Evaluation of Heat Insulation and Surface Resistivity of Mineral Lightweight Aggregate Concrete(MLAC)
Abstract
1. Introduction
2. Experimental Procedure
2.1. Test Material
2.2. Test Variables and Mixture Ratios
3. Results and Analysis
3.1. Mixture Design
3.2. Fresh Properties
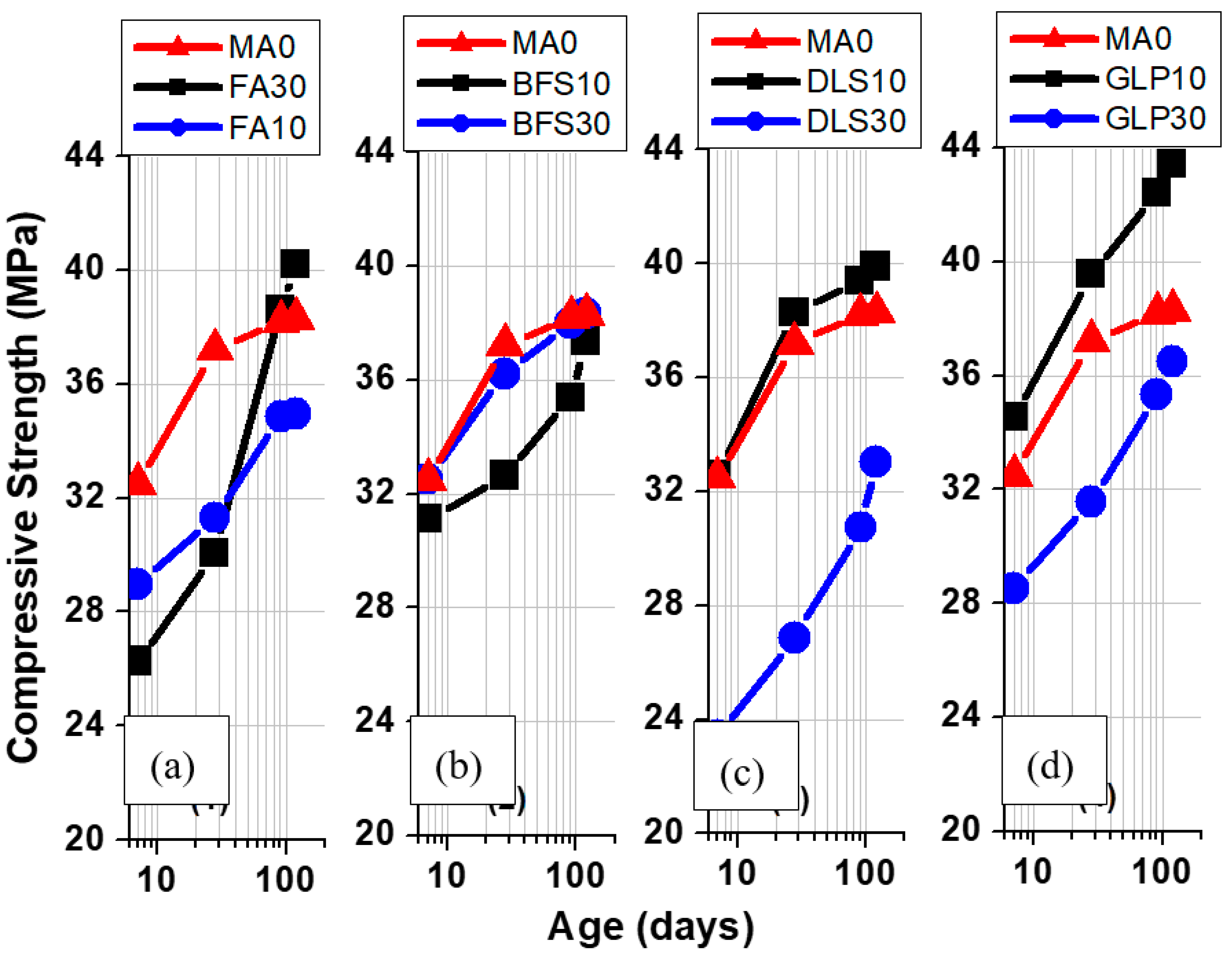
3.3. Compressive Strength
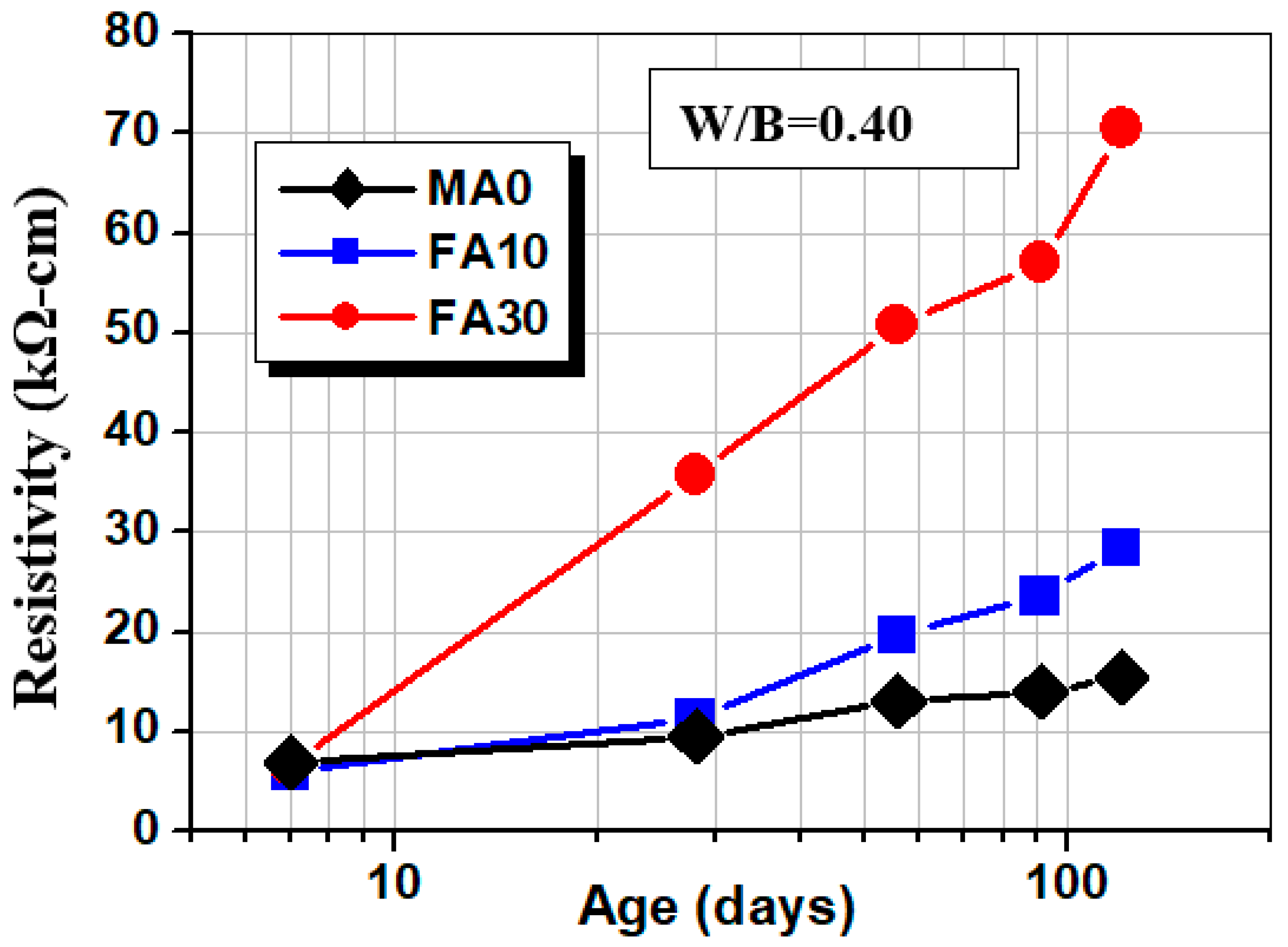
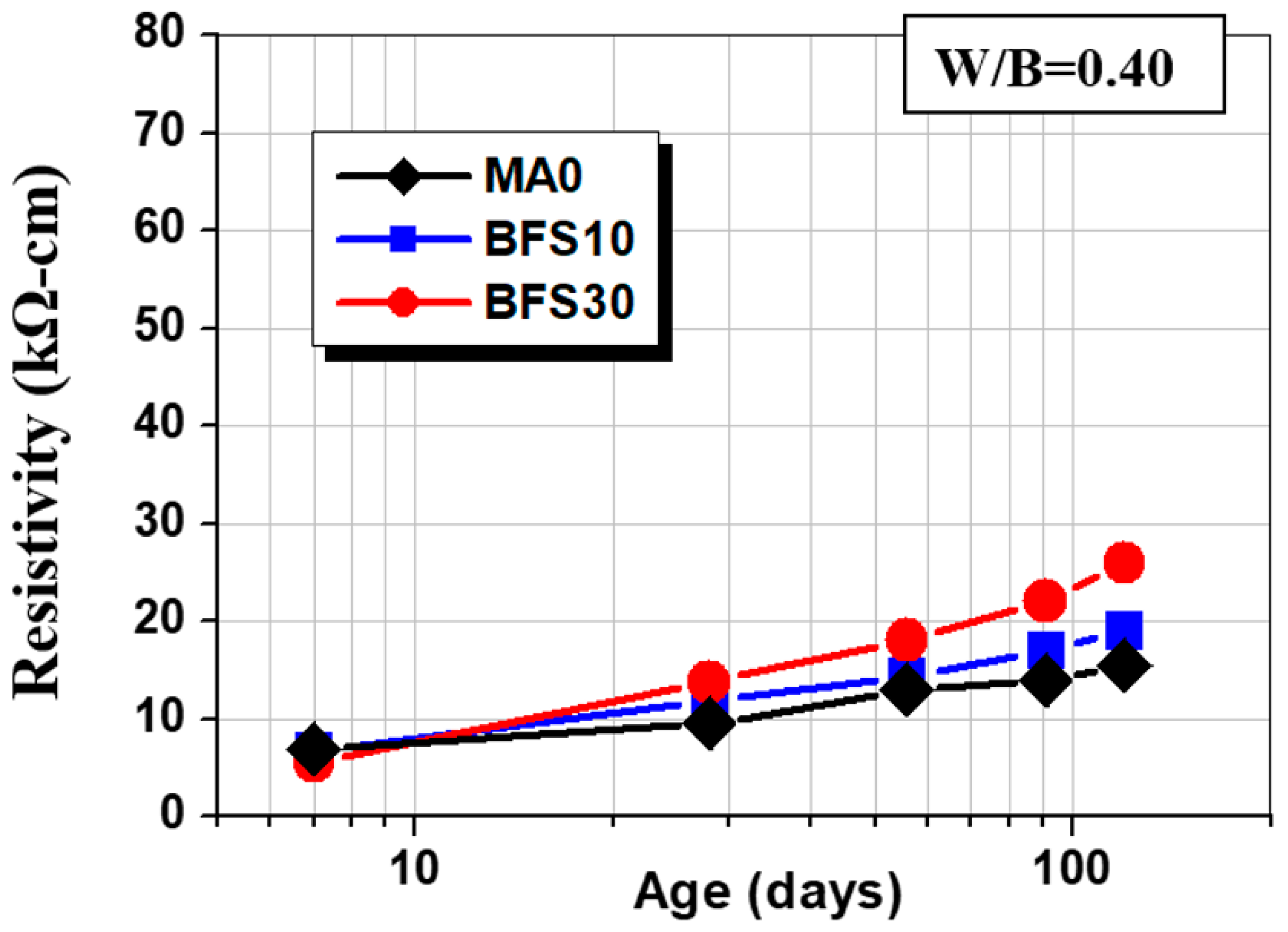
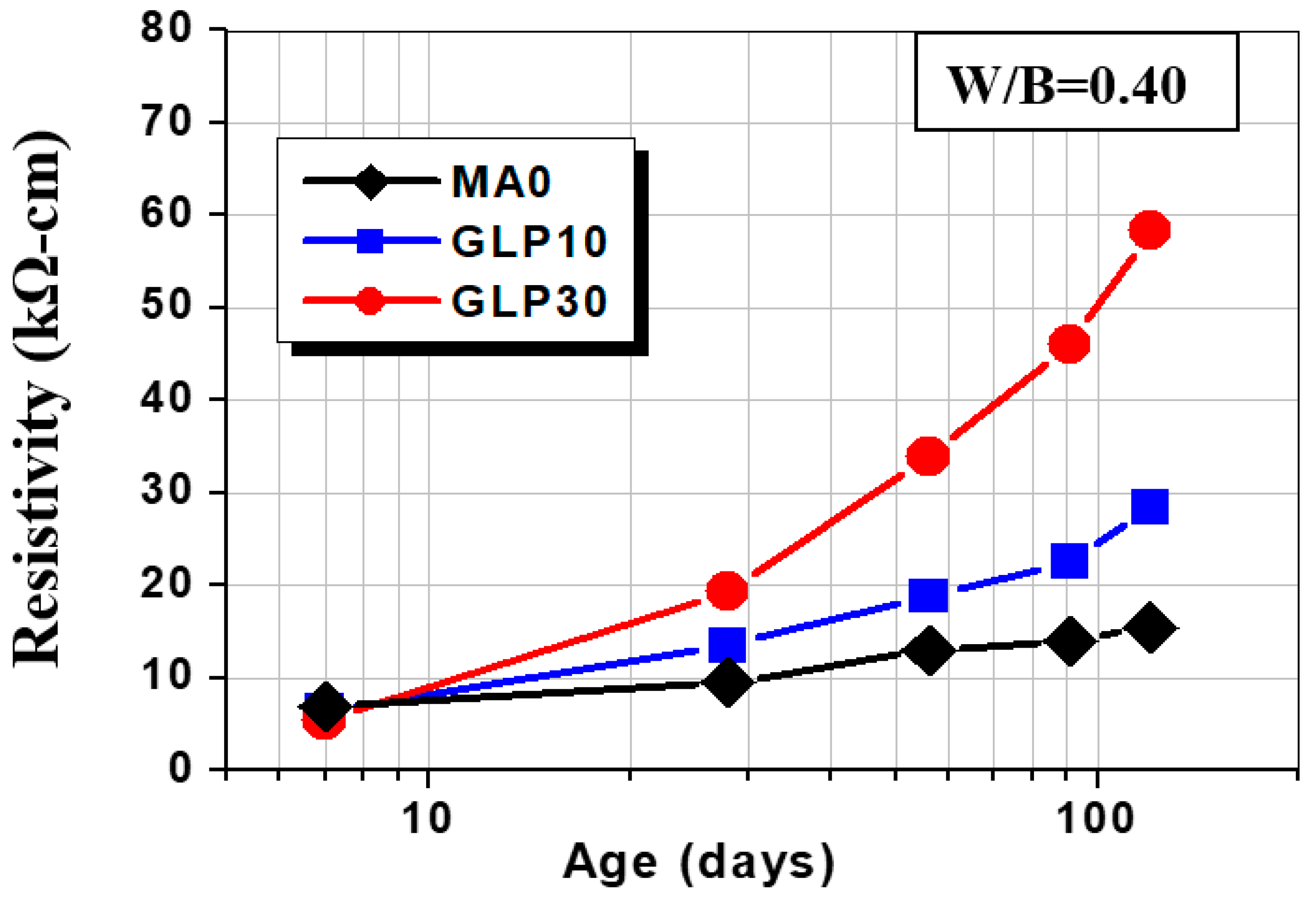
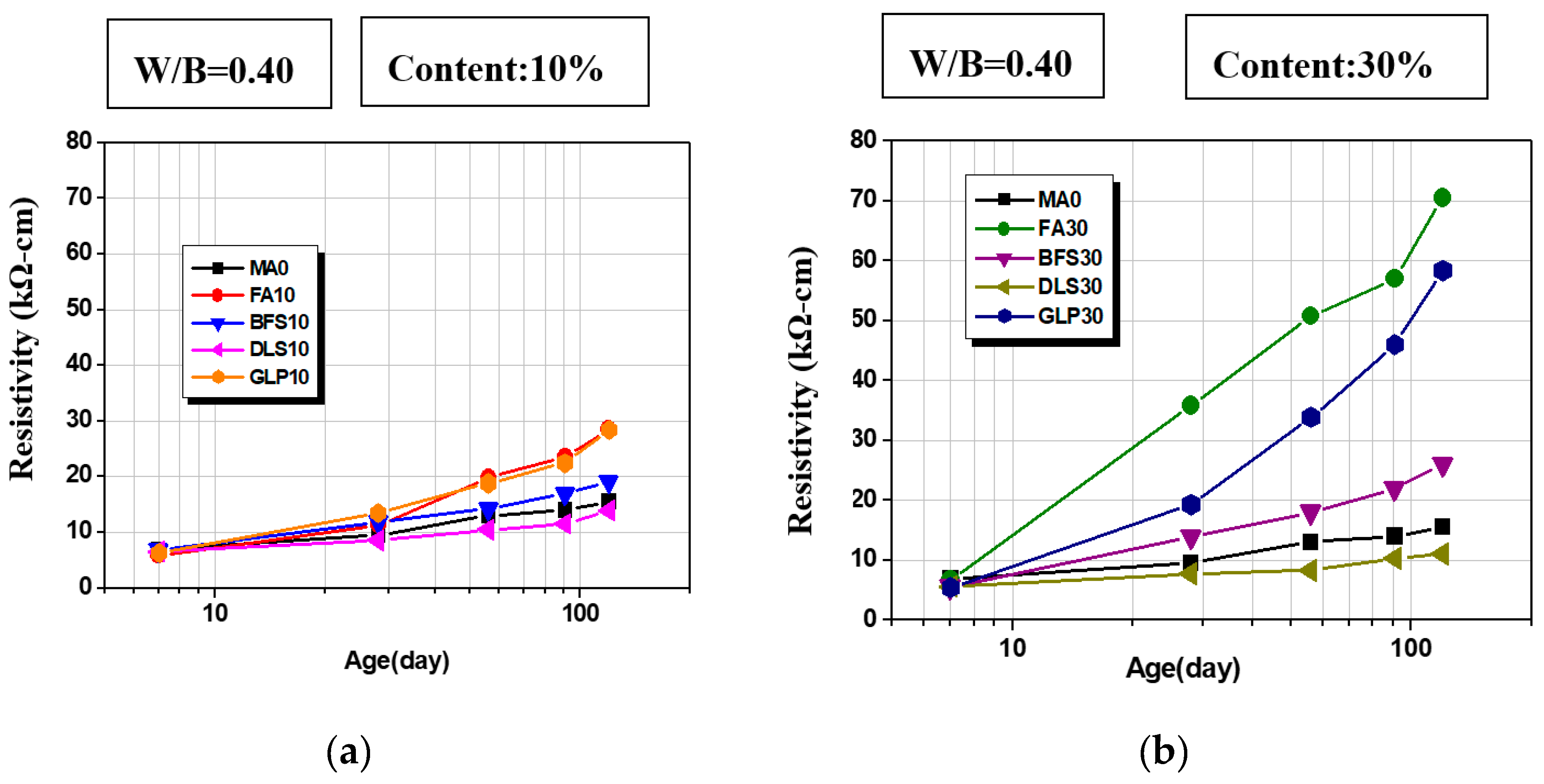
3.4. Surface Resistivity
3.5. Pozzolan Reactivity
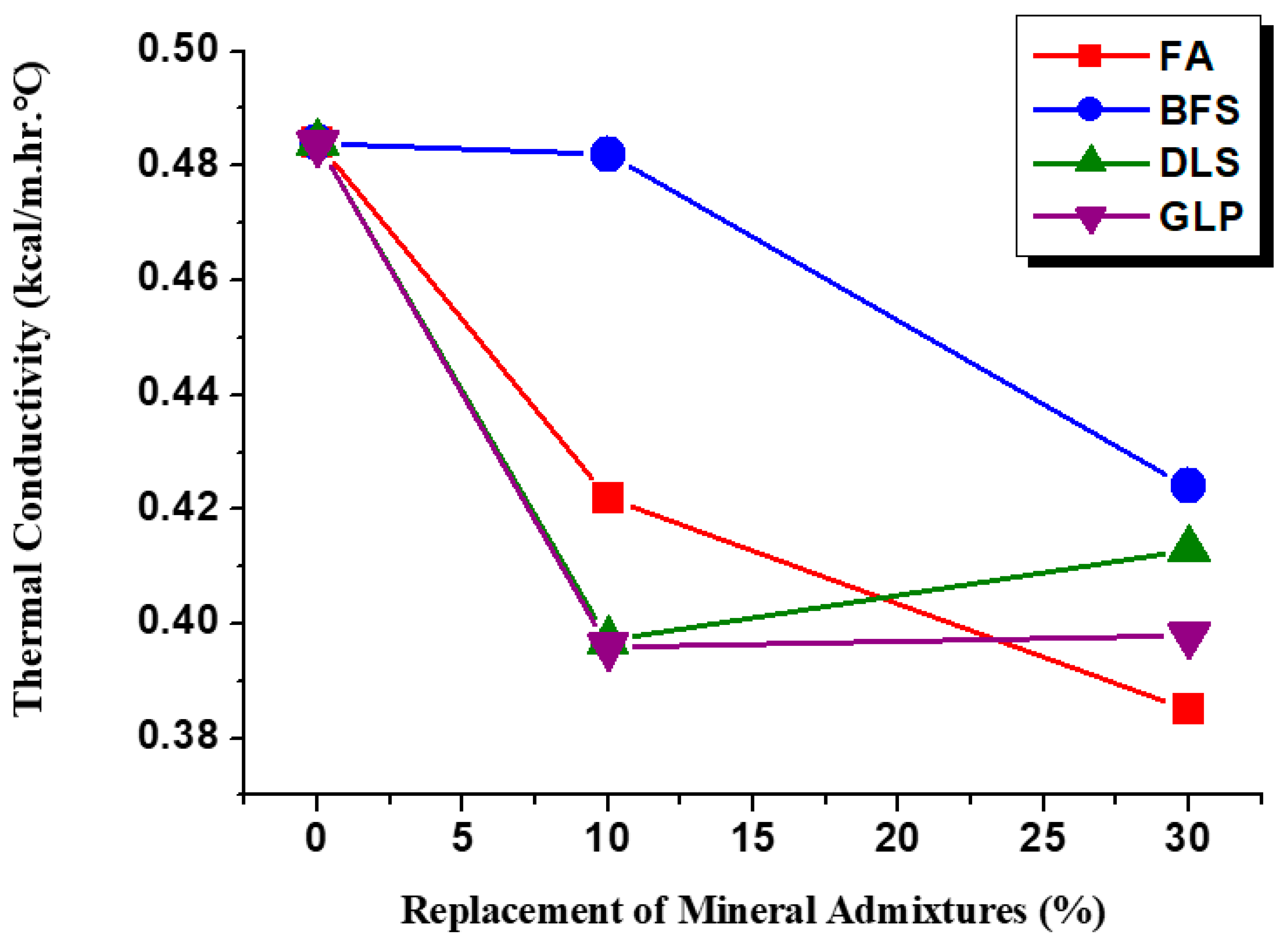
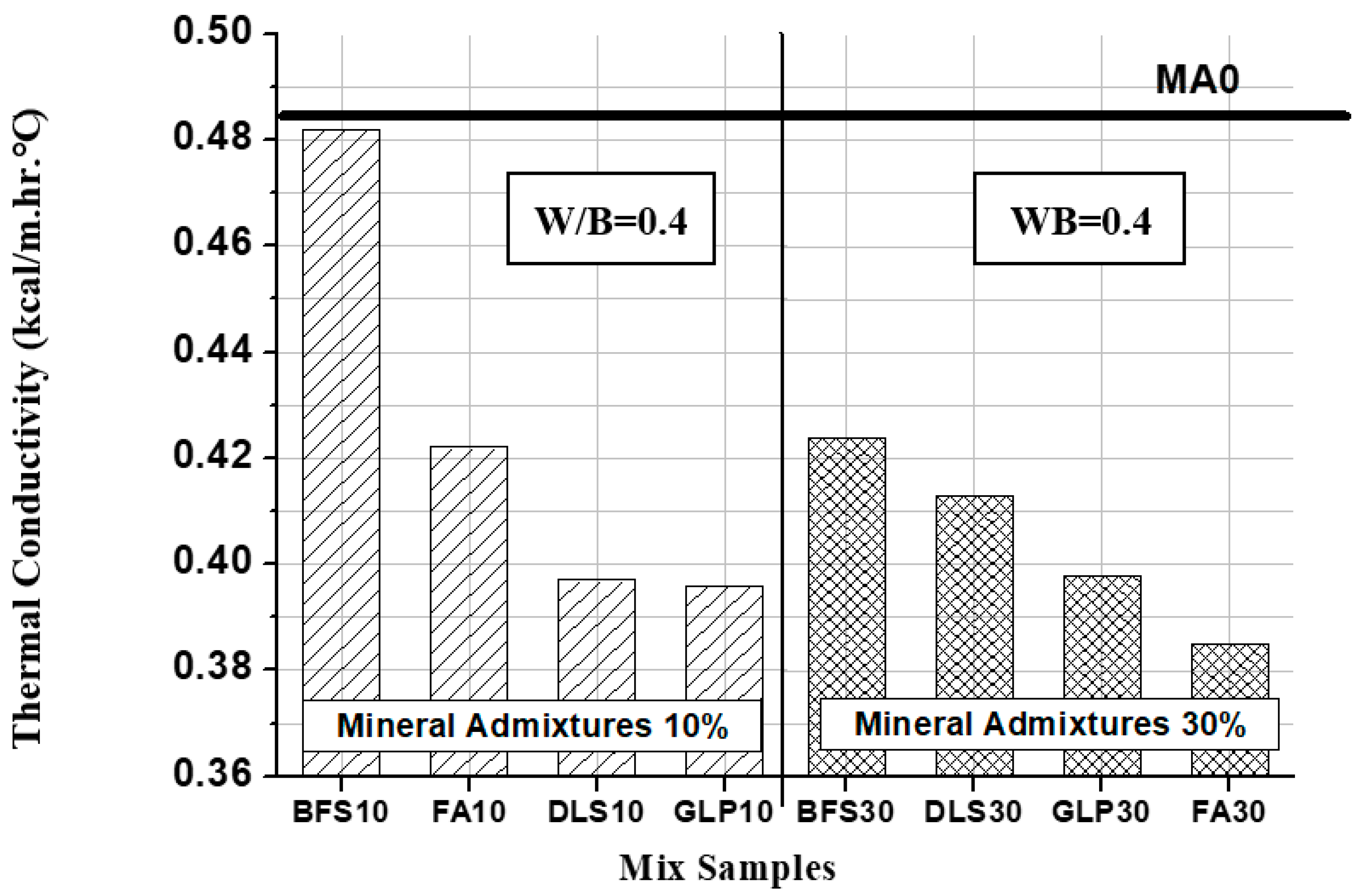
3.6. Thermal Conductivity Analysis
- (1)
- Changes due to mineral admixture
- (2)
- Differences in unit weight
- (3)
- Changes in the water absorption ratio
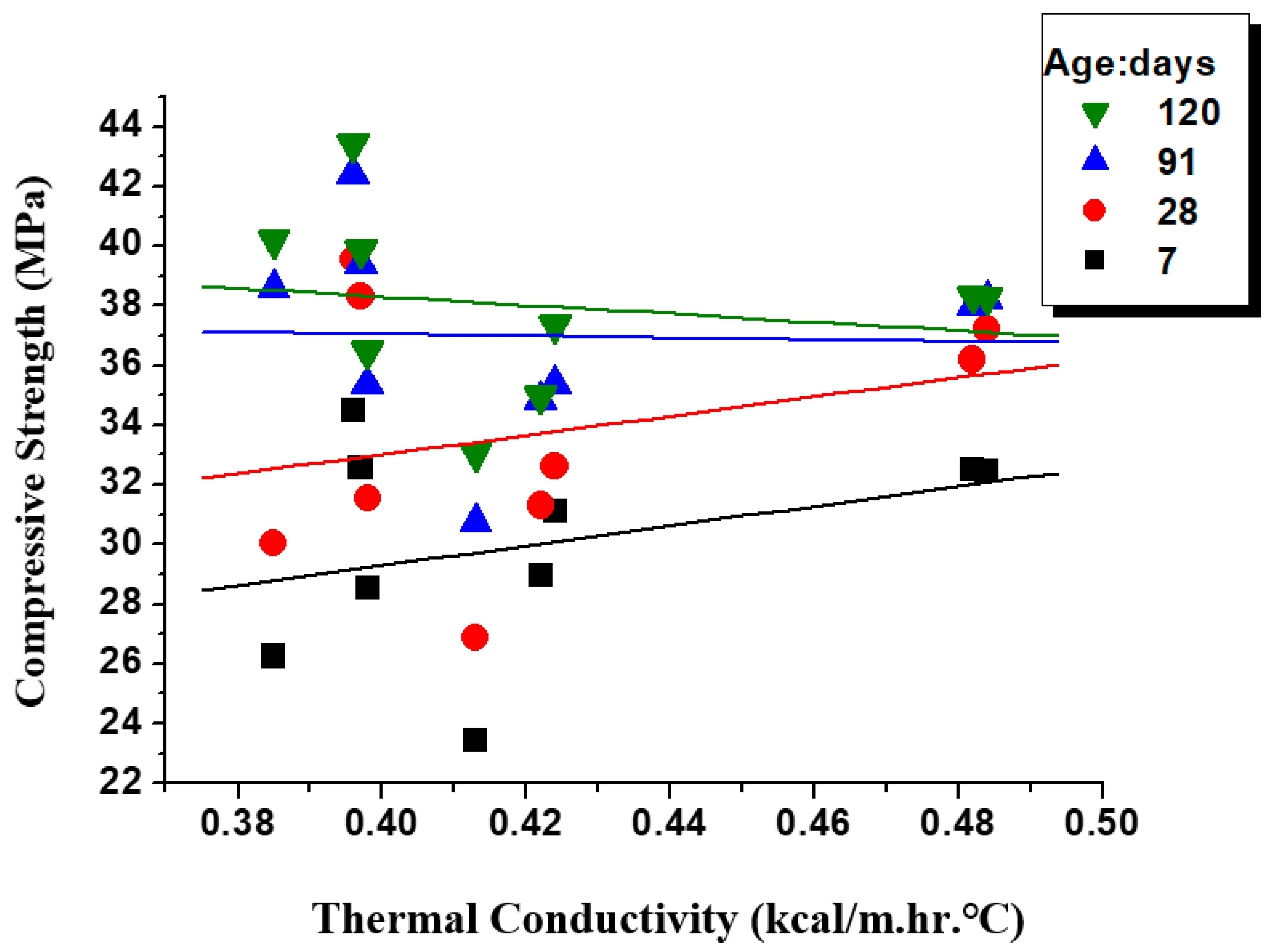
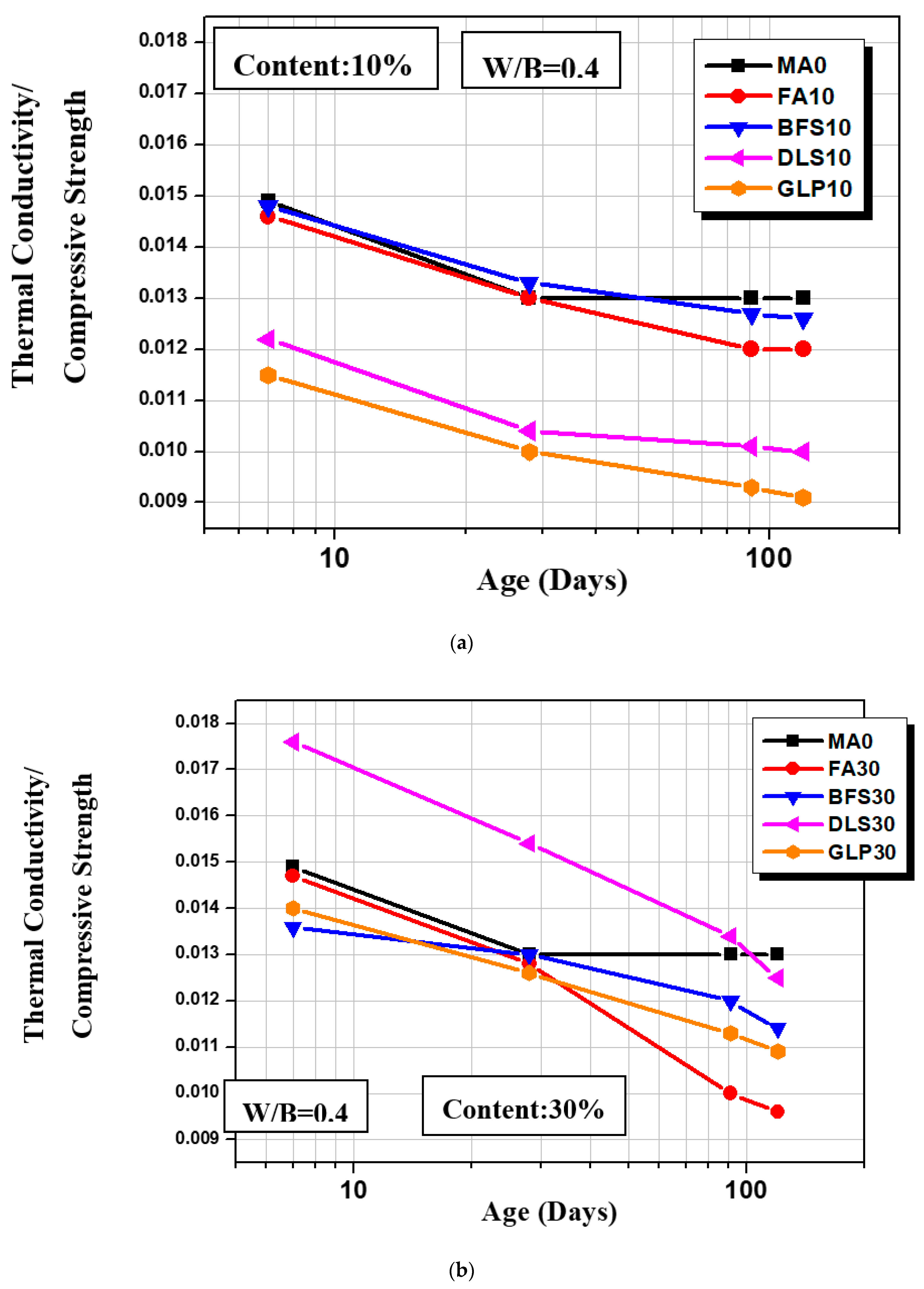
3.7. Relationship of Compressive Strength and Thermal Conductivity
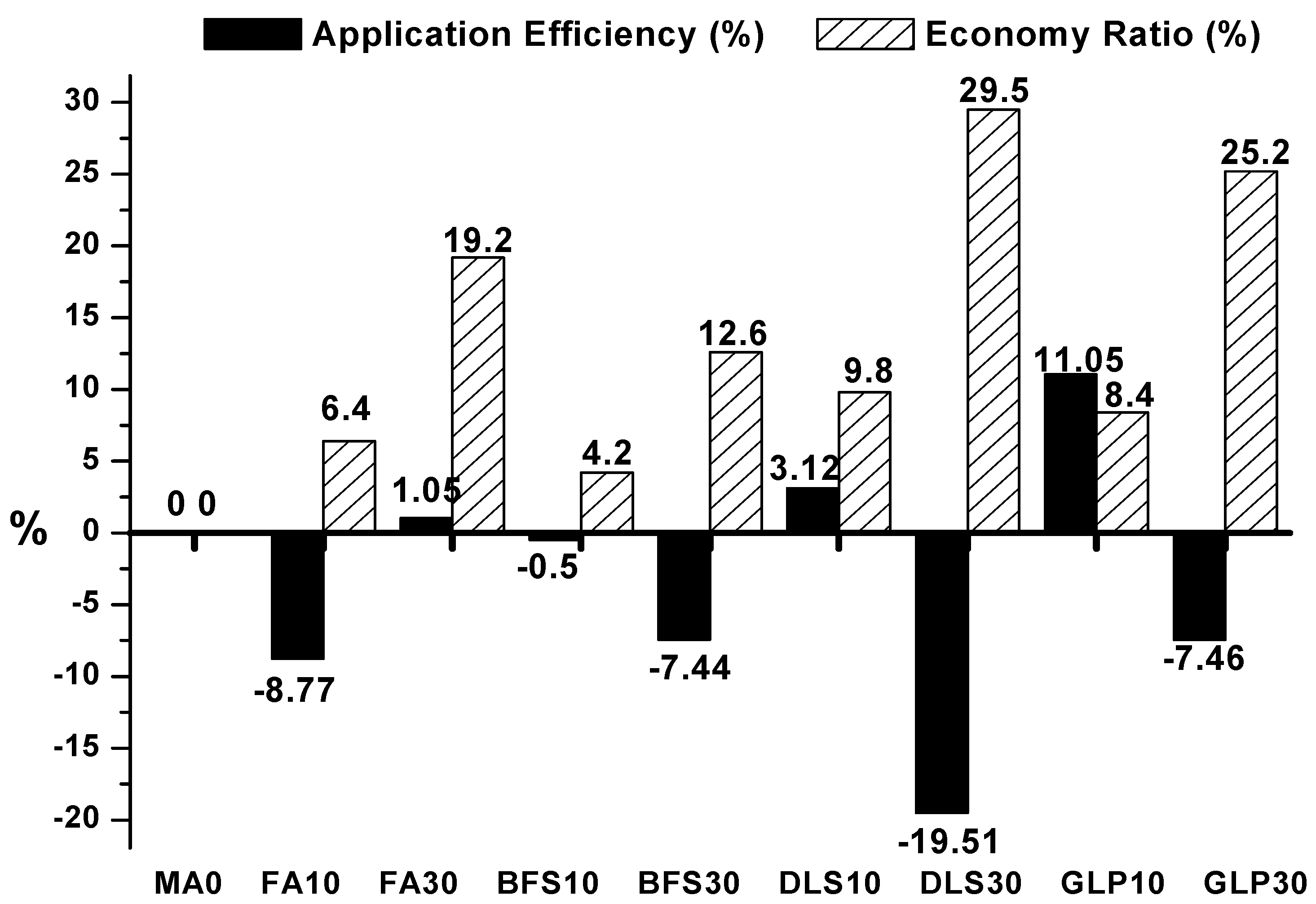
3.8. Application Efficiency of Mineral Admixtures
3.9. CO2 Reduction Benefit
4. Conclusions
- After adding various mineral admixtures, the slump was still above 75 mm. Some hydrated mineral admixtures may have increased the paste viscosity and thus undermined the slump; however, all samples complied with the designed slump of 75 mm–100 mm.
- The compressive strengths of FA10, BFS30, DLS30, and GLP30 at 28d were lower than that of the controlled sample (MA0), at 91%, 98%, 86%, and 95% of that of MAO, respectively. The compressive strengths of FA30, BFS10, DLS10, and GLP10 were higher than that of the controlled sample, at 105%, 100%, 104%, and 113% of that of MA0, respectively. It shows that the strength of lightweight aggregate concrete varies with the types and properties of mineral admixtures and the amount of addition.
- The addition of fly ash, slag, and glass powder contributes to resistivity; FA30 has the highest resistivity, which is 455% of the control group at the age of 120 days; GLP30 takes second place, 377% of the control group, because the two admixtures consume a lot of cement hydration product CH−, changing the ion concentration in concrete. The main reason is that these two admixtures consume a large amount of cement hydration product CH− and change the internal ion concentration of concrete.
- At 7 d, samples with 30% mineral admixtures had significantly lower reactivity indices, and their compressive strengths were also lower than those of samples with 10% mineral admixtures. After 28 d, except for FA10, pozzolan reactivity indices of samples with 30% mixture ratios were higher than those of samples with 10% mixture ratios, indicating that the strength is improved at later ages.
- Adding mineral admixtures to lightweight aggregate concrete could decrease the thermal conductivity K value by about 0.4–25.7%. FA30 exhibited the optimum heat insulation, which was 79.5% of that of the controlled sample, followed by GLP10, GLP30, and DLS10 (82%). FA10, BFS10, and BFS30 showed no significant effects, indicating that the mineral admixture hydration product properties have a significant effect on the thermal conductivity of lightweight aggregate concrete.
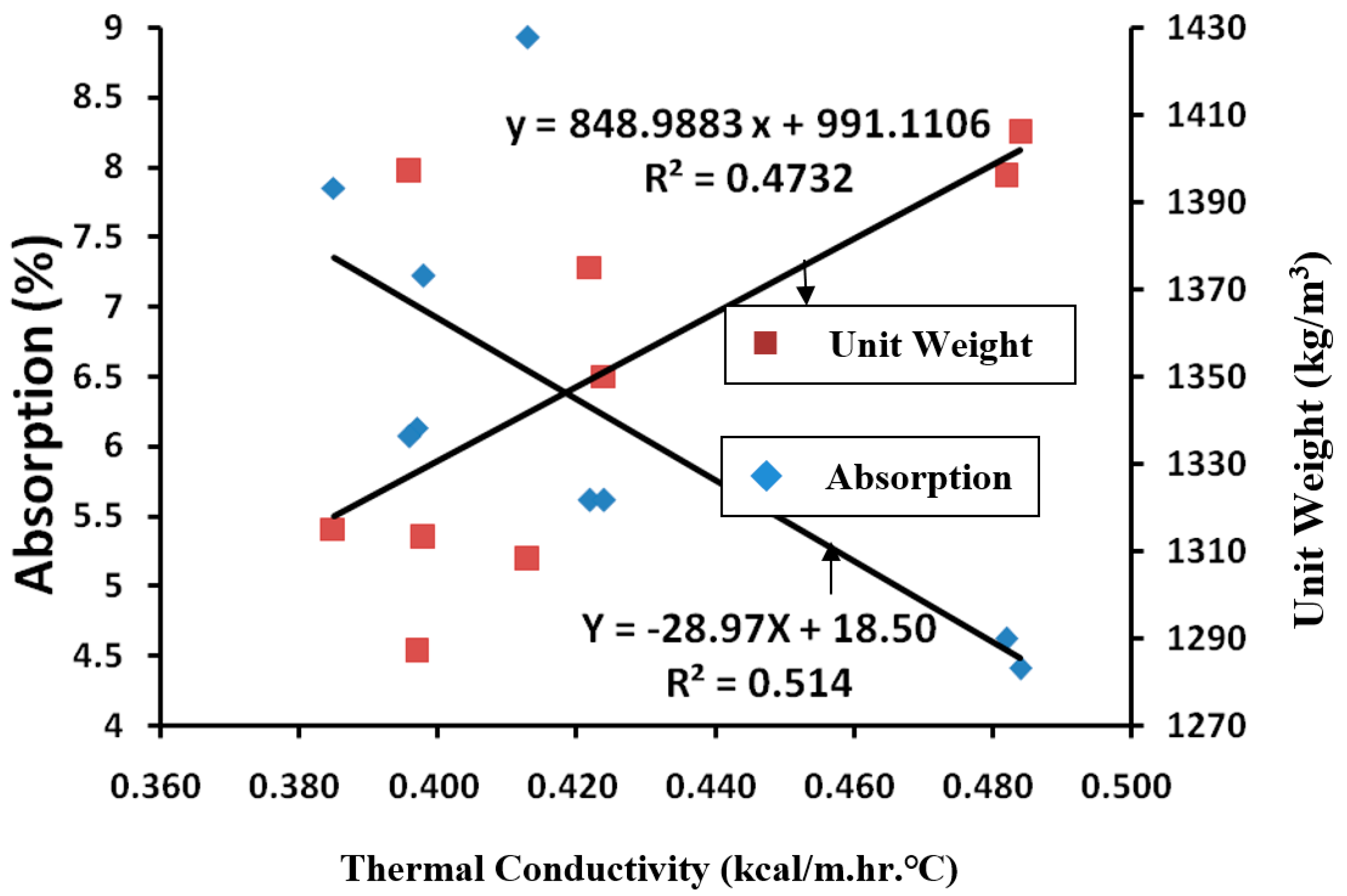
- MA0 have the highest unit weight and thermal conductivity, and FA30 was the lowest of both but had unit weight and thermal conductivity with a slight correlation of R2 = 0.4732. It shows that the properties of hydration products of mineral admixture have a great influence on the thermal conductivity of lightweight aggregate concrete.
- The controlled sample had the lowest water absorption ratio. A greater water absorption ratio would lead to greater porosity and smaller thermal conductivity. The water absorption ratios of samples with an addition of mineral admixtures were 105–202% of that of the controlled sample, indicating that samples with mineral admixtures have better heat insulation.
- Among the samples with the addition of 10% mineral admixtures, GLP10 showed a higher TC/CS ratio, with excellent heat insulation and compressive strength. As for samples with the addition of 30% mineral admixtures, their TC/CS ratios at 7d were less than that of MA0, but at a later age, their TC/CS ratios were higher than that of MA0. The TC/CS ratio of FA30 decreased most significantly.
- The addition of mineral admixtures to the lightweight aggregate concrete can reduce the cost by 4.2~29.5%; the cost saved by the mineral admixtures varies with the material source, processing mode, and market demand. The mineral admixture replacement ratio of 10–30% can reduce the CO2 emission rate by as high as 11.1–42.9%.
Author Contributions
Funding
Conflicts of Interest
References
- Wu, H.H. Discussing the Improvement of the Function of Outer Wall Heat Insulation from the Perspective of Energy-Conserving Architecture. Master’s Thesis, Department of Bioenvironmental Systems Engineering, National Taiwan University, Taipei, Taiwan, 2002. [Google Scholar]
- Dondi, M.; Cappelletti, P.; D’Amore, M.; de Gennaro, R.; Graziano, S.F.; Langella, A.; Raimondo, M.; Zanelli, C. Lightweight aggregates from waste materials: Reappraisal of expansion behavior and prediction schemes for bloating. Constr. Build. Mater. 2016, 127, 394–409. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; González-Corrochano, B.; Azcárate, J.A.; Anselmo Acosta, L.R. Assessment of crystalline phase changes and glass formation by Rietveld-XRD method on ceramic lightweight aggregates sintered from mineral and polymeric wastes. Ceram. Int. 2018, 44, 11840–11851. [Google Scholar] [CrossRef]
- Caprai, V.; Gauvin, F.; Schollbach, K.; Brouwers, H.J.H. MSWI bottom ash as binder replacement in wood cement composites. Constr. Build. Mater. 2019, 196, 672–680. [Google Scholar] [CrossRef]
- Tung-Tsan, C.; Wei-Chien, W.; Her-Yung, W. Mechanical properties and ultrasonic velocity of lightweight aggregate concrete containing mineral powder materials. Constr. Build. Mater. 2020, 258, 119–550. [Google Scholar]
- Wang, H.Y. Study on durability of densified high-performance lightweight aggregate concrete. J. Comput. Concr. 2007, 4, 499–510. [Google Scholar] [CrossRef]
- Wang, H.Y.; Tsai, K.C. Engineering properties of lightweight aggregate concrete made from dredged silt. Cem. Concr. Compos. 2006, 28, 481–485. [Google Scholar] [CrossRef]
- Rafat, S. Performance characteristics of high-volume Class F fly ash concrete. Cem. Concr. Res. 2008, 34, 487–493. [Google Scholar]
- Moripolou, A.; Bakolas, A.; Aggelakopoulou, E. Evaluation of pozzolanic activity of natural and artificial pozzolans by thermal analysis. Thermochem. Acta 2004, 420, 135–140. [Google Scholar] [CrossRef]
- Cabrera, J.; Rojas, M.F. Mechanism of hydration of the metakaolin–lime–water system. Cem. Concr. Res. 2001, 311, 77–182. [Google Scholar] [CrossRef]
- Roszczynialski, W. Determination of pozzolanic activity of materials by thermal analysis. J. Therm. Anal. Calorim 2002, 70, 387–392. [Google Scholar] [CrossRef]
- Ubbriaco, P.; Bruno, P.; Traini, A.; Calabrese, D. Fly ash reactivity- formation of hydrate phases. J. Therm. Anal. Calorim 2001, 66, 293–305. [Google Scholar] [CrossRef]
- Rojas, M.F.; Cabrera, J. The effect of temperature on the hydration rate and stability of the hydration phases of metakaolin–lime–water systems. Cem. Concr. Res. 2002, 32, 133–138. [Google Scholar] [CrossRef]
- Al-Jabri, K.S.; Hisada, M.; Al-Oraimi, S.K.; Al-Saidy, A.H. Copper slag as sand replacement for high performance concrete. Cem. Concr. Compos. 2009, 31, 483–488. [Google Scholar] [CrossRef]
- Bhikshma, V.; Nitturkar, K.; Venkatesham, Y. Investigations on mechanical properties of high strength silica fume concrete, Asian. J. Civ. Eng. 2009, 10, 335–346. [Google Scholar]
- Ghavidel, R.; Madandoust, R. Mechanical properties of concrete containing waste glass powder and rice husk ash. BioSyst. Eng. 2013, 116, 113–119. [Google Scholar]
- Standard Concrete Mix Proportioning-Guidelines; Indian IS 10262; Indian Standard: New Delhi, India, 2009.
- Kumar, G.V.; Vishaliny, M.H.; Govindarajulu, D. Studies on glass powder as partial replacement of cement in concrete production. J. Emerg. Technol. Adv. Eng. 2013, 3, 2250–2459. [Google Scholar]
- Madheswaran, C.K.; Abily, P.S.; Dattatreya, J.K.; Rajamane, N.P. Studies on the use of copper slag as replacement material for river sand in building constructions. J. Inst. Eng. Ind. Ser. A 2014, 95, 169–177. [Google Scholar] [CrossRef]
- Obilade, I.O. Use of rice husk ash as partial replacement for cement in concrete. J. Eng. Appl. Sci. 2014, 5, 11–16. [Google Scholar]
- Patil, D.M.; Sangle, K.K. Experimental investigation of waste glass powder as partial replacement of cement in concrete. J. Adv. Technol. Civ. Eng. 2013, 2, 112–117. [Google Scholar]
- Qamruddin, M.; Kalurkar, L.G. Effect of unprocessed rice husk ash as a cementitious material in concrete (a comparison with silica fume). J. Civ. Eng. Technol. 2013, 4, 240–245. [Google Scholar]
- Schwarz, N.; Cam, H.; Neithalath, N. Influence of a fine glass powder on the durability characteristics of concrete and its comparison to fly ash. Cem. Concr. Compos. 2008, 30, 486–496. [Google Scholar] [CrossRef]
- Shekhawat, B.S.; Aggarwal, V. Utilization of waste glass powder in a concrete-a literature review. J. Innovat. Res. Sci. Eng. Technol. 2014, 3, 14823–14826. [Google Scholar]
- Vasudevan, G.; Pillay, S.G.K. Performance of using waste glass powder in concrete as a replacement of cement. Am. J. Eng. Res. 2013, 2, 175–181. [Google Scholar]
- Wankhede1, P.R.; Fulari, V.A. Effect of fly ash on properties of concrete. J. Emerg. Technol. Adv. Eng. 2014, 4, 284–289. [Google Scholar]
- Wei, W.; Weide, Z.; Guowei, M. Optimum content of copper slag as a fine aggregate in high strength concrete. Mater. Des. 2010, 31, 2878–2883. [Google Scholar]
- Kurpinska, M.; Ferenc, T. Experimental and numerical investigation of mechanical properties of lightweight concretes (LWCs) with various aggregates. Materials 2020, 13, 3474. [Google Scholar] [CrossRef]
- Kou, S.C.; Xing, F. The effect of recycled glass powder and reject fly ash on the mechanical properties of fiber-reinforced ultralight performance concrete. Adv. Mater. Sci. Eng. 2012, 8. [Google Scholar] [CrossRef]
- Berge, B. The Ecology of Building Materials, 2nd ed.; Architectural Press-Elsevier: Cambridge, MA, USA, 2009. [Google Scholar]
- Pelisser, F.; Barcelos, A.; Santos, D.; Peterson, M.; Bernandin, A.M. Lightweight concrete production with low Portland cement consumption. J. Clean. Prod. 2012, 23, 68–74. [Google Scholar] [CrossRef]
- Akcaozoglu, S.; Atis, C.D. Effect of granulated blast furnace slag and fly ash addition on the strength properties of lightweight mortars containing waste PET aggregates. Constr. Build. Mater. 2011, 25, 4052–4058. [Google Scholar] [CrossRef]
- Kockal, N.U.; Ozturan, T. Strength and elastic properties of structural lightweight concretes. Mater. Des. 2011, 32, 2396–2403. [Google Scholar] [CrossRef]
- Ducman, V.; Mirtic, B. The applicability of different waste materials for the production of lightweight aggregates. Waste Manag. (Oxford) 2009, 29, 2361–2368. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, L.; Xu, J.; Li, Y. Mechanical properties of expanded polystyrene lightweight aggregate concrete and brick. Constr. Build. Mater. 2012, 1, 32–38. [Google Scholar] [CrossRef]
- Trtik, P.; Munch, B.; Weiss, W.J.; Kaestner, A.; Jerjen, I.; Josic, L.; Lehman, E.; Lura, P. Release of internal curing water from lightweight aggregates in cement paste investigated by neutron and X-ray tomography. Nucl. Instrum. Methods A 2011, 651, 244–249. [Google Scholar] [CrossRef]
- Madandoust, R.; Ranjbar, M.M.; Mousavi, S.Y. An investigation on the fresh properties of self-compacted lightweight concrete containing expanded polystyrene. Constr. Build. Mater. 2011, 25, 3721–3731. [Google Scholar] [CrossRef]
- Shannag, M.J. Characteristics of lightweight concrete containing mineral admixtures. Constr. Build. Mater. 2011, 25, 658–662. [Google Scholar] [CrossRef]
- Zhang, H. Building Materials in Civil Engineering; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- El-Gamal, S.M.A.; Hashem, F.S.; Amin, M.S. Thermal resistance of hardened cement pastes containing vermiculite and expanded vermiculite. J. Therm. Anal. Calorim 2012, 109, 217–226. [Google Scholar] [CrossRef]
- Koksal, F.; Gencel, O.; Brostow, W.; Lobland Hagg, H.E. Effect of high temperature on mechanical and physical properties of lightweight cement based refractory including expanded vermiculite. Mater. Res. Innov. 2012, 16, 7–13. [Google Scholar] [CrossRef]
- Hossain, K.M.A.; Ahmed, S.; Lachemi, M. Lightweight concrete incorporating pumice based blended cement and aggregate: Mechanical and durability characteristics. Constr. Build. Mater. 2011, 25, 1186–1195. [Google Scholar] [CrossRef]
- Castro, J.; Keiser, L.; Golias, M.; Weiss, J. Absorption and desorption properties of fine lightweight aggregate for application to internally cured concrete mixtures. Cem. Concr. Compos. 2011, 33, 1001–1008. [Google Scholar] [CrossRef]
- Sengul, O.; Azizi, S.; Karaosmanoglu, F.; Tasdemir, M.A. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 43, 671–676. [Google Scholar] [CrossRef]
- Ismail, A.; Elmaghraby, M.; Mekky, H. Engineering properties, microstructure and strength development of lightweight concrete containing pumice aggregates. Int. J. Dordr. 2012, 31, 1465–1476. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeon, J.H.; Lee, H.K. Workability, and mechanical, acoustic and thermal properties of lightweight aggregate concrete with a high volume of entrained air. Constr. Build. Mater. 2012, 29, 193–200. [Google Scholar] [CrossRef]
- Kismi, M.; Poullain, P.; Mounanga, P. Transient thermal response of lightweight cementitious composites made with polyurethane foam waste. Int. J. Thermophys. 2012, 33, 1239–1258. [Google Scholar] [CrossRef]
- Uzala, B.; Turanl, L.; Yücelc, H.; Göncüoğlu, M.C.; Çulfaz, A. Pozzolanic activity of clinoptilolite: A comparative study with silica fume, fly ash and a non-zeolitic natural pozzolan. Cem. Conr. Res. 2010, 40, 398–404. [Google Scholar] [CrossRef]
- Demirboga, R.; Kan, A. Thermal conductivity and shrinkage properties of modified waste polystyrene aggregate concretes. Constr. Build. Mater. 2012, 35, 730–734. [Google Scholar] [CrossRef]
- Pereira-de-Oliveira, L.A.; Castro-Gomes, J.P.; Santos, P.M.S. The potential pozzolanic activity of glass and red-clay ceramic waste as cement mortars components. Constr. Build. Mater. 2012, 31, 197–203. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Chemical reactions of glass cullet used as cement component. J. Mater. Civ. Eng. 2001, 13, 412–417. [Google Scholar] [CrossRef]
- Bajad, M.N.; Modhera, C.D.; Desai, A.K. Effect of glass on strength of concrete subjected to sulphate attack. Int. J. Civ. Eng. Res. Dev. 2011, 1, 1–13. [Google Scholar]
- Meena, A.; Singh, R. Comparative Study of Waste Glass Powder as Pozzolanic Material in Concrete. Bachelor’s Thesis, Department of Civil Engineering, National Institute of Technology, Rourkela, India, 2012; p. 46. [Google Scholar]
- Nassar, R.; Soroushian, P. Field investigation of concrete incorporating milled waste glass. J. Solid Waste Technol. Manag. 2011, 37, 307–319. [Google Scholar] [CrossRef]
- Federico, L. Waste Glass—A Supplementary Cementitious Material. Ph.D. Thesis, Department of Civil Engineering, McMaster University, Hamilton, ON, Canada, 2013; p. 99. [Google Scholar]
- ASTM Chemical Shrinkage of Hydraulic Cement Paste; ASTM C 1608; ASTM: Conshohocken, PA, USA, 2012; 5p.
- Sarkar, S.; Halder, A.; Bishnoi, S. Shrinkage in concretes containing fly ash. In Proceedings of the UKIERI Concrete Congress, Jalandhar, India, 2–5 March 2013. [Google Scholar]
- ASTM. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM C 109M; ASTM: Conshohocken, PA, USA, 2012; p. 10. [Google Scholar]
- ASTM. Measurement of Rate of Absorption of Water by Hydraulic Cement Concretes; ASTM C 1585; ASTM: Conshohocken, PA, USA, 2011. [Google Scholar]
- Habib, U.; Ramazan, D.; Remzi, S.; Rustem, G. The effects of different cement dosages, slumps, and pumice aggregate ratios on the thermal conductivity and density of concrete. Cem. Concr. Res. 2004, 34, 845–848. [Google Scholar]
- Ramazan, D.; Ibrahim, T.; Mehmet, B.K. Thermo-mechanical properties of concrete containing high-volume mineral admixtures. Build. Environ. 2007, 42, 349–354. [Google Scholar]
- Bederina, M.; Marmoret, L.; Mezreb, K.; Khenfer, M.M.; Bali, A. Que’neudec M. Effect of the addition of wood shavings on thermal conductivity of sand concretes: Experimental study and modeling. Constr. Build. Mater. 2007, 21, 662–668. [Google Scholar] [CrossRef]
- Ramazan, D. Thermal conductivity and compressive strength of concrete incorporation with mineral admixtures. Build. Environ. 2007, 42, 67–71. [Google Scholar]
- Kima, K.H.; Jeonb, S.E.; Kimb, J.K.; Sungchul, Y. An experimental study on thermal conductivity of concrete. Cem. Concr. Res. 2003, 33, 363–371. [Google Scholar] [CrossRef]
- Ahmad, S.; Aimin, X. Performance of glass powder as a pozzolanic material in concrete: A field trial on concrete slabs. Cem. Concr. Res. 2006, 36, 457–468. [Google Scholar]
- Hsu, T.H. Evolution of fly ash and bottom ash application and latest development technology. In Proceedings of the Taiwan Concrete Technology and Application Proseminar, Kaohsiung, Taiwan, 12 December 2006; pp. 165–184. [Google Scholar]
- Hwang, C.L. High Performance Concrete Theory and Practice; Chan’s Arch-Publishing Co: Bedford St, UK, 2017. [Google Scholar]
- Li, K.H.; Kao, P. Impact of Nano-silica powder SiO2 and CaCO3 on concrete property. J. China Railw. Soc. 2006, 28, 131–136. [Google Scholar]
- Lin, I.F. A Study on Fire Resistance of Concrete with Mix Lightweight Aggregates. Master’s Thesis, Department of Civil Engineering, Nation Chung Hsing University, Taichung, Taiwan, 2004. [Google Scholar]
- Shin, K.Y.; Kim, S.B.; Kim, J.H.; Chung, M.; Jung, P.S. Thermo-physical properties and transient heat transfer of concrete at elevated temperatures. Nuclear Eng. Des. 2002, 212, 223–241. [Google Scholar] [CrossRef]
- Weng, M.W. The Feasibility of Desulphurization/Granulated Slag Resources Recovery on the Controlled Low Strength Material without Portland Cement. Master’s Thesis, Department of Civil Engineering, National Kaohsiung University of Applied Sciences, Kaohsiung, Taiwan, 2007. [Google Scholar]
- Wu, J.Y. The Study on the Macro and Micro Property of the High-Silicon Powder Waste Glass Mortar. Master’s Thesis, Department of Civil Engineering, National Kaohsiung University of Applied Sciences, Kaohsiung, Taiwan, 2007. [Google Scholar]
- Ramazan, D.; Rustem, G. The effects of expanded perlite aggregate, silica fume and fly ash on the thermal conductivity of lightweight concrete. Cem. Concr. Res. 2003, 33, 723–727. [Google Scholar]
- Huang, S.L. Pozzolan Concrete Instruction Manual; Sinotech Engineering Consultants: Nanjing, China, 2007. [Google Scholar]




| Component | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | LoI | Specific Gravity (g/cm3) | Fineness (m2/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Cement (%) | 20.74 | 4.65 | 3.1 | 62.85 | 3.43 | 2.36 | 2.11 | 3.15 | 344 |
| FA (%) | 48.26 | 38.23 | 4.58 | 2.84 | 2.92 | 0.75 | 5.38 | 2.2 | 393 |
| BFS (%) | 35.47 | 13.71 | 0.33 | 41 | 6.6 | — | 0.95 | 2.9 | 417 |
| DLS (%) | 12.16 | 3.13 | 6.21 | 47.42 | — | — | — | 2.42 | 395 |
| GLP (%) | 62.48 | 16.76 | 9.41 | 2.7 | 0.2 | — | — | 2.46 | 385 |
| Specific Gravity | Water Absorption in 30 min (%) | Water Absorption in 24 h (%) | Maximum Size (in) | Finess Modulus (FM) | Drum Strength (MPa) | Dry-Rodded Unit Weight (kg/m3) | |
|---|---|---|---|---|---|---|---|
| Coarse Aggregates | 1.66 | 4.02 | 19.2 | 3/8 | 7.46 | 15.4 | 1104 |
| Fine Aggregates | 1.06 | 6.4 | 13.4 | 3/5 | 4.33 | — | 702 |
| Unit: kg/m3 | |||||
|---|---|---|---|---|---|
| Content (%) | Cement | Mineral Admixtures * | Water | Lightweight Aggregate | |
| Coarse Aggregates | Fine Aggregates | ||||
| 0 | 540 | — | 216 | 586 | 249 |
| 10 | 486 | 54 | 216 | 586 | 249 |
| 30 | 378 | 162 | 216 | 586 | 249 |
| Age (Days) | MA0 | FA * (%) | BFS * (%) | DLS * (%) | GLP * (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 30 | 10 | 30 | 10 | 30 | 10 | 30 | ||
| 7 | 32.5 | 29.0 | 26.3 | 32.5 | 31.1 | 32.6 | 23.4 | 34.5 | 28.5 |
| 28 | 37.2 | 31.3 | 30.1 | 36.2 | 32.6 | 38.3 | 26.9 | 39.5 | 31.6 |
| 91 | 38.2 | 34.8 | 38.6 | 38.0 | 35.4 | 39.4 | 30.7 | 42.4 | 35.3 |
| 120 | 38.3 | 35.0 | 40.2 | 38.3 | 37.4 | 39.9 | 33.0 | 43.4 | 36.5 |
| Slump (mm) | 95 | 100 | 110 | 85 | 75 | 80 | 70 | 85 | 75 |
| Sample | Cement(%) (1) | Compressive Strength (MPa) (2) | Strength/Cement Ratio (3) | Pozzolan Reaction (4) | Coefficient of Strength/Cement Ratio (5) | Offer of Pozzolan (6) | Indicator of Pozzolan Activity (7) | |
|---|---|---|---|---|---|---|---|---|
| 7 day | MA0 | 100 | 32.48 | 0.325 | 0 | 1 | 0 | — |
| FA10 | 90 | 28.96 | 0.322 | −0.003 | 0.990 | −1.0 | −0.10 | |
| FA30 | 70 | 26.26 | 0.375 | 0.050 | 1.154 | 13.4 | 0.45 | |
| BFS10 | 90 | 32.51 | 0.361 | 0.036 | 1.111 | 10.0 | 1.00 | |
| BFS30 | 70 | 31.11 | 0.444 | 0.119 | 1.367 | 26.9 | 0.90 | |
| DLS10 | 90 | 32.59 | 0.362 | 0.037 | 1.114 | 10.2 | 1.02 | |
| DLS30 | 70 | 23.43 | 0.335 | 0.010 | 1.030 | 2.9 | 0.10 | |
| GLP10 | 90 | 34.53 | 0.384 | 0.059 | 1.181 | 15.3 | 1.53 | |
| GLP30 | 70 | 28.53 | 0.408 | 0.083 | 1.254 | 20.3 | 0.68 | |
| 28 day | MA0 | 100 | 37.21 | 0.372 | 0 | 1 | 0 | — |
| FA10 | 90 | 31.31 | 0.348 | 0.023 | 1.070 | 6.6 | 0.66 | |
| FA30 | 70 | 30.05 | 0.429 | 0.104 | 1.321 | 24.3 | 0.81 | |
| BFS10 | 90 | 36.2 | 0.402 | 0.077 | 1.238 | 19.2 | 1.92 | |
| BFS30 | 70 | 32.63 | 0.466 | 0.141 | 1.434 | 30.3 | 1.01 | |
| DLS10 | 90 | 38.29 | 0.425 | 0.100 | 1.309 | 23.6 | 2.36 | |
| DLS30 | 70 | 26.87 | 0.384 | 0.059 | 1.181 | 15.3 | 0.51 | |
| GLP10 | 90 | 39.54 | 0.439 | 0.114 | 1.352 | 26.0 | 2.60 | |
| GLP30 | 70 | 31.56 | 0.451 | 0.126 | 1.387 | 27.9 | 0.93 | |
| Note | (2)/(1) | (3)-0.325 | (3)/0.325 | (4)/(3) | (6)/[100-(1)] | |||
| (3)-0.372 | (3)/0.372 | |||||||
| Sample | Cement(%) (1) | Compressive Strength (MPa) (2) | Strength/Cement Ratio (3) | Pozzolan Reaction (4) | Coefficient of Strength/Cement Ratio (5) | Offer of Pozzolan (6) | Indicator of Pozzolan Activity (7) | |
| 91 day | MA0 | 100 | 38.19 | 0.382 | 0 | 1 | 0 | — |
| FA10 | 90 | 34.84 | 0.387 | 0.005 | 1.013 | 1.3 | 0.13 | |
| FA30 | 70 | 38.59 | 0.551 | 0.169 | 1.443 | 30.7 | 1.02 | |
| BFS10 | 90 | 38 | 0.422 | 0.040 | 1.105 | 9.5 | 0.95 | |
| BFS30 | 70 | 35.35 | 0.505 | 0.123 | 1.322 | 24.4 | 0.81 | |
| DLS10 | 90 | 39.38 | 0.438 | 0.056 | 1.145 | 12.7 | 1.27 | |
| DLS30 | 70 | 30.74 | 0.439 | 0.057 | 1.150 | 13.0 | 0.43 | |
| GLP10 | 90 | 42.41 | 0.471 | 0.089 | 1.234 | 18.9 | 1.89 | |
| GLP30 | 70 | 35.34 | 0.505 | 0.123 | 1.322 | 24.3 | 0.81 | |
| 120 day | MA0 | 100 | 38.28 | 0.383 | 0 | 1 | 0 | — |
| FA10 | 90 | 34.96 | 0.388 | 0.005 | 1.014 | 1.4 | 0.14 | |
| FA30 | 70 | 40.19 | 0.574 | 0.191 | 1.499 | 33.3 | 1.11 | |
| BFS10 | 90 | 38.34 | 0.426 | 0.043 | 1.112 | 10.1 | 1.01 | |
| BFS30 | 70 | 37.35 | 0.534 | 0.151 | 1.393 | 28.2 | 0.94 | |
| DLS10 | 90 | 39.89 | 0.443 | 0.060 | 1.157 | 13.6 | 1.36 | |
| DLS30 | 70 | 33.04 | 0.472 | 0.089 | 1.232 | 18.9 | 0.63 | |
| GLP10 | 90 | 43.43 | 0.483 | 0.100 | 1.260 | 20.6 | 2.06 | |
| GLP30 | 70 | 36.49 | 0.521 | 0.138 | 1.361 | 26.5 | 0.88 | |
| Note | (2)/(1) | (3)-0.382 | (3)/0.382 | (4)/(3) | (6)/[100-(1)] | |||
| (3)-0.383 | (3)/0.383 |
| Sample | MA0 | FA (%) | BFS (%) | DLS (%) | GLP (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 30 | 10 | 30 | 10 | 30 | 10 | 30 | ||
| TC (kcal/m.h. °C) | 0.484 | 0.422 | 0.385 | 0.482 | 0.424 | 0.397 | 0.413 | 0.396 | 0.398 |
| Unit Weight (kg/m3) | 1406 | 1375 | 1315 | 1396 | 1350 | 1287 | 1308 | 1397 | 1313 |
| Absorption (%) | 4.41 | 5.61 | 7.85 | 4.62 | 5.61 | 6.13 | 8.93 | 6.07 | 7.22 |
| NO. | Cement (kg/m3) | Mineral Admixtures (kg/m3) | CSE (MPa/kg) (1) * | Application Efficiency (%) (2) * | |||
|---|---|---|---|---|---|---|---|
| 7 day | 28 day | 91 day | 120 day | ||||
| MA0 | 540 | 0 | 0.060 | 0.069 | 0.071 | 0.071 | — |
| FA10 | 486 | 54 | 0.060 | 0.064 | 0.072 | 0.072 | −8.77 |
| FA30 | 378 | 162 | 0.069 | 0.079 | 0.102 | 0.106 | +1.05 |
| BFS10 | 486 | 54 | 0.067 | 0.074 | 0.078 | 0.079 | −0.50 |
| BFS30 | 378 | 162 | 0.082 | 0.086 | 0.094 | 0.099 | −7.44 |
| DLS10 | 486 | 54 | 0.067 | 0.079 | 0.081 | 0.082 | +3.12 |
| DLS30 | 378 | 162 | 0.062 | 0.071 | 0.081 | 0.087 | −19.51 |
| GLP10 | 486 | 54 | 0.071 | 0.081 | 0.087 | 0.089 | +11.05 |
| GLP30 | 378 | 162 | 0.075 | 0.083 | 0.093 | 0.097 | −7.46 |
| No. | Cement (kg/m3) | Mineral Admixtures (kg/m3) | Price of Cementitious Materials (NTD/m3) (1) | Economy (NTD) (2) | Economy Ratio (%) (3) | |
|---|---|---|---|---|---|---|
| Cement | Mineral Admixtures | |||||
| MA0 | 540 | 0 | 1350 | 0 | — | — |
| FA10 | 486 | 54 | 1215 | 48.6 | 86.4 | 6.4 |
| FA30 | 378 | 162 | 945 | 145.8 | 259.2 | 19.2 |
| BFS10 | 486 | 54 | 1215 | 78.3 | 56.7 | 4.2 |
| BFS30 | 378 | 162 | 945 | 234.9 | 170.1 | 12.6 |
| DLS10 | 486 | 54 | 1215 | 2.16 | 133 | 9.8 |
| DLS30 | 378 | 162 | 945 | 6.48 | 398.5 | 29.5 |
| GLP10 | 486 | 54 | 1215 | 21.6 | 113.4 | 8.4 |
| GLP30 | 378 | 162 | 945 | 64.8 | 340.2 | 25.2 |
| W/C | W/B | The Ratio of Mineral Admixtures Content (%) | Cement Content (kg/m3) | Mineral Admixtures Content (kg/m3) | Reduced CO2 Emission (ton/m3) | Reduced Ratio of CO2 (%/per m3 con.) |
|---|---|---|---|---|---|---|
| 0.40 | 0.40 | 0 | 540 | 0 | — | — |
| 0.44 | 0.40 | 10 | 486 | 54 | 0.0459 | 11.1 |
| 0.57 | 0.40 | 30 | 378 | 162 | 0.1377 | 42.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-N.; Chen, T.-T.; Hung, C.-C.; Wang, H.-Y. Evaluation of Heat Insulation and Surface Resistivity of Mineral Lightweight Aggregate Concrete(MLAC). Appl. Sci. 2020, 10, 7871. https://doi.org/10.3390/app10217871
Chang J-N, Chen T-T, Hung C-C, Wang H-Y. Evaluation of Heat Insulation and Surface Resistivity of Mineral Lightweight Aggregate Concrete(MLAC). Applied Sciences. 2020; 10(21):7871. https://doi.org/10.3390/app10217871
Chicago/Turabian StyleChang, Jung-Nan, Tung-Tsan Chen, Chang-Chi Hung, and Her-Yung Wang. 2020. "Evaluation of Heat Insulation and Surface Resistivity of Mineral Lightweight Aggregate Concrete(MLAC)" Applied Sciences 10, no. 21: 7871. https://doi.org/10.3390/app10217871
APA StyleChang, J.-N., Chen, T.-T., Hung, C.-C., & Wang, H.-Y. (2020). Evaluation of Heat Insulation and Surface Resistivity of Mineral Lightweight Aggregate Concrete(MLAC). Applied Sciences, 10(21), 7871. https://doi.org/10.3390/app10217871






