; Bianchi, A.; Musci, A. Surgically assisted rapid palatal expansion vs. segmental Le Fort I osteotomy: Transverse stability over a 2-year period. J. Cranio Maxillofac. Surg. 2009, 37, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, R.; Gander, E.; Hoffmann, J.; Engel, M. Surgical management of cross-bites in orthognathic surgery: Surgically assisted rapid maxillary expansion (SARME) versus two-piece maxilla. J. Cranio Maxillofac. Surg. 2015, 43, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Sicher, H. Oral Anatomy; CV Mosby: St. Louis, MO, USA, 1963. [Google Scholar]
- Pollock, R.A. Craniomaxillofacial Buttresses. Anatomy and Operative Repair; Thieme Medical Publishers, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Işeri, H.; Tekkaya, A.E.; Bilgiç, Ö.S. Biomechanical effects of rapid maxillary expansion on the craniofacial skeleton, studied by the finite element method. Eur. J. Orthod. 1998, 20, 347–356. [Google Scholar] [CrossRef]
- Jafari, A.; Shetty, K.; Kumar, M. Study on stress distribution and displacement of various craniofacial structures following application of transverse orthopedic forces—A three-dimensional FEM study. Angle Orthod. 2003, 73, 12–20. [Google Scholar] [CrossRef]
- Holberg, M.D.M.D.C. Effects of Rapid Maxillary Expansion on the Cranial Base—An FEM-Analysis. J. Orofac. Orthop. Fortschr. Kieferorthop. 2005, 66, 54–66. [Google Scholar] [CrossRef]
- Silverstein, K.; Quinn, P.D. Surgically-assisted rapid palatal expansion for management of transverse maxillary deficiency. J. Oral Maxillofac. Surg. 1997, 55, 725–727. [Google Scholar] [CrossRef]
- Sangsari, A.H.; Sadr-Eshkevari, P.; Al-Dam, A.; Friedrich, R.E.; Freymiller, E.; Rashad, A. Surgically Assisted Rapid Palatomaxillary Expansion with or without Pterygomaxillary Disjunction: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2016, 74, 338–348. [Google Scholar] [CrossRef]
- Günbay, T.; Akay, M.C.; Günbay, S.; Aras, A.; Koyuncu, B.Ö.; Sezer, B. Transpalatal Distraction Using Bone-Borne Distractor: Clinical Observations and Dental and Skeletal Changes. J. Oral Maxillofac. Surg. 2008, 66, 2503–2514. [Google Scholar] [CrossRef]
- Lanigan, D.T.; Mintz, S.M. Complications of surgically assisted rapid palatal expansion: Review of the literature and report of a case. J. Oral Maxillofac. Surg. 2002, 60, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Handelman, C.S.; Wang, L.; BeGole, E.A.; Haas, A.J. Nonsurgical rapid maxillary expansion in adults: Report on 47 cases using the Haas expander. Angle Orthod. 2000, 70, 129–144. [Google Scholar] [PubMed]
- Pinto, P.X.; Mommaerts, M.Y.; Wreakes, G.; Jacobs, W.V. Immediate postexpansion changes following the use of the transpalatal distractor. J. Oral Maxillofac. Surg. 2001, 59, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, N.; Oktay, H. Effects of rapid maxillary expansion on nasal breathing and some noso-respiratory and breathing problems in growing children: A literature review. Int. J. Ped. Otorhinolaryng. 2008, 72, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Landes, C.A.; Laudemann, K.; Schübel, F.; Petruchin, O.; Mack, M.; Kopp, S.; Sader, R.A. Comparison of Tooth- and Bone-Borne Devices in Surgically Assisted Rapid Maxillary Expansion by Three-Dimensional Computed Tomography Monitoring. J. Craniofacial Surg. 2009, 20, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Laudemann, K.; Petruchin, O.; Mack, M.G.; Kopp, S.; Sader, R.; Landes, C.A. Evaluation of surgically assisted rapid maxillary expansion with or without pterygomaxillary disjunction based upon preoperative and post-expansion 3D computed tomography data. Oral Maxillofac. Surg. 2009, 13, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Manerikar, R.; Sinha, R. Surgical Management of Transverse Maxillary Deficiency in Adults. J. Maxillofac. Oral Surg. 2010, 9, 241–246. [Google Scholar] [CrossRef]
- Matteini, C.; Mommaerts, M.Y. Posterior transpalatal distraction with pterygoid disjunction: A short-term model study. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 498–502. [Google Scholar] [CrossRef]
- Holberg, C.; Steinhäuser, S.; Rudzki, I. Surgically assisted rapid maxillary expansion: Midfacial and cranial stress distribution. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 776–782. [Google Scholar] [CrossRef]
- Holberg, C.; Rudzki-Janson, I. Stresses at the cranial base induced by rapid maxillary expansion. Angle Orthod. 2006, 76, 543–550. [Google Scholar]
- Lagravère, M.O.; Major, P.; Flores-Mir, C. Dental and skeletal changes following surgically assisted rapid maxillary expansion. Int. J. Oral Maxillofac. Surg. 2006, 35, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.; Moura, L.B.; Trento, G.; Holzinger, D.; Gabrielli, M.A.C.; Pereira-Filho, V.A. Surgically assisted rapid maxillary expansion: A systematic review of complications. Int. J. Oral Maxillofac. Surg. 2020, 49, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Maspero, C.; Maspero, C.; Cavagnetto, D.; El Morsi, M.; Fama, A.; Farronato, M. Available Technologies, Applications and Benefits of Teleorthodontics. A Literature Review and Possible Applications during the COVID-19 Pandemic. J. Clin. Med. 2020, 9, 1891. [Google Scholar] [CrossRef] [PubMed]


| Variable | Young’s Modulus [MPa] | Poisson’s Ratio |
|---|---|---|
| Compact bone | 13,700 | 0.26 |
| Cancellous bone | 1370 | 0.3 |
| Enamel | 80,000 | 0.26 |
| Dentin | 20,000 | 0.15 |
| Stainless steel | 200,000 | 0.3 |
| Model 1—the model used for finite element analysis—without osteotomy | Model 2—the model used for finite element analysis—sagittal osteotomy |
| Model 3—the model used for finite element analysis—transversal osteotomy modo Le Fort I without PMJ separation | Model 4—the model used for finite element analysis—transversal osteotomy modo Le Fort I with PMJ separation |
| Model 5—the model used for finite element analysis—sagittal osteotomy with transversal osteotomy modo Le Fort I without PMJ separation | Model 6—the model used for finite element analysis—sagittal osteotomy with transversal osteotomy modo Le Fort I with PMJ separation |
| Anatomical Structures | Model I No Surgery | Model II | Model III | Model IV | Model V | Model VI |
|---|---|---|---|---|---|---|
| Nasofrontal suture | 2.2 | 5.5 | 1.1 | 1.1 | 4.4 | 2.2 |
| Zygomaticomaxillary suture | 3.3 | 5.5 | 4.4 | 1.1 | 4.4 | 2.2 |
| Arcus superciliaris—brow ridge | 2.2 | 8.8 | 2.2 | 2.2 | 7.7 | 3.3 |
| Zygomaticofrontal suture | 4.4 | 7.7 | 3.3 | 2.2 | 7.7 | 3.3 |
| Palatal suture anterior region | >25 | 1.1 | >25 | >25 | 1.1 | 1.1 |
| Palatal suture posterior region | 13.8 | 1.1 | 13.8 | 13.8 | 1.1 | 1.1 |
| Supraorbital margin | 2.2 | 4.4 | 2.2 | 1.1 | 4.4 | 2.2 |
| Infraorbital margin | 4.4 | 5.5 | 2.2 | 1.1 | 7.7 | 2.2 |
| Apertura piriformis—the lowest point | 5.5 | 1.1 | >10 | >10 | 1.1 | 1.1 |
| Anterior wall of maxillary sinus | >10 | 4.4 | 7.7 | 1.1 | 3.3 | 2.2 |
| Zygomaticoalveolar crest | >10 | >10 | 5.5 | 3.3 | 5.5 | 3.3 |
| Processus alveolaris of maxillae regio incisors and canine | 7.7 | 1.1 | 10 | >10 | 1.1 | 1.1 |
| Processus alveolaris of maxillae regio premolars | >10 | 8.8 | >10 | >10 | 7.7 | 3.3 |
| Processus alveolaris of maxillae regio molars | 5.5 | 1.1 | 5.5 | 7.7 | 3.3 | 3.3 |
| Crown/collum fifth maxilla tooth | >10 | 5.5 | >10 | >10 | 5.5 | 4.4 |
| Variable | Model I | Model II | Model III | Model IV | Model V | Model VI | |
|---|---|---|---|---|---|---|---|
| X | Mesial incisal angle of maxillary tooth 1 | +0.04 | +0.31 | +0.04 | +0.04 | +0.31 | +0.4 |
| Buccal cusp tip of maxillary tooth 5 | +0.04 | +0.22 | +0.04 | +0.13 | +0.31 | +0.31 | |
| Posterolateral surface of the maxilla | +0.04 | +0.22 | +0.04 | +0.04 | +0.22 | +0.22 | |
| Apertura piriformis—the lowest point | +0.04 | +0.22 | +0.04 | +0.04 | +0.22 | +0.22 | |
| Y | Mesial incisal angle of maxillary tooth 1 | −0.05 | +0.05 | −0.5 | −0.5 | +0.05 | +0.07 |
| Buccal cusp tip of maxillary tooth 5 | +0.01 | +0.03 | +0.01 | +0.03 | +0.03 | +0.01 | |
| Posterolateral surface of the maxilla | +0.01 | +0.03 | +0.03 | +0.05 | +0.03 | −0.01 | |
| Apertura piriformis—the lowest point | −0.03 | +0.03 | −0.03 | −0.03 | +0.03 | +0.05 | |
| Z | Mesial incisal angle of maxillary tooth 1 | −0.03 | +0.01 | −0.03 | −0.03 | −0.05 | −0.05 |
| Buccal cusp tip of maxillary tooth 5 | −0.03 | +0.07 | +0.01 | +0.03 | +0.07 | +0.05 | |
| Posterolateral surface of the maxilla | +0.03 | +0.07 | +0.03 | +0.07 | +0.07 | +0.07 | |
| Apertura piriformis—the lowest point | −0.03 | +0.01 | −0.03 | −0.03 | −0.05 | −0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawiślak, E.; Olejnik, A.; Frątczak, R.; Nowak, R. Impact of Osteotomy in Surgically Assisted Rapid Maxillary Expansion Using Tooth-Borne Appliance on the Formation of Stresses and Displacement Patterns in the Facial Skeleton—A Study Using Finite Element Analysis (FEA). Appl. Sci. 2020, 10, 8261. https://doi.org/10.3390/app10228261
Zawiślak E, Olejnik A, Frątczak R, Nowak R. Impact of Osteotomy in Surgically Assisted Rapid Maxillary Expansion Using Tooth-Borne Appliance on the Formation of Stresses and Displacement Patterns in the Facial Skeleton—A Study Using Finite Element Analysis (FEA). Applied Sciences. 2020; 10(22):8261. https://doi.org/10.3390/app10228261
Chicago/Turabian StyleZawiślak, Ewa, Anna Olejnik, Roman Frątczak, and Rafał Nowak. 2020. "Impact of Osteotomy in Surgically Assisted Rapid Maxillary Expansion Using Tooth-Borne Appliance on the Formation of Stresses and Displacement Patterns in the Facial Skeleton—A Study Using Finite Element Analysis (FEA)" Applied Sciences 10, no. 22: 8261. https://doi.org/10.3390/app10228261
APA StyleZawiślak, E., Olejnik, A., Frątczak, R., & Nowak, R. (2020). Impact of Osteotomy in Surgically Assisted Rapid Maxillary Expansion Using Tooth-Borne Appliance on the Formation of Stresses and Displacement Patterns in the Facial Skeleton—A Study Using Finite Element Analysis (FEA). Applied Sciences, 10(22), 8261. https://doi.org/10.3390/app10228261





