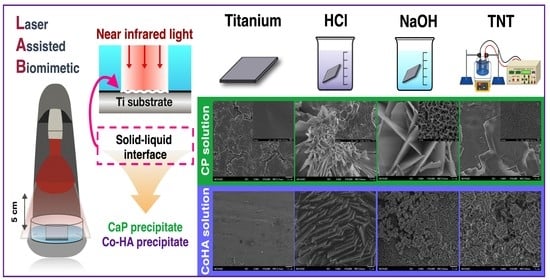
Effect of Bioactivity of Surface Topography and Coating Forming by Infrared Light-Induced on Titanium for Bone Repair
Abstract
1. Introduction
2. Experimental Procedures
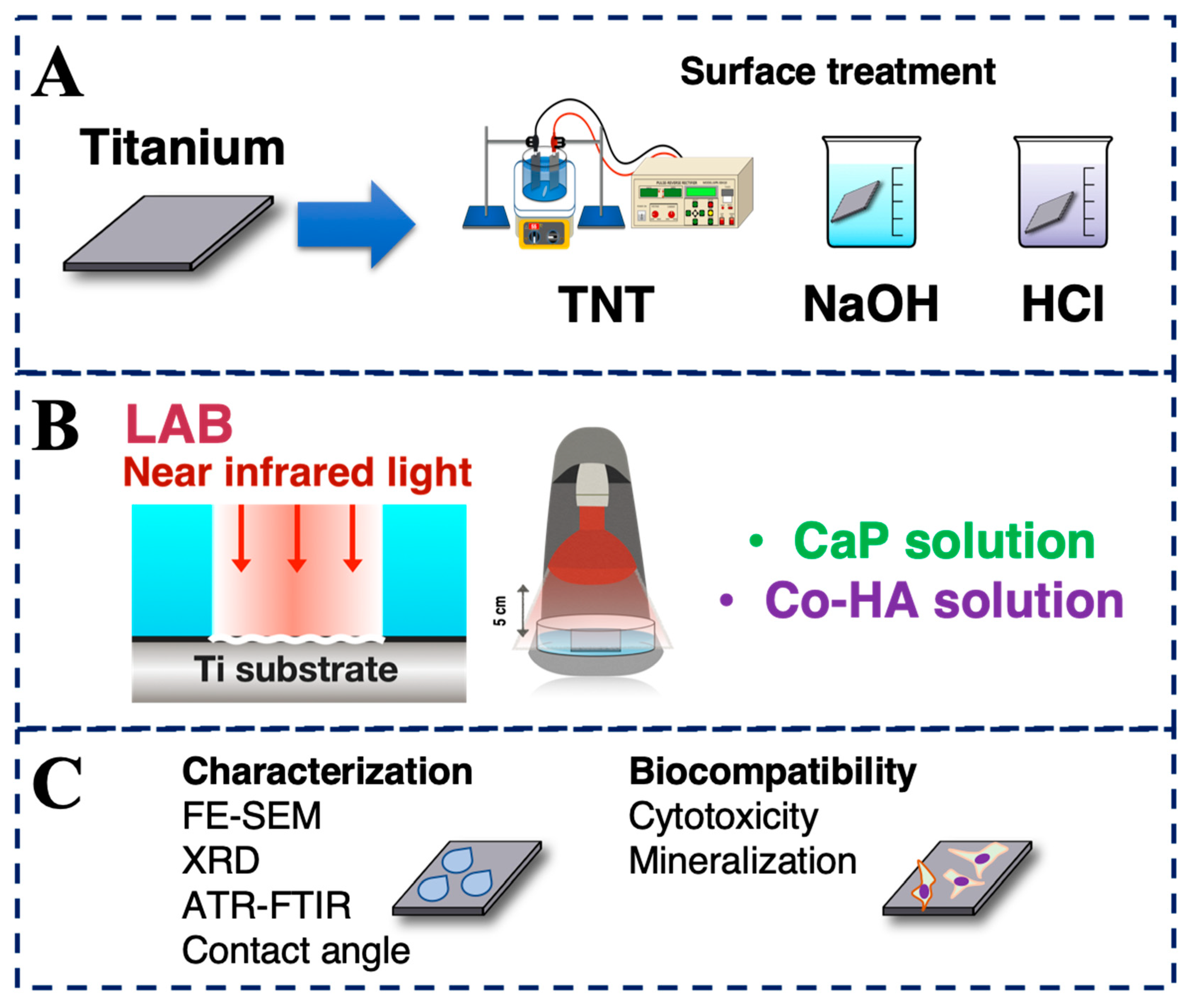
2.1. Surface Treatment of Titanium
2.2. LAB Preparation
2.3. Characterization
2.4. Hydrophilicity Test
2.5. Biocompatibility
2.6. Bioactivity
2.7. Statistical Analysis
3. Results and Discussion
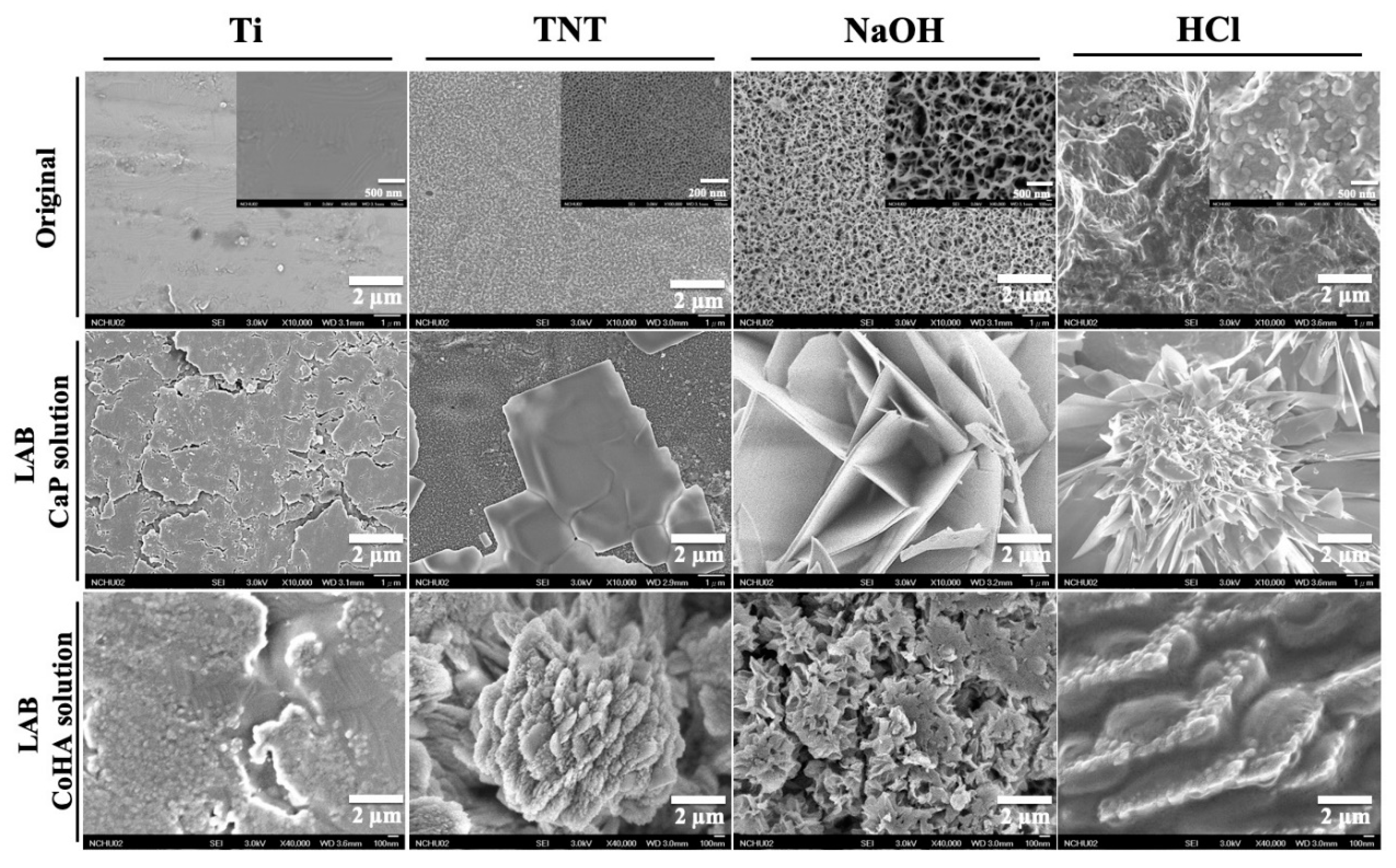
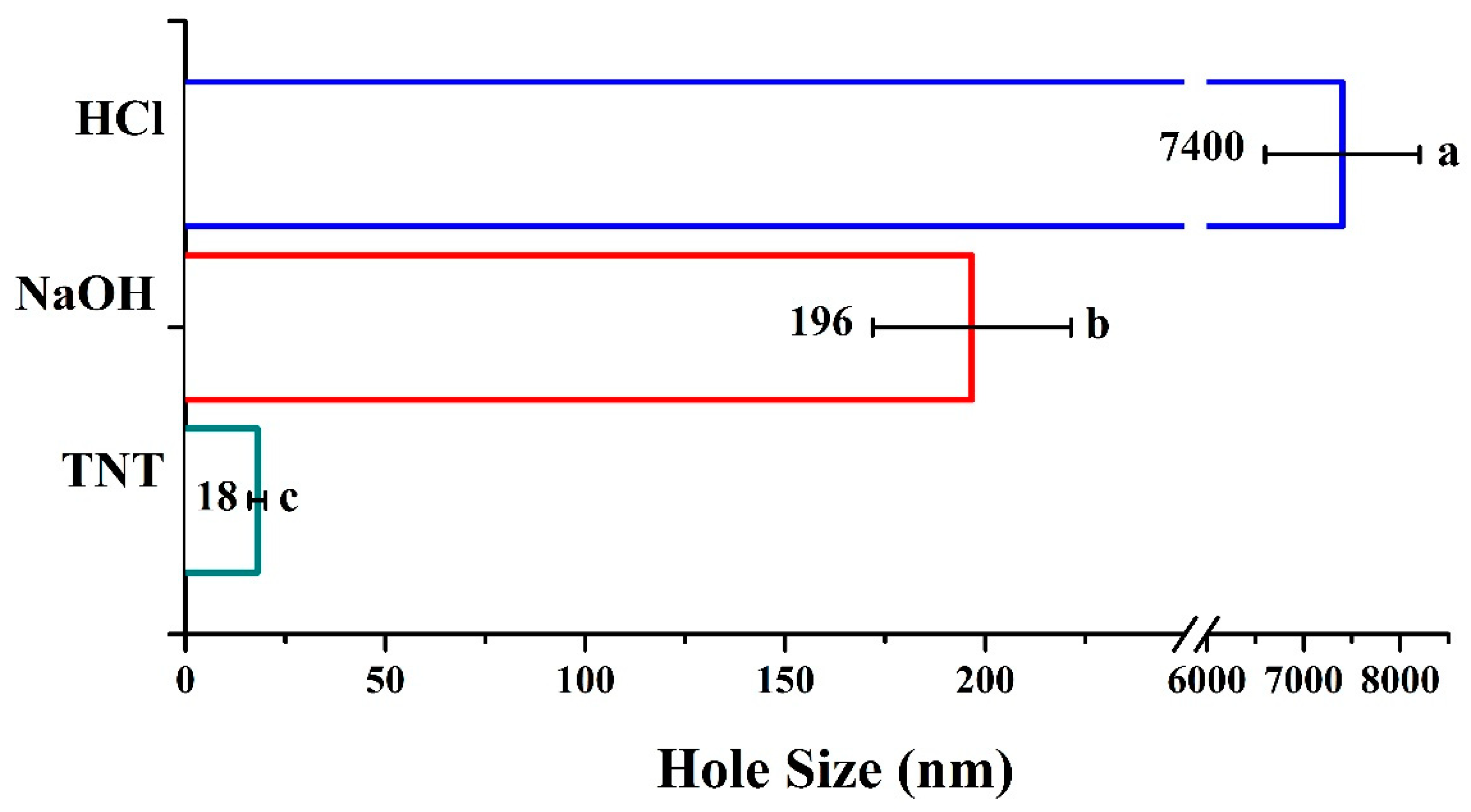
3.1. Surface Treatment of Titanium
3.2. Hydrophilicity Test
3.3. Characterization
3.4. Biocompatibility
3.5. Bioactivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isa, N.N.C.; Mohd, Y.; Yury, N. Electrochemical deposition and characterization of hydroxyapatite (hap) on titanium substrate. APCBEES Procedia 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, D.; Shivaram, A.; Tarafder, S.; Bandyopadhyay, A. Calcium phosphate coated 3d printed porous titanium with nanoscale surface modification for orthopedic and dental applications. Mater. Des. 2018, 151, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Rath, S.; Anand, A.; Ghosh, N.; Das, L.; Kokate, S.B.; Dixit, P.; Majhi, S.; Rout, N.; Singh, S.P.; Bhattacharyya, A. Cobalt chloride-mediated protein kinase calpha (pkcα) phosphorylation induces hypoxia-inducible factor 1α (hif1α) in the nucleus of gastric cancer cell. Biochem. Biophys. Res. Commun. 2016, 471, 205–212. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chuang, C.-C.; Wang, P.-T.; Tang, C.-M. A comparative study on the direct and pulsed current electrodeposition of cobalt-substituted hydroxyapatite for magnetic resonance imaging application. Materials 2018, 12, 116. [Google Scholar] [CrossRef]
- Niu, B.; Shi, P.; Shanshan, E.; Wei, D.; Li, Q.; Chen, Y. Preparation and characterization of ha sol–gel coating on mao coated az31 alloy. Surf. Coat. Technol. 2016, 286, 42–48. [Google Scholar] [CrossRef]
- De Groot, K.; Geesink, R.; Klein, C.P.A.T.; Serekian, P. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef]
- Cotell, C.M. Pulsed laser deposition and processing of biocompatible hydroxylapatite thin films. Appl. Surf. Sci. 1993, 69, 140–148. [Google Scholar] [CrossRef]
- Torrisi, L.; Setola, R. Thermally assisted hydroxyapatite obtained by pulsed-laser deposition on titanium substrates. Thin Solid Films 1993, 227, 32–36. [Google Scholar] [CrossRef]
- Mahanti, M.; Nakamura, M.; Pyatenko, A.; Sakamaki, I.; Koga, K.; Oyane, A. The mechanism underlying calcium phosphate precipitation on titanium via ultraviolet, visible, and near infrared laser-assisted biomimetic process. J. Phys. D Appl. Phys. 2016, 49, 1–10. [Google Scholar] [CrossRef]
- Oyane, A.; Sakamaki, I.; Shimizu, Y.; Kawaguchi, K.; Koshizaki, N. Liquid-phase laser process for simple and area-specific calcium phosphate coating. J. Biomed. Mater. Res. A 2012, 100, 2573–2580. [Google Scholar] [CrossRef]
- Oyane, A.; Matsuoka, N.; Koga, K.; Shimizu, Y.; Nakamura, M.; Kawaguchi, K.; Koshizaki, N.; Sogo, Y.; Ito, A.; Unuma, H. Laser-assisted biomimetic process for surface functionalization of titanium metal. Colloids Interface Sci. Commun. 2015, 4, 5–9. [Google Scholar] [CrossRef]
- Oyane, A.; Sakamaki, I.; Pyatenko, A.; Nakamura, M.; Ishikawa, Y.; Shimizu, Y.; Kawaguchi, K.; Koshizaki, N. Laser-assisted calcium phosphate deposition on polymer substrates in supersaturated solutions. R. Soc. Chem. 2014, 4, 53645–53648. [Google Scholar] [CrossRef]
- Pecheva, E.; Petrov, T.; Lungu, C.; Montgomery, P.; Pramatarova, L. Stimulated in vitro bone-like apatite formation by a novel laser processing technique. Chem. Eng. J. 2008, 137, 144–153. [Google Scholar] [CrossRef]
- Ivanoff, C.-J.; Widmark, G.; Hallgren, C.; Sennerby, L.; Wennerberg, A. Histologic evaluation of the bone integration of tio2 blasted and turned titanium microimplants in humans. Clin. Oral Implants Res. 2001, 12, 128–134. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Cheng, C.H.; HO, W.F.; Lai, C.H.; Hsu, H.C.; Wu, S.C. Apatite formation on a nano-porous titanium alloy surface treated with aqueous naoh. J. Sci. Eng. Technol. 2010, 6, 1–7. [Google Scholar]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Matsushita, T.; Kokubo, T.; Nakamura, T. Osteoinductive porous titanium implants: Effect of sodium removal by dilute hcl treatment. Biomaterials 2006, 27, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-H.; Finõnes, R.R.; Daraio, C.; Chen, L.-H.; Jin, S. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Lai, Y.; Lin, C. Electrochemically multi-anodized tio2 nanotube arrays for enhancing hydrogen generation by photoelectrocatalytic water splitting. Electrochim. Acta 2010, 55, 4776–4782. [Google Scholar] [CrossRef]
- Sasidharan, M.; Oyane, A.; Kim, H.-M.; Kokubo, T.; Ito, A. Biomimetic coating of laminin-apatite composite on titanium metal and its excellent cell-adhesive properties. Adv. Mater. 2004, 16, 1071–1074. [Google Scholar]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S. Ca, p-rich layer formed on high-strenght bioactive glass-ceramic a-w. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Preparation of bioactive ti and its alloys via simple chemical surface treatment. Biomed. Mater. Res. Part A 1996, 32, 409–417. [Google Scholar] [CrossRef]
- Liang, J.; Xu, S.; Shen, M.; Cheng, B.; Li, Y.; Liu, X.; Qin, D.; Bellare, A.; Kong, L. Osteogenic activity of titanium surfaces with hierarchical micro/nano-structures obtained by hydrofluoric acid treatment. Int. J. Nanomed. 2017, 12, 1317–1328. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oshida, Y.; Andres, C.J.; Barco, M.T. Surface characterizations of variously treated titanium materials. Int. J. Oral Maxillofac. Implant. 2001, 16, 333–342. [Google Scholar]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified sla titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Masahashi, N.; Semboshi, S.; Ohtsu, N.; Oku, M. Microstructure and superhydrophilicity of anodic tio2 films on pure titanium. Thin Solid Films 2008, 516, 7488–7496. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- He, Q.; Huang, Z. Controlled synthesis and morphological evolution of dendritic porous microspheres of calcium phosphates. J. Porous Mater. 2008, 16, 683–689. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Toyama, T.; Nakashima, K.; Yasue, T. Hydrothermal synthesis of β-tricalcium phosphate from amorphous calcium phosphate. J. Ceram. Soc. Jpn. 2002, 110, 716–721. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Zhang, M. Characterization and thermal behavior of calcium deficient hydroxyapatite whiskers with various ca/p ratios. Mater. Chem. Phys. 2011, 126, 642–648. [Google Scholar] [CrossRef]
- Lamberti, A.; Chiodoni, A.; Shahzad, N.; Bianco, S.; Quaglio, M.; Pirri, C. Ultrafast room-temperature crystallization of TiO2 nanotubes exploiting water-vapor treatment. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Ji, H.; Ding, C. Effect of ti-oh formation on bioactivity of vacuum plasma sprayed titanium coating after chemical treatment. Surf. Coat. Technol. 2007, 202, 494–498. [Google Scholar] [CrossRef]
- Granja, P.L.; Ribeiro, C.C.; de Jeso, B.; Baquey, C.; Barbosa, M.A. Mineralization of regenerated cellulose hydrogels. J. Mater. Sci. Mater. Med. 2001, 12, 785–791. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Y.; Jia, S.; Huang, Y.; Gao, C.; Wan, Y. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater. Lett. 2006, 60, 1710–1713. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Nie, L.; Suo, J.; Zou, P.; Feng, S. Preparation and properties of biphasic calcium phosphate scaffolds multiply coated with ha/plla nanocomposites for bone tissue engineering applications. J. Nanomater. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Tanaka, T.; Kojima, I.; Ohse, T.; Ingelfinger, J.R.; Adler, S.; Fujita, T.; Nangaku, M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab. Investig. 2005, 85, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, N.; Ajduković, Z.; Savić, V.; Najman, S.; Mihailović, D.; Vasiljević, P.; Stojanović, Z.; Uskoković, V.; Uskoković, D. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J. Mater. Sci. Mater. Med. 2013, 24, 343–354. [Google Scholar] [CrossRef]
- Svenskaya, Y.; Parakhonskiy, B.V.; Haase, A.; Atkin, V.; Lukyanets, E.; Gorin, D.; Antolini, R. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys. Chem. 2013, 182, 11–15. [Google Scholar] [CrossRef]
- Kramer, E.; Itzkowitz, E.; Wei, M. Synthesis and characterization of cobalt-substituted hydroxyapatite powders. Ceram. Int. 2014, 40, 13471–13480. [Google Scholar] [CrossRef]
- Gopi, D.; Karthika, A.; Nithiya, S.; Kavitha, L. In vitro biological performance of minerals substituted hydroxyapatite coating by pulsed electrodeposition method. Mater. Chem. Phys. 2014, 144, 75–85. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J.; Shinjo, R. X-ray diffraction is a promising tool to characterize coral skeletons. Adv. Mater. Phys. Chem. 2013, 3, 120–125. [Google Scholar] [CrossRef]
- Lin, W.; Chuang, C.; Yao, C.; Tang, C. Effect of cobalt precursors on cobalt-hydroxyapatite used in bone regeneration and MRI. J. Dent. Res. 2020. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef]
- Scarano, A.; Crocetta, E.; Quaranta, A.; Lorusso, F. Influence of the thermal treatment to address a better osseointegration of ti6al4v dental implants: Histological and histomorphometrical study in a rabbit model. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar]
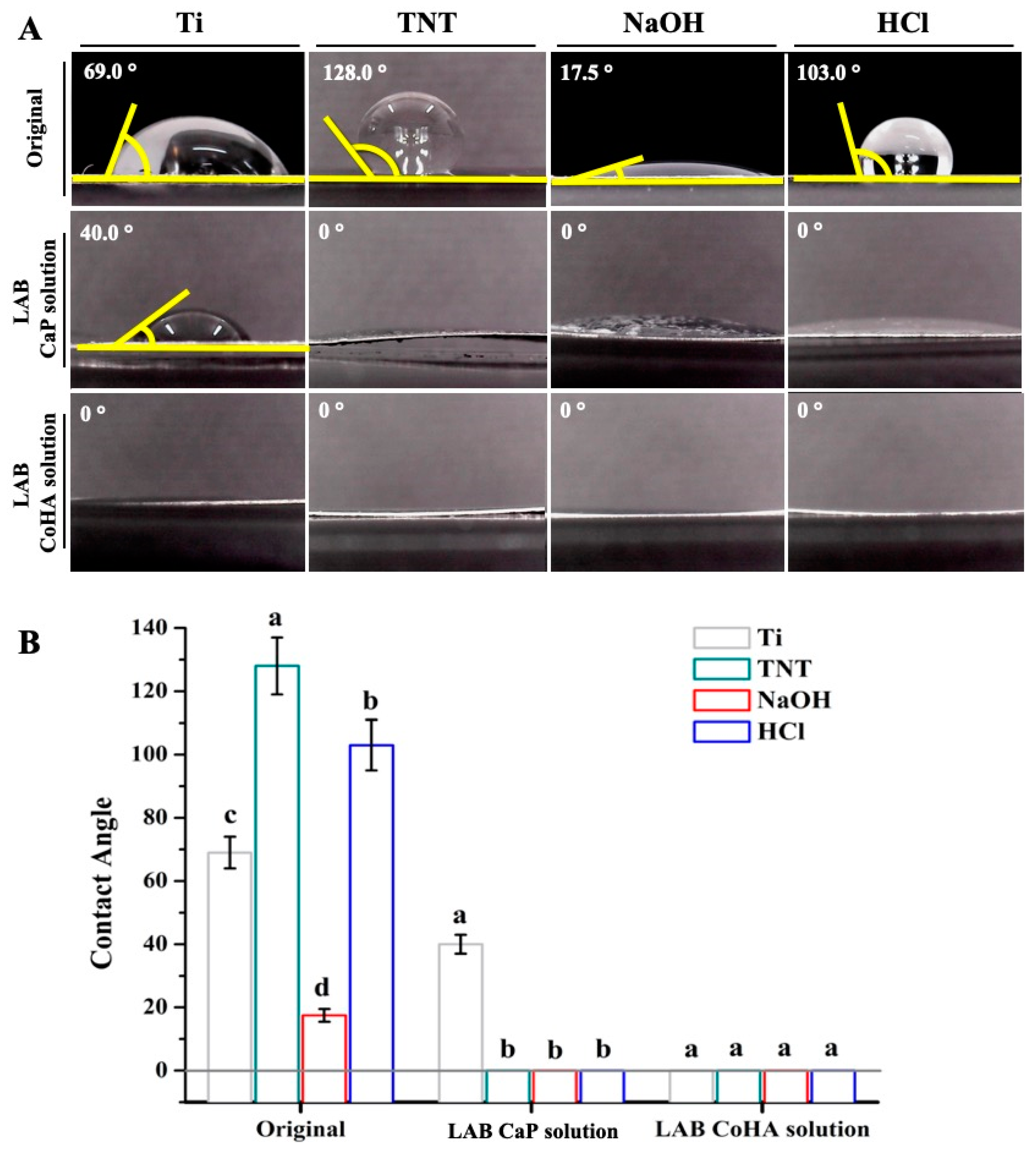
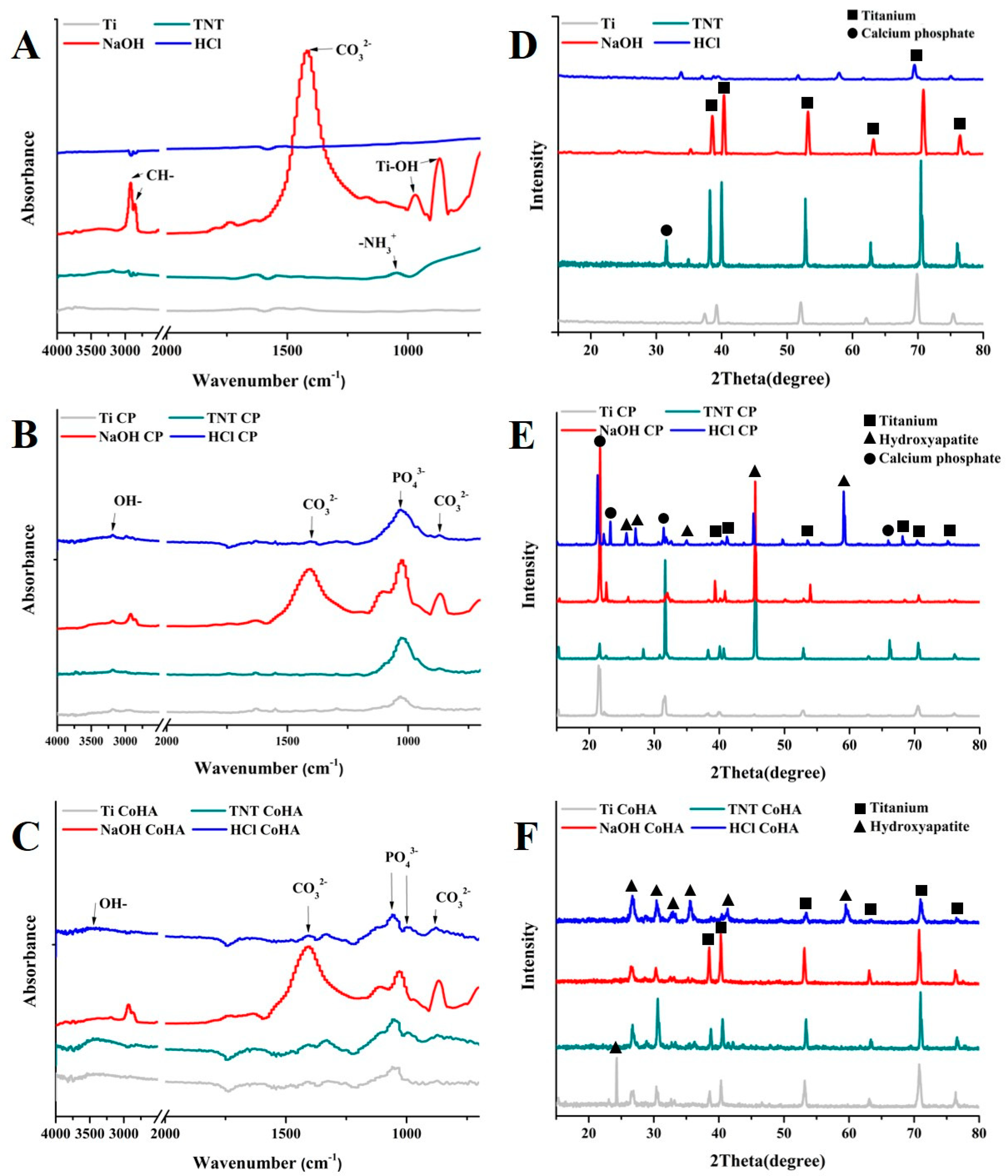
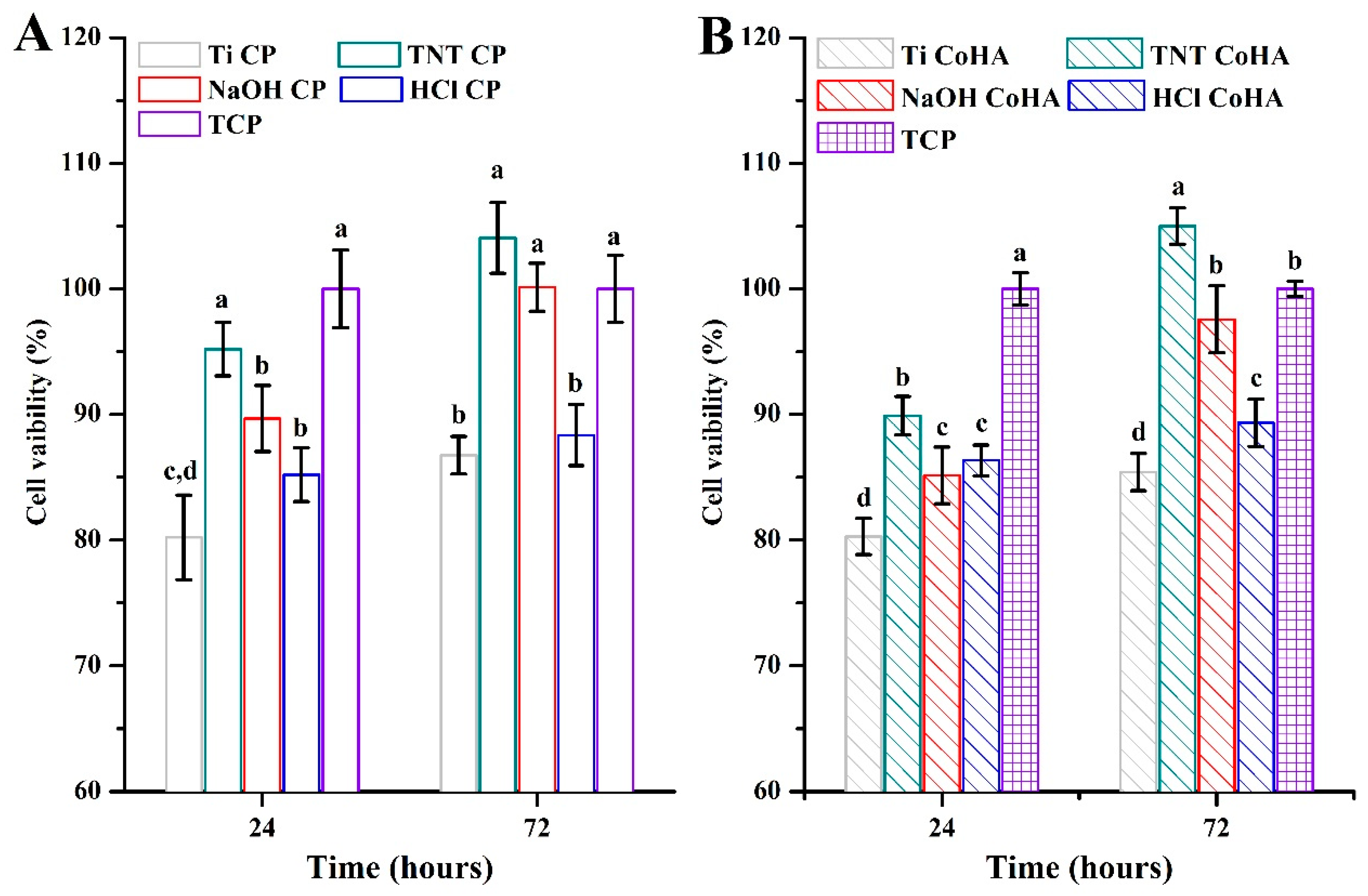
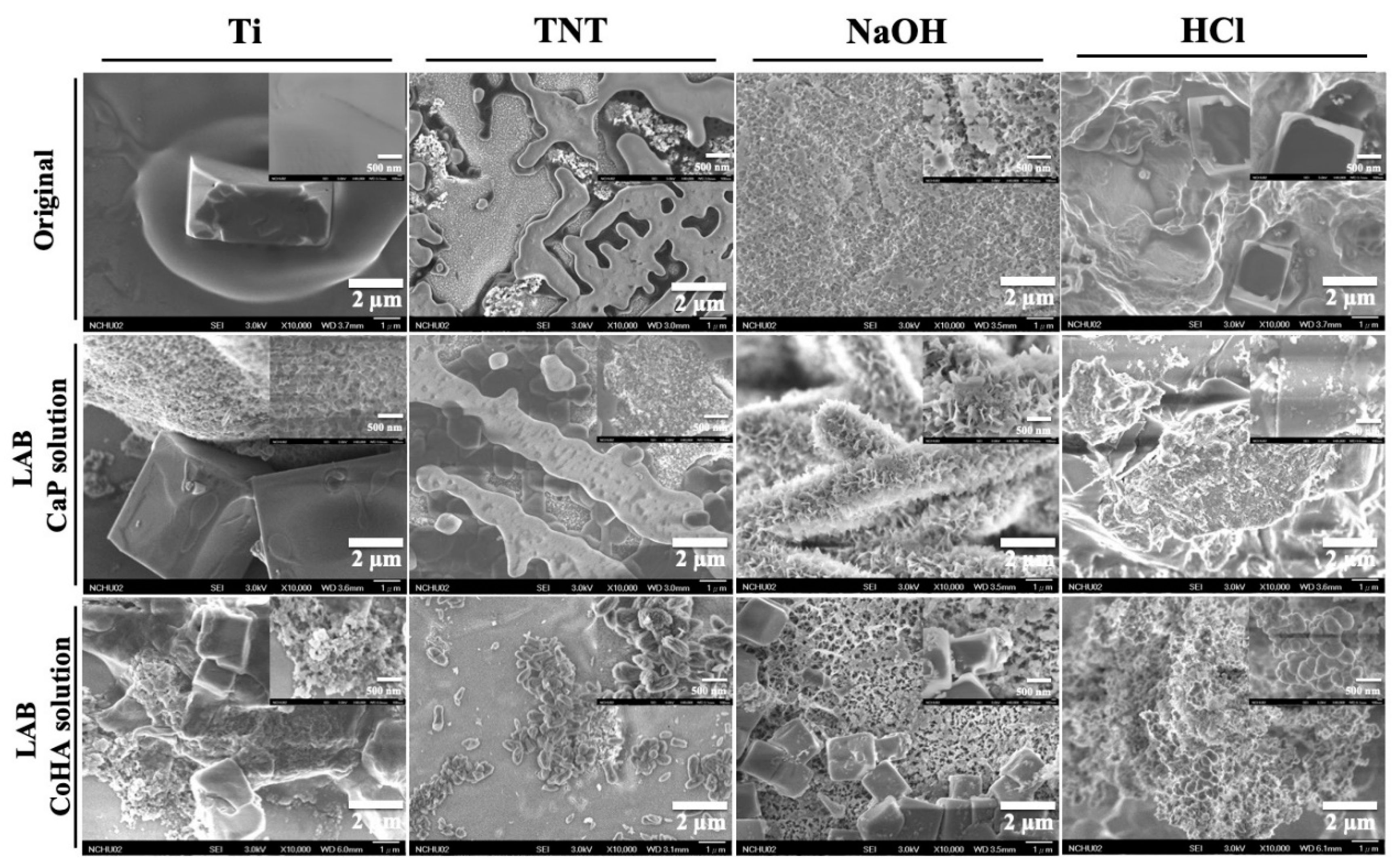

| Sample | Original | LAB | |
|---|---|---|---|
| CaP Solution | CoHA Solution | ||
| Control group | Ti | Ti CP | Ti CoHA |
| Anodic oxidation | TNT | TNT CP | TNT CoHA |
| Alkali treatment | NaOH | NaOH CP | NaOH CoHA |
| Acid treatment | HCl | HCl CP | HCl CoHA |
| Atomic% | C | Ti | O | Na | F | Ca | P | Ca/P | Co | Ca + Co/P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Original | Ti | 12.88 | 87.12 | - | - | - | N.D | ||||
| TNT | 7.92 | 29.83 | 48.57 | - | 13.68 | ||||||
| NaOH | 5.61 | 31.45 | 58.91 | 4.03 | - | ||||||
| HCl | - | 100 | - | - | - | ||||||
| LAB CaP solution | Ti | 11.50 | 83.93 | 1.65 | - | - | 1.5 | 1.4 | 1.07 | N.D | N.D |
| TNT | 3.00 | 16.10 | 37.16 | - | - | 2.18 | 1.81 | 1.20 | |||
| NaOH | 7.01 | 6.69 | 68.32 | 1.14 | - | 8.65 | 7.37 | 1.17 | |||
| HCl | 3.67 | 18.42 | 63.95 | - | - | 7.46 | 6.50 | 1.15 | |||
| LAB CoHA solution | Ti | 13.23 | 86.77 | - | - | - | - | - | - | - | - |
| TNT | - | - | 69.31 | 1.50 | - | 12.52 | 15.81 | 0.79 | 0.41 | 0.82 | |
| NaOH | - | - | 68.06 | 1.82 | - | 12.97 | 15.68 | 0.83 | 0.65 | 0.87 | |
| HCl | 0.61 | - | 68.27 | 3.14 | - | 11.19 | 13.17 | 0.85 | 0.66 | 0.90 | |
| Atomic% | Ti | Ca | P | Ca/P | Co | Ca + Co/P | |
|---|---|---|---|---|---|---|---|
| Original | Ti | 28.71 | 0.82 | 0.77 | 1.06 | N.D | |
| TNT | 28.02 | 0.49 | 0.41 | 1.20 | |||
| NaOH | 14.88 | 6.88 | 6.11 | 1.13 | |||
| HCl | 32.24 | 2.14 | 1.76 | 1.22 | |||
| LAB CaP solution | Ti | 23.45 | 1.17 | 1.02 | 1.15 | N.D | |
| TNT | 1.00 | 8.67 | 6.03 | 1.44 | |||
| NaOH | 3.48 | 9.50 | 7.80 | 1.22 | |||
| HCl | 3.23 | 8.90 | 6.80 | 1.31 | |||
| LAB CoHA solution | Ti | 26.73 | 2.97 | 3.68 | 0.81 | 0.55 | 0.96 |
| TNT | 15.92 | 4.22 | 3.21 | 1.31 | 1.15 | 1.67 | |
| NaOH | 23.03 | 2.18 | 1.75 | 1.24 | 0.78 | 1.69 | |
| HCl | 12.10 | 14.20 | 11.76 | 1.21 | 3.09 | 1.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-M.; Fan, F.-Y.; Lin, W.-T.; Wang, L.; Lin, W.-C. Effect of Bioactivity of Surface Topography and Coating Forming by Infrared Light-Induced on Titanium for Bone Repair. Appl. Sci. 2020, 10, 8158. https://doi.org/10.3390/app10228158
Tang C-M, Fan F-Y, Lin W-T, Wang L, Lin W-C. Effect of Bioactivity of Surface Topography and Coating Forming by Infrared Light-Induced on Titanium for Bone Repair. Applied Sciences. 2020; 10(22):8158. https://doi.org/10.3390/app10228158
Chicago/Turabian StyleTang, Cheng-Ming, Fang-Yu Fan, Wei-Ting Lin, Liping Wang, and Wei-Chun Lin. 2020. "Effect of Bioactivity of Surface Topography and Coating Forming by Infrared Light-Induced on Titanium for Bone Repair" Applied Sciences 10, no. 22: 8158. https://doi.org/10.3390/app10228158
APA StyleTang, C.-M., Fan, F.-Y., Lin, W.-T., Wang, L., & Lin, W.-C. (2020). Effect of Bioactivity of Surface Topography and Coating Forming by Infrared Light-Induced on Titanium for Bone Repair. Applied Sciences, 10(22), 8158. https://doi.org/10.3390/app10228158