Tracking the Local Structure Change during the Photoabsorption Processes of Photocatalysts by the Ultrafast Pump-Probe XAFS Method
Abstract
Featured Application
Abstract
1. Introduction
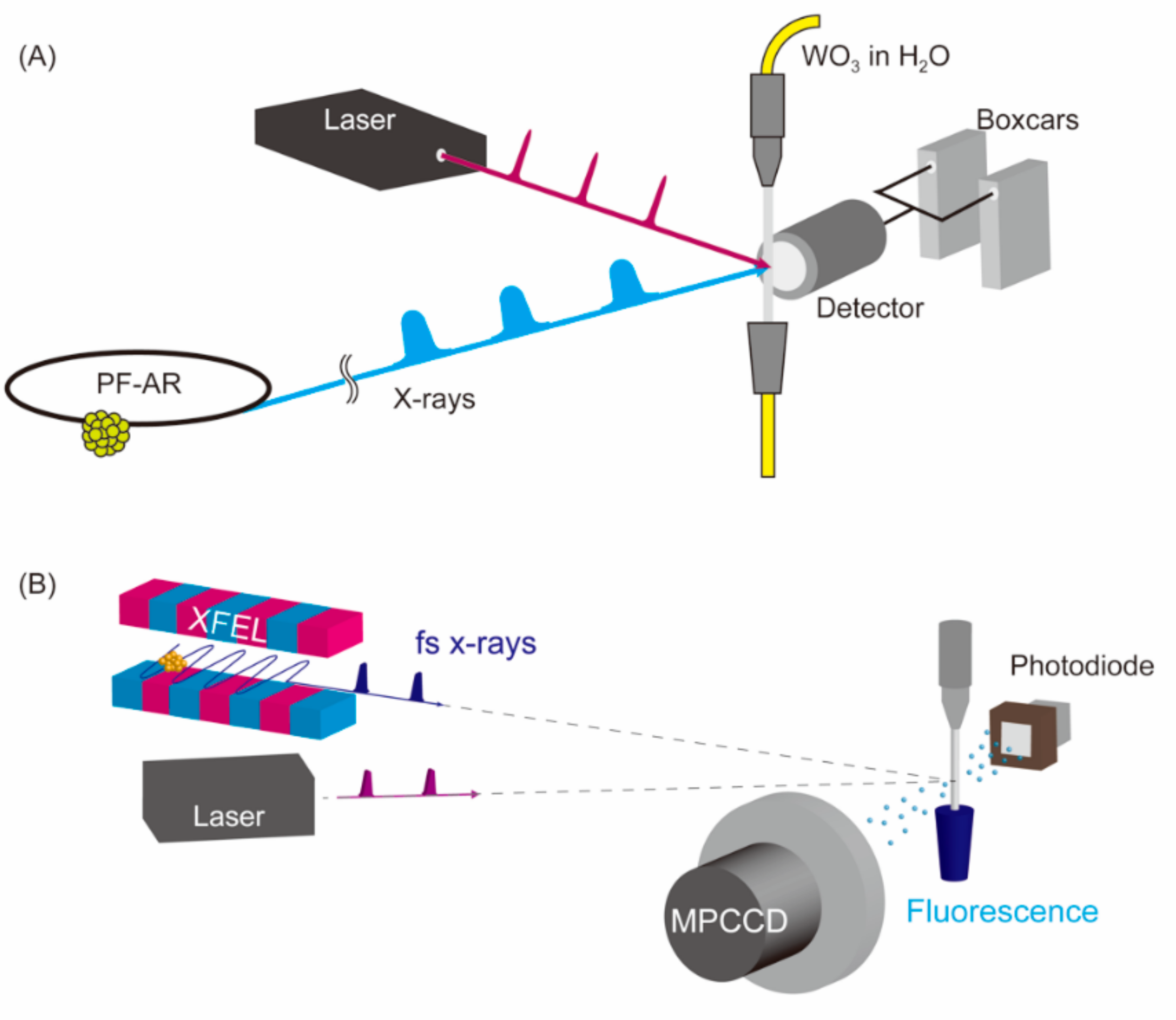
2. Materials and Methods
3. Results
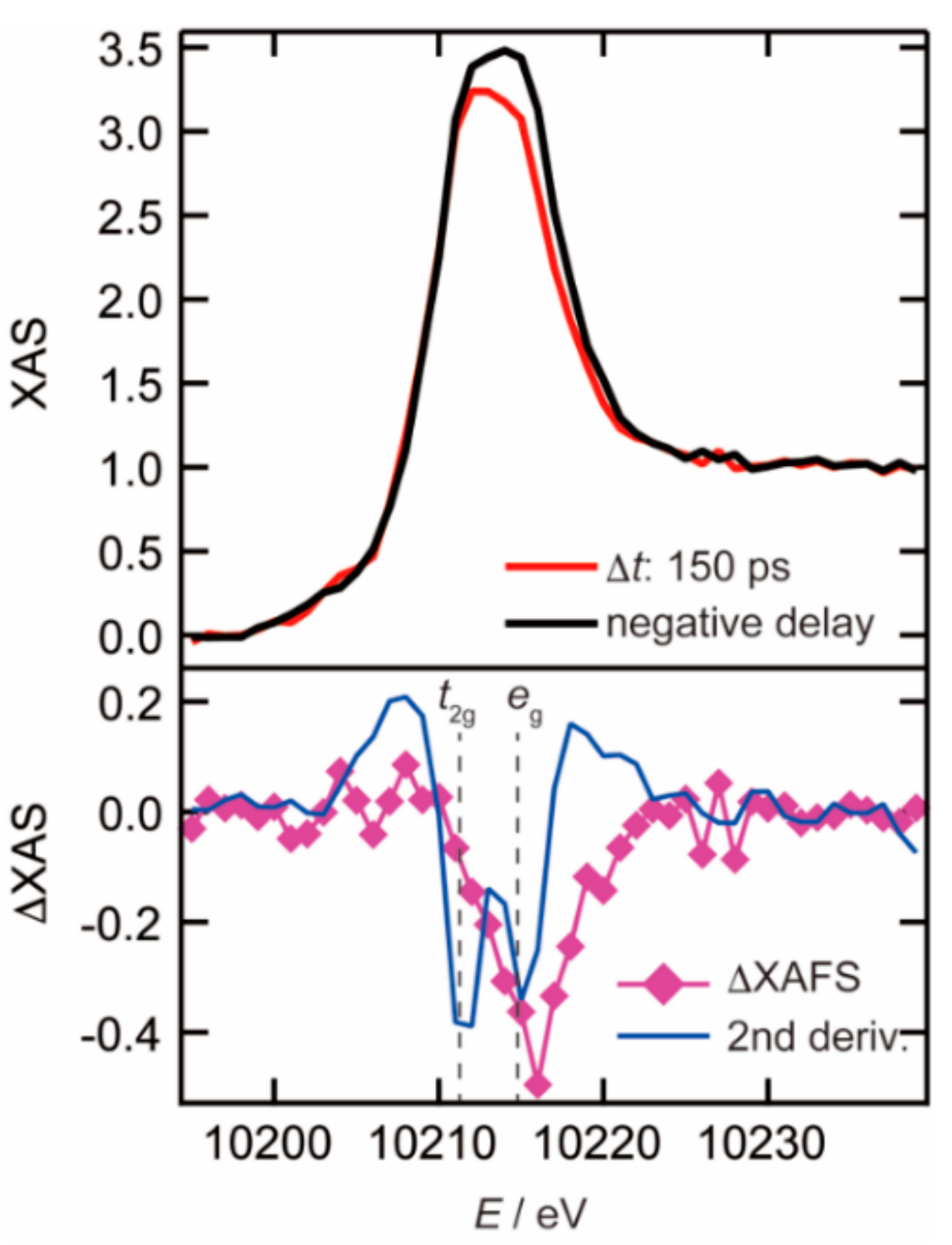
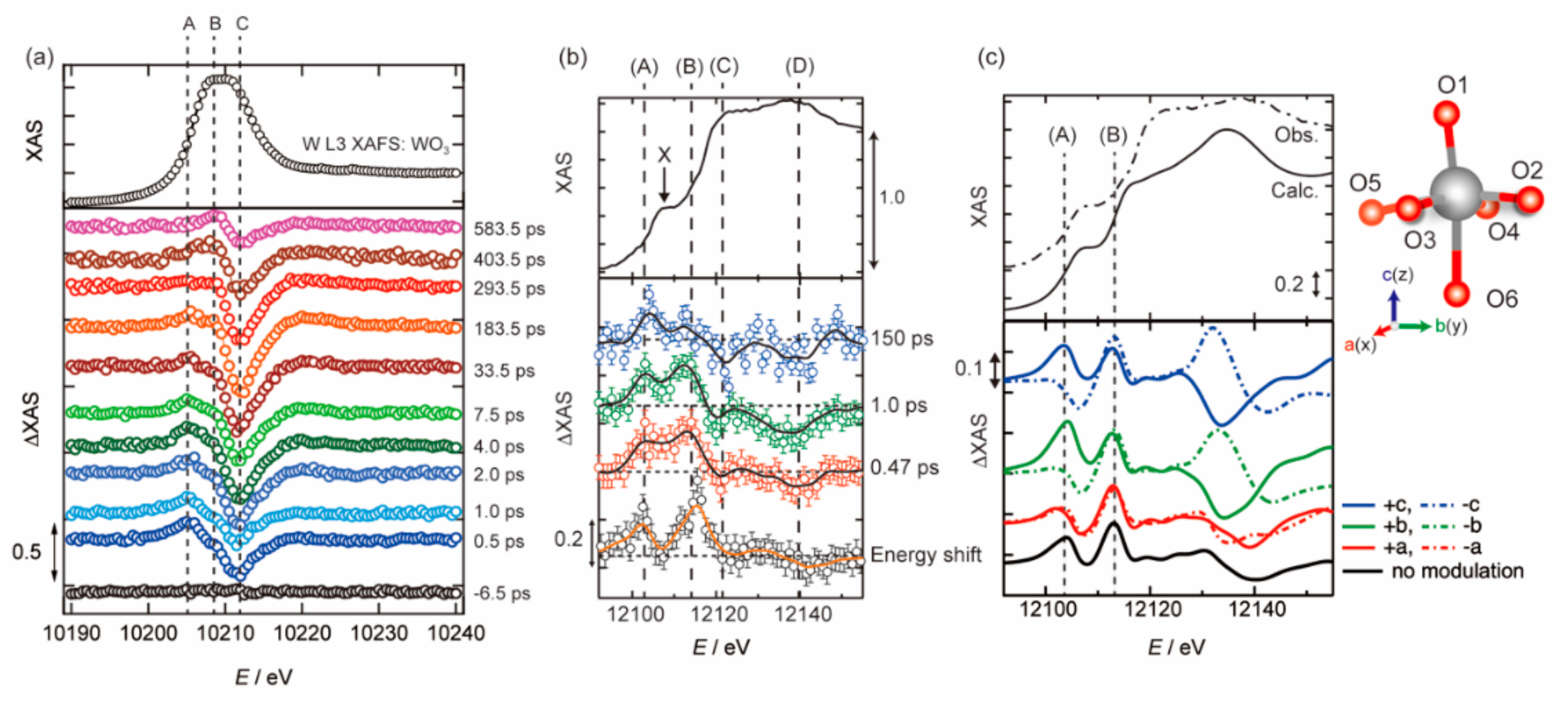
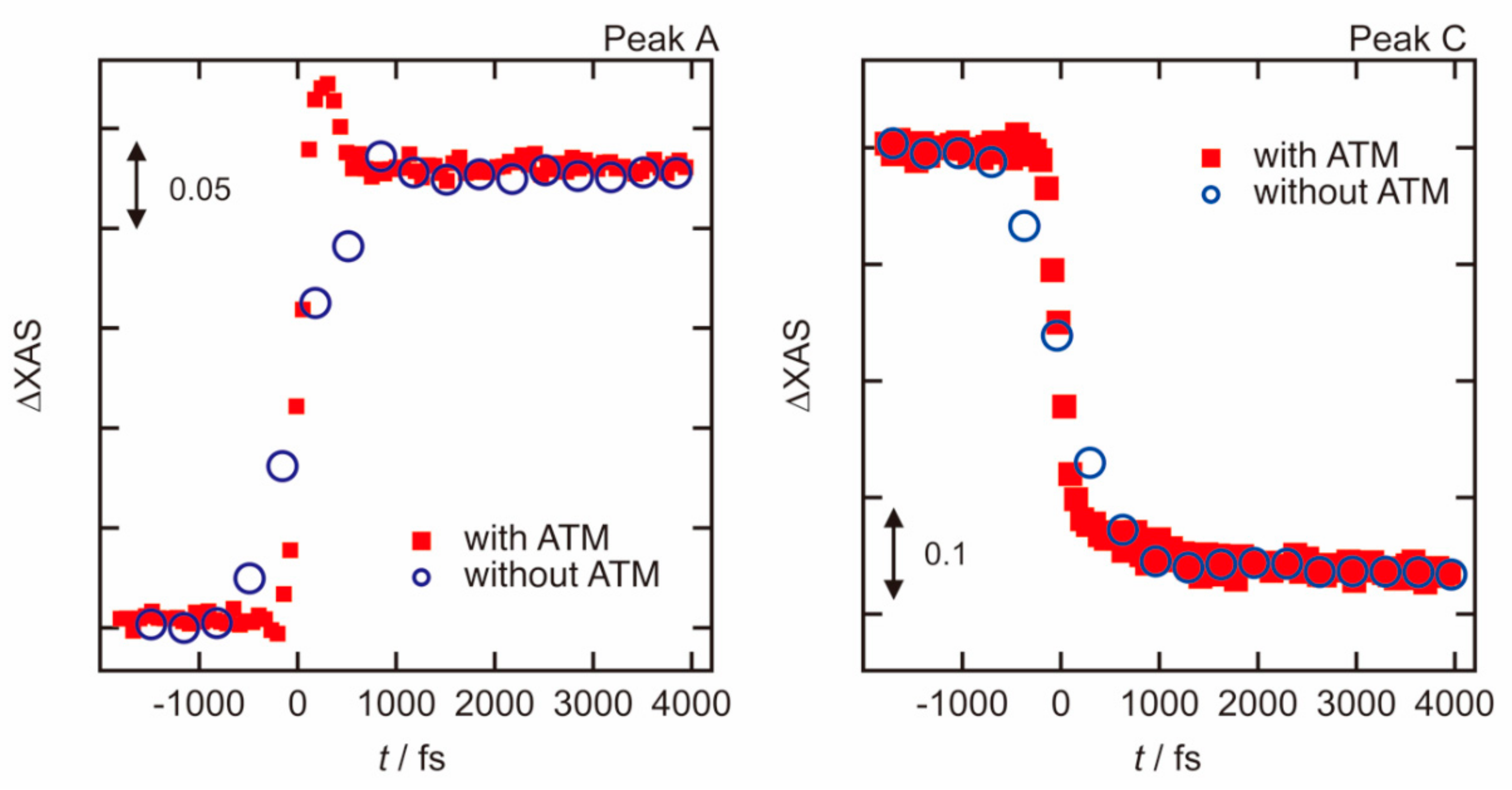
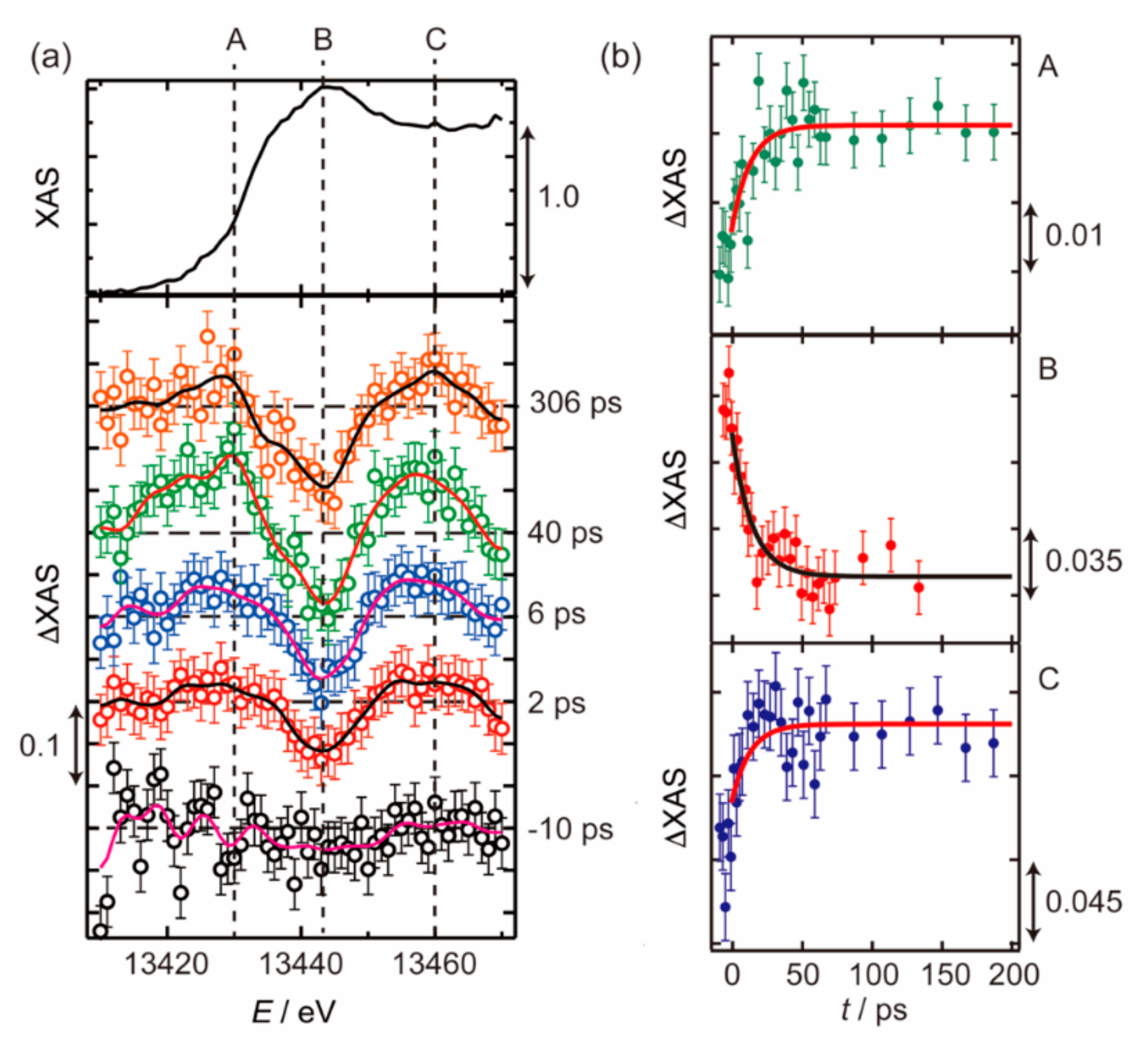
3.1. Photoexcited States of WO3 in Femtosecond and Nanosecond Regions
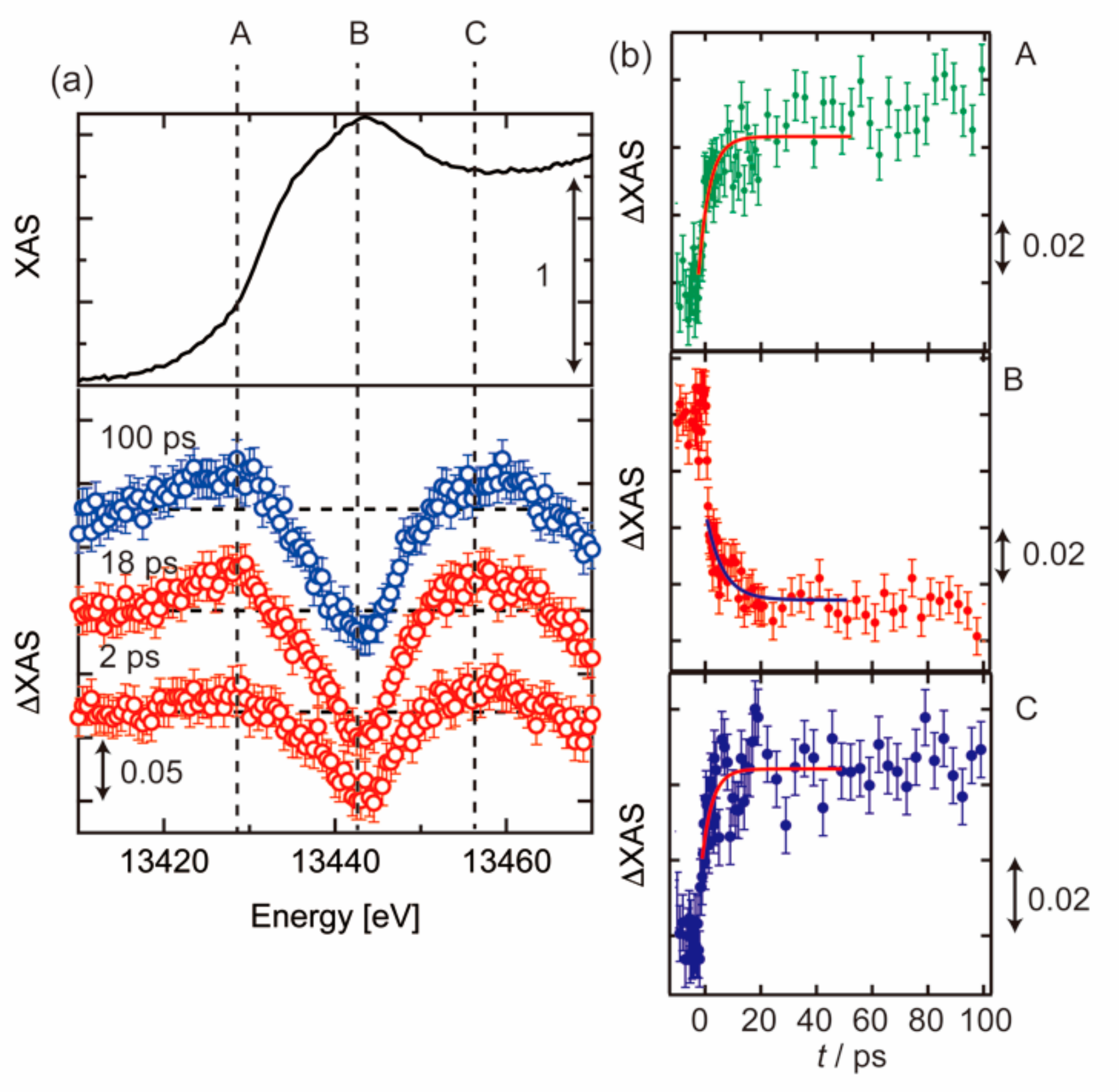
3.2. Photoexcited States of BiVO4
4. Summary and Perspective
4.1. Photoexcited States of Photocatalysts
4.2. Advantage of Pump-Probe XAFS Techniques and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frahm, R. New method for time dependent X-ray absorption studies. Rev. Sci. Instrum. 1989, 60, 2515. [Google Scholar] [CrossRef]
- Matsushita, T.; Phizackerley, R.P. A Fast X-ray Absorption Spectrometer for Use with Synchrotron Radiation. Jpn. J. Appl. Phys. 1981, 20, 2223–2228. [Google Scholar] [CrossRef]
- Sekizawa, O.; Uruga, T.; Takagi, Y.; Nitta, K.; Kato, K.; Tanida, H.; Uesugi, K.; Hoshino, M.; Ikenaga, E.; Takeshita, K.; et al. SPring-8 BL36XU: Catalytic Reaction Dynamics for Fuel Cells. J. Phys. Conf. Ser. 2016, 712, 012142. [Google Scholar] [CrossRef]
- Nachtegaal, M.; Müller, O.; König, C.; Frahm, R. QEXAFS: Techniques and Scientific Applications for Time-Resolved XAS. X-ray Absorpt. X-ray Emiss. Spectrosc. 2016, 155–183. [Google Scholar] [CrossRef]
- Smolentsev, G.; Guda, A.; Zhang, X.; Haldrup, K.; Andreiadis, E.S.; Chavarot-Kerlidou, M.; Canton, S.E.; Nachtegaal, M.; Artero, V.; Sundstrom, V. Pump-Flow-Probe X-ray Absorption Spectroscopy as a Tool for Studying Intermediate States of Photocatalytic Systems. J. Phys. Chem. C 2013, 117, 17367–17375. [Google Scholar] [CrossRef]
- Thiel, D.J.; Līviņš, P.; Stern, E.A.; Lewis, A. Microsecond-resolved XAFS of the triplet excited state of Pt2(P2O5H2)4−4. Nature 1993, 362, 40–43. [Google Scholar] [CrossRef]
- Mills, D.; Lewis, A.; Harootunian, A.; Huang, J.; Smith, B. Time-resolved X-ray absorption spectroscopy of carbon monoxide-myoglobin recombination after laser photolysis. Science 1984, 223, 811–813. [Google Scholar] [CrossRef]
- Pham, V.-T.; Gawelda, W.; Zaushitsyn, Y.; Kaiser, M.; Grolimund, D.; Johnson, S.L.; Abela, R.; Bressler, C.; Chergui, M. Observation of the Solvent Shell Reorganization around Photoexcited Atomic Solutes by Picosecond X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 2007, 129, 1530–1531. [Google Scholar] [CrossRef]
- Nozawa, S.; Adachi, S.; Takahashi, J.I.; Tazaki, R.; Guérin, L.; Daimon, M.; Tomita, A.; Sato, T.; Chollet, M.; Collet, E. Developing 100 ps-resolved X-ray structural analysis capabilities on beamline NW14A at the Photon Factory Advanced Ring. J. Synchrotron Radiat. 2007, 14, 313–319. [Google Scholar] [CrossRef]
- Saes, M.; Bressler, C.; Abela, R.; Grolimund, D.; Johnson, S.L.; Heimann, P.A.; Chergui, M. Observing Photochemical Transients by Ultrafast X-Ray Absorption Spectroscopy. Phys. Rev. Lett. 2003, 90, 047403. [Google Scholar] [CrossRef]
- Jennings, G.; Jäger, W.J.H.; Chen, L.X. Application of a multi-element Ge detector in laser pump/x-ray probe time-domain x-ray absorption fine structure. Rev. Sci. Instrum. 2002, 73, 362. [Google Scholar] [CrossRef]
- Chen, L.X.; Jäger, W.J.H.; Jennings, G.; Gosztola, D.J.; Munkholm, A.; Hessler, J.P. Capturing a Photoexcited Molecular Structure Through Time-Domain X-ray Absorption Fine Structure. Science 2001, 292, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Chergui, M.; Collet, E. Photoinduced Structural Dynamics of Molecular Systems Mapped by Time-Resolved X-ray Methods. Chem. Rev. 2017, 117, 11025–11065. [Google Scholar] [CrossRef]
- Smolentsev, G.; Milne, C.J.; Guda, A.; Haldrup, K.; Szlachetko, J.; Azzaroli, N.; Cirelli, C.; Knopp, G.; Bohinc, R.; Menzi, S.; et al. Taking a snapshot of the triplet excited state of an OLED organometallic luminophore using X-rays. Nat. Commun. 2020, 11, 2131. [Google Scholar] [CrossRef]
- Penfold, T.J.; Szlachetko, J.; Santomauro, F.G.; Britz, A.; Gawelda, W.; Doumy, G.; March, A.M.; Southworth, S.H.; Rittmann, J.; Abela, R.; et al. Revealing hole trapping in zinc oxide nanoparticles by time-resolved X-ray spectroscopy. Nat. Commun. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Parchenko, S.; Paris, E.; McNally, D.; Abreu, E.; Dantz, M.; Bothschafter, E.M.; Reid, A.H.; Schlotter, W.F.; Lin, M.-F.; Wandel, S.F.; et al. Orbital dynamics during an ultrafast insulator to metal transition. Phys. Rev. Res. 2020, 2, 023110. [Google Scholar] [CrossRef]
- Ismail, A.S.M.; Uemura, Y.; Park, S.H.; Kwon, S.; Kim, M.; Elnaggar, H.; Frati, F.; Niwa, Y.; Wadati, H.; Hirata, Y.; et al. Direct observation of the electronic states of photoexcited hematite with ultrafast 2p3d X-ray absorption spectroscopy and resonant inelastic X-ray scattering. Phys. Chem. Chem. Phys. 2020, 22, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Wernet, P.; Kunnus, K.; Josefsson, I.; Rajkovic, I.; Quevedo, W.; Beye, M.; Schreck, S.; Grubel, S.; Scholz, M.; Nordlund, D.; et al. Orbital-specific mapping of the ligand exchange dynamics of Fe(CO)5 in solution. Nature 2015, 520, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Koide, A.; Uemura, Y.; Kido, D.; Wakisaka, Y.; Takakusagi, S.; Ohtani, B.; Niwa, Y.; Nozawa, S.; Ichiyanagi, K.; Fukaya, R.; et al. Photoinduced anisotropic distortion as the electron trapping site of tungsten trioxide by ultrafast W L1-edge X-ray absorption spectroscopy with full potential multiple scattering calculations. Phys. Chem. Chem. Phys. 2020, 22, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Uehara, H.; Niwa, Y.; Nozawa, S.; Sato, T.; Adachi, S.; Ohtani, B.; Takakusagi, S.; Asakura, K. In Situ Picosecond XAFS Study of an Excited State of Tungsten Oxide. Chem. Lett. 2014, 43, 977–979. [Google Scholar] [CrossRef]
- Uemura, Y.; Kido, D.; Wakisaka, Y.; Uehara, H.; Ohba, T.; Niwa, Y.; Nozawa, S.; Sato, T.; Ichiyanagi, K.; Fukaya, R.; et al. Dynamics of photoelectrons and structural changes of tungsten trioxide observed by femtosecond transient XAFS. Angew. Chem. Int. Ed. 2016, 55, 1364–1367. [Google Scholar] [CrossRef]
- Uemura, Y.; Kido, D.; Koide, A.; Wakisaka, Y.; Niwa, Y.; Nozawa, S.; Ichiyanagi, K.; Fukaya, R.; Adachi, S.; Katayama, T.; et al. Capturing local structure modulations of photoexcited BiVO4 by ultrafast transient XAFS. Chem. Commun. 2017, 53, 7314–7317. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Ohtani, B. Hidden but Possibly Fatal Misconceptions in Photocatalysis Studies: A Short Critical Review. Catalysts 2016, 6, 192. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Rittmann-Frank, M.H.; Milne, C.J.; Rittmann, J.; Reinhard, M.; Penfold, T.J.; Chergui, M. Mapping of the Photoinduced Electron Traps in TiO2 by Picosecond X-ray Absorption Spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 5858–5862. [Google Scholar] [CrossRef]
- Obara, Y.; Ito, H.; Ito, T.; Kurahashi, N.; Thürmer, S.; Tanaka, H.; Katayama, T.; Togashi, T.; Owada, S.; Yamamoto, Y. Femtosecond time-resolved X-ray absorption spectroscopy of anatase TiO2 nanoparticles using XFEL. Struct. Dyn. 2017, 4, 044033. [Google Scholar] [CrossRef]
- Abe, R.; Takami, H.; Murakami, N.; Ohtani, B. Pristine Simple Oxides as Visible Light Driven Photocatalysts: Highly Efficient Decomposition of Organic Compounds over Platinum-Loaded Tungsten Oxide. J. Am. Chem. Soc. 2008, 130, 7780–7781. [Google Scholar] [CrossRef]
- Abe, R.; Takata, T.; Sugihara, H.; Domen, K. Photocatalytic overall water splitting under visible light by TaON and WO3 with an IO3−/I− shuttle redox mediator. Chem. Commun. 2005, 3829–3831. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Iwase, A.; Kudo, A. Photoelectrochemical water splitting using visible-light-responsive BiVO4 fine particles prepared in an aqueous acetic acid solution. J. Mater. Chem. 2010, 20, 7536–7542. [Google Scholar] [CrossRef]
- Sato, T.; Nozawa, S.; Tomita, A.; Hoshino, M.; Koshihara, S.; Fujii, H.; Adachi, S. Coordination and Electronic Structure of Ruthenium(II)-tris-2,2′-bipyridine in the Triplet Metal-to-Ligand Charge-Transfer Excited State Observed by Picosecond Time-Resolved Ru K-Edge XAFS. J. Phys. Chem. C 2012, 116, 14232–14236. [Google Scholar] [CrossRef]
- Nozawa, S.; Sato, T.; Chollet, M.; Ichiyanagi, K.; Tomita, A.; Fujii, H.; Adachi, S.-I.; Koshihara, S.-Y. Direct Probing of Spin State Dynamics Coupled with Electronic and Structural Modifications by Picosecond Time-Resolved XAFS. J. Am. Chem. Soc. 2010, 132, 61–63. [Google Scholar] [CrossRef]
- Kim, J.G.; Nozawa, S.; Kim, H.; Choi, E.H.; Sato, T.; Kim, T.W.; Kim, K.H.; Ki, H.; Kim, J.; Choi, M.; et al. Mapping the emergence of molecular vibrations mediating bond formation. Nature 2020, 582, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, J.G.; Nozawa, S.; Sato, T.; Oang, K.Y.; Kim, T.W.; Ki, H.; Jo, J.; Park, S.; Song, C.; et al. Direct observation of bond formation in solution with femtosecond X-ray scattering. Nature 2015, 518, 385–389. [Google Scholar] [CrossRef]
- Ichiyanagi, K.; Takagi, S.; Kawai, N.; Fukaya, R.; Nozawa, S.; Nakamura, K.G.; Liss, K.-D.; Kimura, M.; Adachi, S. Microstructural deformation process of shock-compressed polycrystalline aluminum. Sci. Rep. 2019, 9, 7604. [Google Scholar] [CrossRef]
- Sato-Tomita, A.; Shibayama, N. Size and Shape Controlled Crystallization of Hemoglobin for Advanced Crystallography. Crystals 2017, 7, 282. [Google Scholar] [CrossRef]
- Nozawa, S. Upgrade the Beamline PF-AR NW14A for the High-Repetition-Rate X-Ray Pump-Probe Experiments. In Proceedings of the Mechanical Engineering Design of Synchrotron Radiation Equipment and Instrumentation Conference, Barcelona, Spain, 11–16 September 2017; pp. 351–352. [Google Scholar]
- Yabashi, M.; Katayama, T. XFEL. In XAFS Techniques for Catalysts, Nanomaterals and Surfaces; Iwasawa, Y., Asakura, K., Tada, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; p. 63. [Google Scholar]
- Tono, K.; Togashi, T.; Inubushi, Y.; Sato, T.; Katayama, T.; Ogawa, K.; Ohashi, H.; Kimura, H.; Takahashi, S.; Takeshita, K.; et al. Beamline, experimental stations and photon beam diagnostics for the hard x-ray free electron laser of SACLA. New J. Phys. 2013, 15, 083035. [Google Scholar] [CrossRef]
- Ishikawa, T.; Aoyagi, H.; Asaka, T.; Asano, Y.; Azumi, N.; Bizen, T.; Ego, H.; Fukami, K.; Fukui, T.; Furukawa, Y.; et al. A compact X-ray free-electron laser emitting in the sub-ångström region. Nat. Photonics 2012, 6, 540–544. [Google Scholar] [CrossRef]
- Katayama, T.; Nozawa, S.; Umena, Y.; Lee, S.; Togashi, T.; Owada, S.; Yabashi, M. A versatile experimental system for tracking ultrafast chemical reactions with X-ray free-electron lasers. Struct. Dyn. 2019, 6, 054302. [Google Scholar] [CrossRef]
- Katayama, T.; Northey, T.; Gawelda, W.; Milne, C.J.; Vankó, G.; Lima, F.A.; Bohinc, R.; Németh, Z.; Nozawa, S.; Sato, T.; et al. Tracking multiple components of a nuclear wavepacket in photoexcited Cu(I)-phenanthroline complex using ultrafast X-ray spectroscopy. Nat. Commun. 2019, 10, 3606. [Google Scholar] [CrossRef]
- Ogi, Y.; Obara, Y.; Katayama, T.; Suzuki, Y.I.; Liu, S.Y.; Bartlett, N.C.M.; Kurahashi, N.; Karashima, S.; Togashi, T.; Inubushi, Y.; et al. Ultraviolet photochemical reaction of [Fe(III)(C2O4)3]3− in aqueous solutions studied by femtosecond time-resolved X-ray absorption spectroscopy using an X-ray free electron laser. Struct. Dyn. 2015, 2, 034901. [Google Scholar] [CrossRef]
- Kinschel, D.; Bacellar, C.; Cannelli, O.; Sorokin, B.; Katayama, T.; Mancini, G.F.; Rouxel, J.R.; Obara, Y.; Nishitani, J.; Ito, H.; et al. Femtosecond X-ray emission study of the spin cross-over dynamics in haem proteins. Nat. Commun. 2020, 11, 4145. [Google Scholar] [CrossRef]
- Katayama, T.; Hirano, T.; Morioka, Y.; Sano, Y.; Osaka, T.; Owada, S.; Togashi, T.; Yabashi, M. X-ray optics for advanced ultrafast pump-probe X-ray experiments at SACLAThis article will form part of a virtual special issue on X-ray free-electron lasers. J. Synchrotron Radiat. 2019, 26, 333–338. [Google Scholar] [CrossRef]
- Kameshima, T.; Ono, S.; Kudo, T.; Ozaki, K.; Kirihara, Y.; Kobayashi, K.; Inubushi, Y.; Yabashi, M.; Horigome, T.; Holland, A.; et al. Development of an X-ray pixel detector with multi-port charge-coupled device for X-ray free-electron laser experiments. Rev. Sci. Instrum. 2014, 85, 033110. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Tono, K.; Yabashi, M.; Togashi, T.; Sato, T.; Inubushi, Y.; Omodani, M.; Kirihara, Y.; Matsushita, T.; Kobayashi, K.; et al. A photodiode amplifier system for pulse-by-pulse intensity measurement of an X-ray free electron laser. Rev. Sci. Instrum. 2012, 83, 043108. [Google Scholar] [CrossRef]
- Katayama, T.; Inubushi, Y.; Obara, Y.; Sato, T.; Togashi, T.; Tono, K.; Hatsui, T.; Kameshima, T.; Bhattacharya, A.; Ogi, Y.; et al. Femtosecond x-ray absorption spectroscopy with hard x-ray free electron laser. Appl. Phys. Lett. 2013, 103, 131105. [Google Scholar] [CrossRef]
- Katayama, T.; Owada, S.; Togashi, T.; Ogawa, K.; Karvinen, P.; Vartiainen, I.; Eronen, A.; David, C.; Sato, T.; Nakajima, K.; et al. A beam branching method for timing and spectral characterization of hard X-ray free-electron lasers. Struct. Dyn. 2016, 3, 034301. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Uesigi, T.; Abe, Y.; Sayama, K.; Arakawa, H. The photocatalytic oxidation of water to O2 over pure CeO2, WO3, and TiO2 using Fe3+ and Ce4+ as electron acceptors. Appl. Catal. A 2001, 205, 117–128. [Google Scholar] [CrossRef]
- Asakura, H.; Shishido, T.; Yamazoe, S.; Teramura, K.; Tanaka, T. Structural Analysis of Group V, VI, and VII Metal Compounds by XAFS. J. Phys. Chem. C 2011, 115, 23653–23663. [Google Scholar] [CrossRef]
- Yamazoe, S.; Hitomi, Y.; Shishido, T.; Tanaka, T. XAFS Study of Tungsten L1- and L3-Edges: Structural Analysis of WO3 Species Loaded on TiO2 as a Catalyst for Photooxidation of NH3. J. Phys. Chem. C 2008, 112, 6869–6879. [Google Scholar] [CrossRef]
- Hatada, K.; Hayakawa, K.; Benfatto, M.; Natoli, C.R. Full-potential multiple scattering theory with space-filling cells for bound and continuum states. J. Phys. Condens. Matter 2010, 22, 185501. [Google Scholar] [CrossRef][Green Version]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Yu, J.; Kudo, A. Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Berglund, S.P.; Rettie, A.J.E.; Hoang, S.; Mullins, C.B. Incorporation of Mo and W into nanostructured BiVO4 films for efficient photoelectrochemical water oxidation. Phys. Chem. Chem. Phys. 2012, 14, 7065–7075. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Sharp, I.D.; Cooper, J.K.; Toma, F.M.; Buonsanti, R. Bismuth Vanadate as a Platform for Accelerating Discovery and Development of Complex Transition-Metal Oxide Photoanodes. ACS Energy Lett. 2017, 2, 139–150. [Google Scholar] [CrossRef]
- Walsh, A.; Yan, Y.; Huda, M.N.; Al-Jassim, M.M.; Wei, S.-H. Band Edge Electronic Structure of BiVO4: Elucidating the Role of the Bi s and V d Orbitals. Chem. Mater. 2009, 21, 547–551. [Google Scholar] [CrossRef]
- Cooper, J.K.; Gul, S.; Toma, F.M.; Chen, L.; Glans, P.-A.; Guo, J.; Ager, J.W.; Yano, J.; Sharp, I.D. Electronic Structure of Monoclinic BiVO4. Chem. Mater. 2014, 26, 5365–5373. [Google Scholar] [CrossRef]
- Aiga, N.; Jia, Q.; Watanabe, K.; Kudo, A.; Sugimoto, T.; Matsumoto, Y. Electron–Phonon Coupling Dynamics at Oxygen Evolution Sites of Visible-Light-Driven Photocatalyst: Bismuth Vanadate. J. Phys. Chem. C 2013, 117, 9881–9886. [Google Scholar] [CrossRef]
- Ravensbergen, J.; Abdi, F.F.; van Santen, J.H.; Frese, R.N.; Dam, B.; van de Krol, R.; Kennis, J.T.M. Unraveling the Carrier Dynamics of BiVO4: A Femtosecond to Microsecond Transient Absorption Study. J. Phys. Chem. C 2014, 118, 27793–27800. [Google Scholar] [CrossRef]
- Butler, K.T.; Dringoli, B.J.; Zhou, L.; Rao, P.M.; Walsh, A.; Titova, L.V. Ultrafast carrier dynamics in BiVO4 thin film photoanode material: Interplay between free carriers, trapped carriers and low-frequency lattice vibrations. J. Mater. Chem. A 2016, 4, 18516–18523. [Google Scholar] [CrossRef]
- Ziwritsch, M.; Müller, S.; Hempel, H.; Unold, T.; Abdi, F.F.; van de Krol, R.; Friedrich, D.; Eichberger, R. Direct Time-Resolved Observation of Carrier Trapping and Polaron Conductivity in BiVO4. ACS Energy Lett. 2016, 1, 888–894. [Google Scholar] [CrossRef]
- Grigioni, I.; Stamplecoskie, K.G.; Selli, E.; Kamat, P.V. Dynamics of Photogenerated Charge Carriers in WO3/BiVO4 Heterojunction Photoanodes. J. Phys. Chem. C 2015, 119, 20792–20800. [Google Scholar] [CrossRef]
- Nan, J.; John, C.H.S. Can near-edge structure of the Bi L 3 edge determine the formal valence states of Bi? J. Phys. Condens. Matter 2006, 18, 8029. [Google Scholar]
- Rettie, A.J.E.; Chemelewski, W.D.; Emin, D.; Mullins, C.B. Unravelling Small-Polaron Transport in Metal Oxide Photoelectrodes. J. Phys. Chem. Lett. 2016, 7, 471–479. [Google Scholar] [CrossRef]
- Katz, J.E.; Zhang, X.; Attenkofer, K.; Chapman, K.W.; Frandsen, C.; Zarzycki, P.; Rosso, K.M.; Falcone, R.W.; Waychunas, G.A.; Gilbert, B. Electron Small Polarons and Their Mobility in Iron (Oxyhydr)oxide Nanoparticles. Science 2012, 337, 1200–1203. [Google Scholar] [CrossRef]
- Carneiro, L.M.; Cushing, S.K.; Liu, C.; Su, Y.; Yang, P.; Alivisatos, A.P.; Leone, S.R. Excitation-wavelength-dependent small polaron trapping of photoexcited carriers in α-Fe2O3. Nat. Mater. 2017, 16, 819–825. [Google Scholar] [CrossRef]
- Bedja, I.; Hotchandani, S.; Kamat, P.V. Photoelectrochemistry of quantized tungsten trioxide colloids: Electron storage, electrochromic, and photoelectrochromic effects. J. Phys. Chem. 1993, 97, 11064–11070. [Google Scholar] [CrossRef]
- Pesci, F.M.; Cowan, A.J.; Alexander, B.D.; Durrant, J.R.; Klug, D.R. Charge Carrier Dynamics on Mesoporous WO3 during Water Splitting. J. Phys. Chem. Lett. 2011, 2, 1900–1903. [Google Scholar] [CrossRef]
- Amano, F.; Ishinaga, E.; Yamakata, A. Effect of Particle Size on the Photocatalytic Activity of WO3 Particles for Water Oxidation. J. Phys. Chem. C 2013, 117, 22584–22590. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.; Min, C.-K.; Eom, I.; Nam, I.; Lee, H.-S.; Kang, H.-S.; Kim, H.-D.; Jang, H.Y.; Kim, S.; et al. PAL-XFEL soft X-ray scientific instruments and X-ray optics: First commissioning results. Rev. Sci. Instrum. 2018, 89, 055105. [Google Scholar] [CrossRef]
- Shavorskiy, A.; Ye, X.; Karslıoğlu, O.; Poletayev, A.D.; Hartl, M.; Zegkinoglou, I.; Trotochaud, L.; Nemšák, S.; Schneider, C.M.; Crumlin, E.J.; et al. Direct Mapping of Band Positions in Doped and Undoped Hematite during Photoelectrochemical Water Splitting. J. Phys. Chem. Lett. 2017, 8, 5579–5586. [Google Scholar] [CrossRef] [PubMed]
- Tohji, K.; Udagawa, Y. Novel approach for structure analysis by x-ray Raman scattering. Phys. Rev. B Condens. Matter 1987, 36, 9410–9412. [Google Scholar] [CrossRef]
- Bergmann, U.; Groenzin, H.; Mullins, O.C.; Glatzel, P.; Fetzer, J.; Cramer, S.P. Carbon K-edge X-ray Raman spectroscopy supports simple, yet powerful description of aromatic hydrocarbons and asphaltenes. Chem. Phys. Lett. 2003, 369, 184–191. [Google Scholar] [CrossRef]
- Sirisit, N.; Kido, D.; Wakisaka, Y.; Ariga-Miwa, H.; Takakusagi, S.; Asakura, K.; Sekizawa, O.; Sakata, T.; Uruga, T.; Iwasawa, Y. Evidence for Multi-Atom Resonance X-ray Raman Spectroscopy—An in situ Low Z-element and Bond-specific X-ray Spectroscopy. e-J. Surf. Sci. Nanotechnol. 2018, 16, 387–390. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemura, Y.; Yokoyama, T.; Katayama, T.; Nozawa, S.; Asakura, K. Tracking the Local Structure Change during the Photoabsorption Processes of Photocatalysts by the Ultrafast Pump-Probe XAFS Method. Appl. Sci. 2020, 10, 7818. https://doi.org/10.3390/app10217818
Uemura Y, Yokoyama T, Katayama T, Nozawa S, Asakura K. Tracking the Local Structure Change during the Photoabsorption Processes of Photocatalysts by the Ultrafast Pump-Probe XAFS Method. Applied Sciences. 2020; 10(21):7818. https://doi.org/10.3390/app10217818
Chicago/Turabian StyleUemura, Yohei, Toshihiko Yokoyama, Tetsuo Katayama, Shunsuke Nozawa, and Kiyotaka Asakura. 2020. "Tracking the Local Structure Change during the Photoabsorption Processes of Photocatalysts by the Ultrafast Pump-Probe XAFS Method" Applied Sciences 10, no. 21: 7818. https://doi.org/10.3390/app10217818
APA StyleUemura, Y., Yokoyama, T., Katayama, T., Nozawa, S., & Asakura, K. (2020). Tracking the Local Structure Change during the Photoabsorption Processes of Photocatalysts by the Ultrafast Pump-Probe XAFS Method. Applied Sciences, 10(21), 7818. https://doi.org/10.3390/app10217818





