1. Introduction
The use of conventional laboratory assays—the Born aggregometry—for the assessment of the platelet function has the disadvantages of being time and resource consuming with poor quality assurance [
1]. Whole blood multiple impedance aggregometry with the Multiplate
® device (Roche, Mannheim, Germany) has shown a number of advantages in the evaluation of the platelet function. Use of whole blood samples avoids the need for the centrifugation step and the eventual activation of platelets during preparation. Moreover, time requirement for measurement is markedly reduced in comparison to Born aggregometry. In addition, the computerized design and the availability of standardized activators simplify the measurements and therefore allow bedside monitoring of the platelet function [
2,
3].
Impedance aggregometry has been demonstrated to be useful in several settings [
4,
5,
6]. In cardiac surgery, an impedance aggregometry-based algorithm was demonstrated to reduce the requirements of blood, plasma and platelet transfusion [
7,
8,
9]. In patients with coronary artery disease and percutaneous coronary intervention, impedance aggregometry was useful for the detection of aspirin responsiveness and resistance [
10]. In 40 patients, who underwent transcatheter aortic valve implantation due to severe aortic stenosis, multiple aggregometry was useful to detect high reactivity despite the use of clopidogrel (in 42%) and aspirin (in 11%) and the risk of thromboembolic events [
6,
11]. In patients with intracranial hemorrhage impedance aggregometry was used to evaluate the need for platelet concentrates [
12].
As whole blood samples subjected to impedance aggregometry are not corrected for platelet count, an effect of platelet count on the readings of the device can be assumed. However, it is the general opinion, that platelet count affects impedance aggregometry values only in thrombocytopenia with less than 100,000 mm
−3 [
13,
14]. In contrast, a recent study demonstrates, that light transmission aggregometry, but not Multiplate
® findings, are independent of platelet count in the physiological range [
15].
In our study we examined the effect of platelet count on impedance aggregometry findings. We hypothesized that impedance aggregometry findings are dependent on platelet count, and that the correction of impedance aggregometry findings for platelet count using the ratio of the variables results in a variable which is independent of platelet count.
2. Materials and Methods
Blood Sampling: After approval of the ethics committee of the University Hospital Essen, blood samples were drawn for the experimental study from 8 probands without a history of antiplatelet therapy intake or thrombotic disorder. For collection, hirudin-containing tubes (2.7 mL, 0.045 mg r-hirudin; S-Monovette®, SARSTEDT, Nümbrecht, Germany) were used.
Blood fractionation: Platelet-rich plasma (PRP) and platelet-poor plasma (PPP), respectively, were obtained by centrifugation of blood samples at 110 g for 15 min or 1800 g for 10 min at 24 °C.
Dilution of platelets: After measuring the platelet concentrations in PRP using a Coulter counter (KX-21N™ Automated Hematology Analyzer, Sysmex, Kobe, Japan), the cell suspension was diluted with PPP to achieve different platelet concentrations. Resultant platelet concentrations were controlled and ranged from 55,000 to 580,000 mm−3 platelets.
Multiple impedance aggregometry: The undiluted and diluted PRP samples were then subjected to multiple electrode impedance aggregometry (Multiplate® analyzer, Roche, Mannheim, Germany) within 30 min to 2 h from blood sampling, and the platelet activator thrombin receptor activating peptide (TRAPtest) was used to induce platelet aggregation.
Statistics: The IBM SPSS statistics package version 22 was used for the statistical analysis. The Pearson correlation coefficient was used for linear correlation analysis. Differences between groups were evaluated after testing for normal distribution (Komolgorov-Smirnov test). A
p-value of less than 0.05 was considered significant. For power-analysis, the software GPower 3.1 and the post hoc point biserial model were used [
16].
3. Results
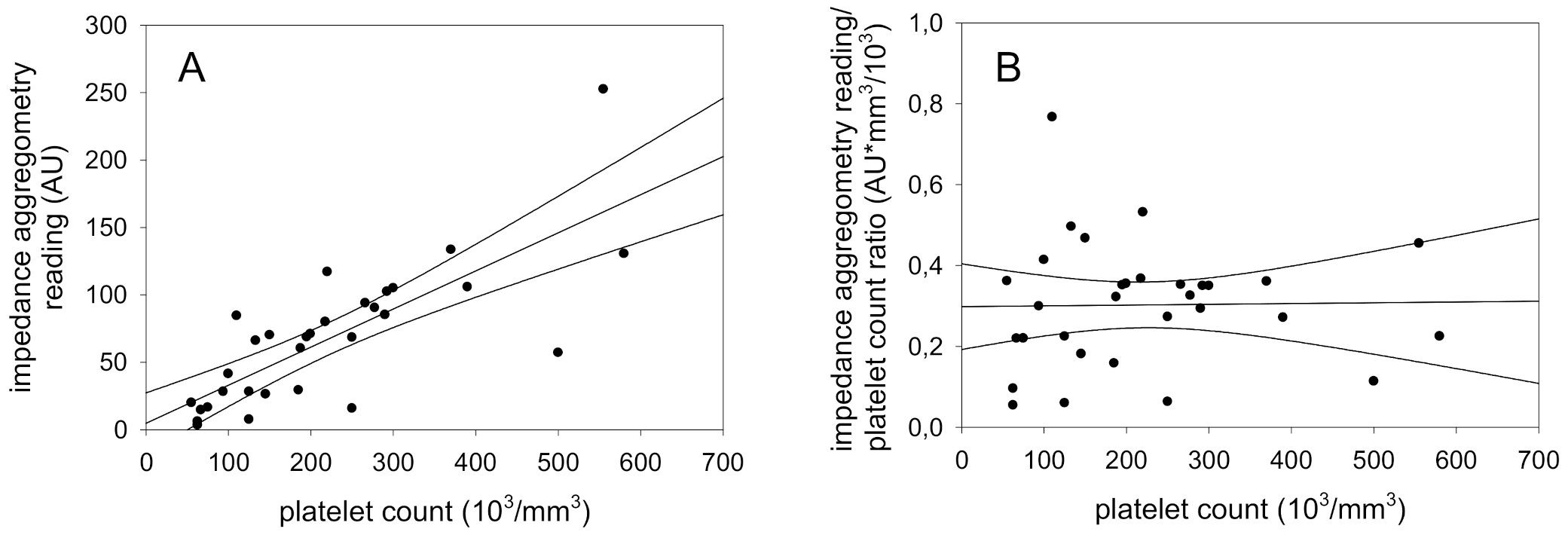
In the present study, platelet-rich plasma was diluted with platelet-poor plasma from the same patient to obtain samples with differing platelet counts but identical aggregation characteristics. Measurement of platelet function with impedance aggregometry and determination of platelet count with a Coulter counter demonstrated that impedance aggregometry readings increased with platelet count. The combined results of 8 experiments are shown in
Figure 1 and demonstrate a significant correlation of 0.78 (
p = 0.0001) in a broad platelet count range (55,000–580,000 mm
−3) As the regression line passes the origin of the graph, we normalized the impedance aggregometry findings for platelet count calculating the ratio of the variables. The results, shown in
Figure 2, demonstrate that the ratio is independent of platelet count (r = 0.78,
p = 0.0001).
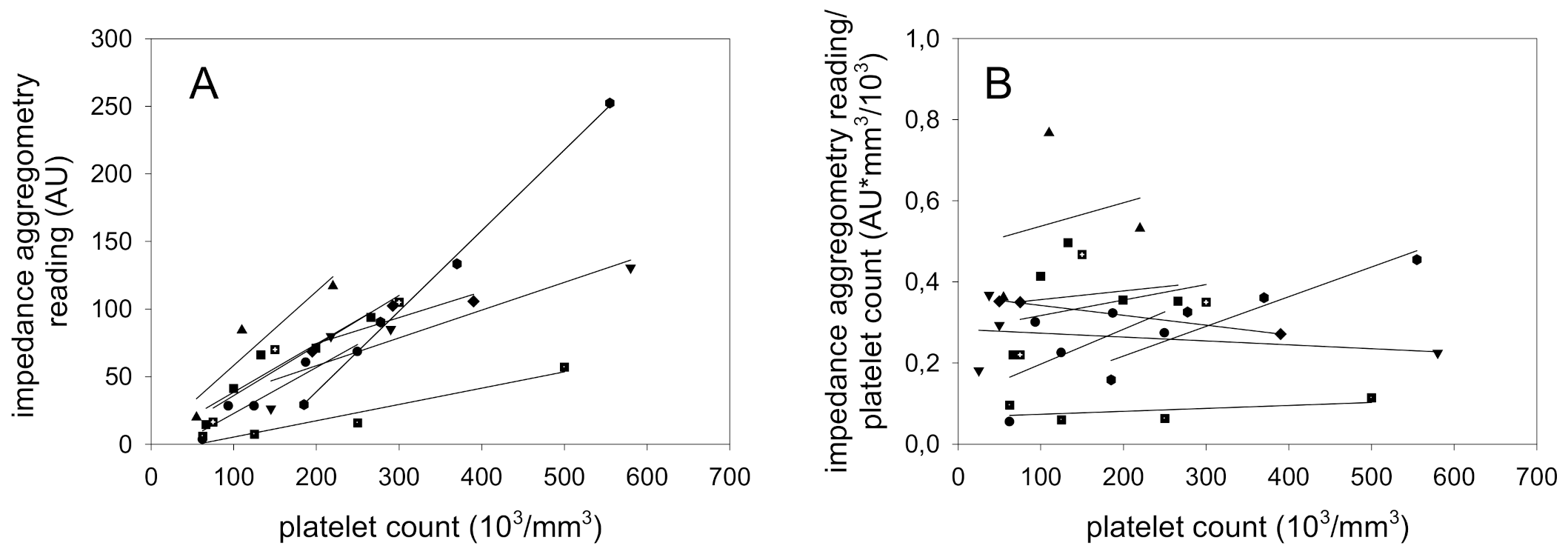
Although
Figure 1A shows a proportionality between the platelet count and impedance aggregometry findings, the variability of some samples was high. To decide whether this finding is due to “biological” differences in platelet function, individual regression curves of the 8 probands were calculated. The results demonstrate that there are marked differences between the healthy probands (
Figure 2A). Normalization of impedance aggregometry findings by the platelet count in each of the 8 probands demonstrated that the ratio is capable to differentiate between the platelet aggregation characteristics independent of the platelet count (
Figure 2B). The proband with the highest responsive platelets demonstrated a more than 5-fold increased aggregation in comparison to the lowest responsive proband.
4. Discussion
The results of the present study demonstrate that the platelet aggregation findings as obtained by impedance aggregometry are critically dependent on the platelet count: a strict proportional relation between both variables was demonstrated in samples with both reduced, normal and elevated platelet count, respectively. Correction can easily be achieved by formation of a ratio of impedance reading and the correspondent platelet count.
The conventional procedure to determine platelet function is the Born aggregometry [
1,
17]. The method determines the transmission of light in a platelet-rich plasma sample upon induction of platelet aggregation by an activator. For this procedure, the platelet count is adjusted to a certain value for reasons of comparability of results. The method is technically demanding, requires a specialized laboratory and is not easy to standardize.
Impedance aggregometry, in contrast, relies on the aggregation of activated platelets to the surface of electrodes. Measurements are performed in anticoagulated whole blood samples and the samples are not adjusted for platelet count before the assay. Several standardized activators are commercially available; thus, a detailed analysis of platelet aggregation characteristics is feasible. Computerized design and the use of unprocessed whole blood enables the determination at the bedside.
The fact that impedance aggregometry uses unprocessed whole blood implicates that there is no correction for platelet count. As shown in the present study, impedance aggregometry readings are dependent on both the platelet function and platelet count. This fact might not be critical when the pattern of aggregation obtained by several activators is compared. For example, clopidogrel leads to a specific reduction of the ADP-induced platelet aggregation, while the response to the remaining activators (arachidonic acid, collagen, thrombin receptor activating peptide) is unaltered. Thus, the differences in impedance aggregometry readings with the different activators might give an impression for eventual action of platelet antagonists. In other clinical situations, the thrombotic risk might likely be defined by both the platelet count and platelet function. However, in other constellations, the interpretation of results might be impossible. For example, either tirofiban or thrombocytopenia can decrease impedance aggregometry findings and the diagnosis of etiology is impossible from the readout. In this case, correction of impedance aggregometry finding will lead to the right diagnosis.
There are, however, some disadvantages of the correction. At first, the complexity of the procedure increases and the use at the bedside might be impossible in some settings. Moreover, errors of both impedance aggregometry and the platelet count measurement increase the combined error, when using the calculation for correction. The combined error will probably increase especially in severe thrombocytopenia and might limit the advantages of correction. One limitation of the present study is the fact that only thrombin receptor activating peptide was used for platelet activation in the present study. However, the suggestion that different activators might lead to divergent platelet count dependencies of impedance aggregometry findings seems rather unlikely.
5. Conclusions
Impedance aggregometry readings obtained with thrombin receptor activating peptide activation of platelets are dependent on the platelet count even in the physiological platelet count range. The readings can be corrected by the ratio of the impedance aggregometry finding and platelet count. Further studies are required to investigate whether the correction results in a better prediction of clinical endpoints.
Author Contributions
Conceptualization, M.H.; methodology, M.H.; software, does not apply; validation, M.H.; formal analysis, M.S. and M.H.; investigation, M.S. and M.H.; resources, M.H.; data curation, M.H.; writing, M.S. and M.H.; writing—review and editing, M.H.; visualization, M.S.; supervision, M.H.; project administration, M.H.; funding acquisition, M.S. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutscher akademischer Austauschdienst—DAAD (German Egyptian Research Long-Term Scholarship Program). We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harrison, P. Assessment of platelet function in the laboratory. Hamostaseologie 2009, 29, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Tóth, O.; Calatzis, A.; Penz, S.; Losonczy, H.; Siess, W. Multiple electrode aggregometry: A new device to measure platelet aggregation in whole blood. Thromb. Haemost. 2006, 96, 781–788. [Google Scholar]
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Csilla, J.; Dirkmann, D.; Dusse, F.; Hanke, A.; Adamzik, M.; Hartmann, M.; Philipp, S.; Weber, A.A.; Rahe-Meyer, N. Messung der thrombozytenfunktion mit point-of-care-methoden. Herz Kardiovaskuläre Erkrankungen 2008, 33, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Christ, G.; Lang, I.M.; Delle-Karth, G.; Huber, K.; Jilma, B. Multiple electrode aggregometry predicts stent thrombosis better than the vasodilator-stimulated phosphoprotein phosphorylation assay. J. Thromb. Haemost. 2010, 8, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schunk, S.; Linn, J.; Peters, N.; Spannagl, M.; Deisenberg, M.; Brückmann, H.; Mayer, T.E. Monitoring of clopidogrel-related platelet inhibition: Correlation of nonresponse with clinical outcome in supra-aortic stenting. Am. J. Neuroradiol. 2008, 29, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Auci, E.; Vetrugno, L.; Riccardi, I.; Brussa, A.; Orso, D.; Baroselli, A.; Gigante, A.; Cecotti, R.; Bassi, F.; Livi, U.; et al. Multiple electrode aggregometry after cardiopulmonary bypass to assess platelet (Dys)-function and transfusion threshold: A concordance study. J. Cardiothorac. Vasc. Anesth. 2020, 24, S1053-0770(20)30602-9, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Jambor, C.; Hanke, A.A.; Dirkmann, D.; Adamzik, M.; Hartmann, M.; Rahe-Meyer, N. Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transfus. Med. Hemother. 2007, 34, 396–411. [Google Scholar] [CrossRef]
- Görlinger, K.; Fries, D.; Dirkmann, D.; Weber, C.F.; Hanke, A.A.; Schöchl, H. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus. Med. Hemother. 2012, 39, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ivandic, B.T.; Giannitsis, E.; Philipp Schlick, P.; Staritz, P.; Katus, H.A.; Hohlfeld, T. Determination of aspirin responsiveness by use of whole blood platelet aggregometry. Clin. Chem. 2007, 53, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Orvin, K.; Eisen, A.; Perl, L.; Zemer-Wassercug, N.; Codner, P.; Assali, A.; Vaknin-Assa, H.; Lev, L.I.; Kornowski, R. Platelet reactivity in patients undergoing transcatheter aortic valve implantation. J. Thromb. Thrombolysis 2015, 42, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Beynon, C.; Sakowitz, O.W.; Unterberg, A.W. Multiple electrode aggregometry in antiplatelet-related intracerebral haemorrhage. J. Clin. Neurosci. 2013, 20, 1805–1806. [Google Scholar] [CrossRef] [PubMed]
- Hanke, A.A.; Roberg, K.; Monaca, E.; Sellmann, T.; Weber, C.F.; Rahe-Meyer, K.; Görlinger, K. Impact of platelet count on results obtained from multiple electrode platelet aggregometry (Multiplate™). Eur. J. Med. Res. 2010, 15, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Stissing, T.; Dridi, N.P.; Ostrowski, S.R.; Bochsen, L.; Johansson, P.I. The influence of low platelet count on whole blood aggregometry assessed by multiplate. Thromb. Hemost. 2011, 17, E211–E217. [Google Scholar] [CrossRef] [PubMed]
- Femia, E.A.; Scavone, M.; Lecchi, A.; Cattaneo, M. Effect of platelet count on platelet aggregation measured with impedance aggregometry (MultiplateTM analyzer) and with light transmission aggregometry. J. Thromb. Haemost. 2013, 11, 2193–2196. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Breddin, H.K. Can platelet aggregometry be standardized? Platelets 2005, 16, 151–158. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).