The Onset Threshold of Cybersickness in Constant and Accelerating Optical Flow
Abstract
1. Introduction
2. Background and Related Work
2.1. Origin of Nauseogenic Stimuli
2.2. Cybersickness Induced by Scene Movements along Different Axes
3. Method
3.1. Participants
3.2. Optical Flow Model
3.3. Staircase Procedure
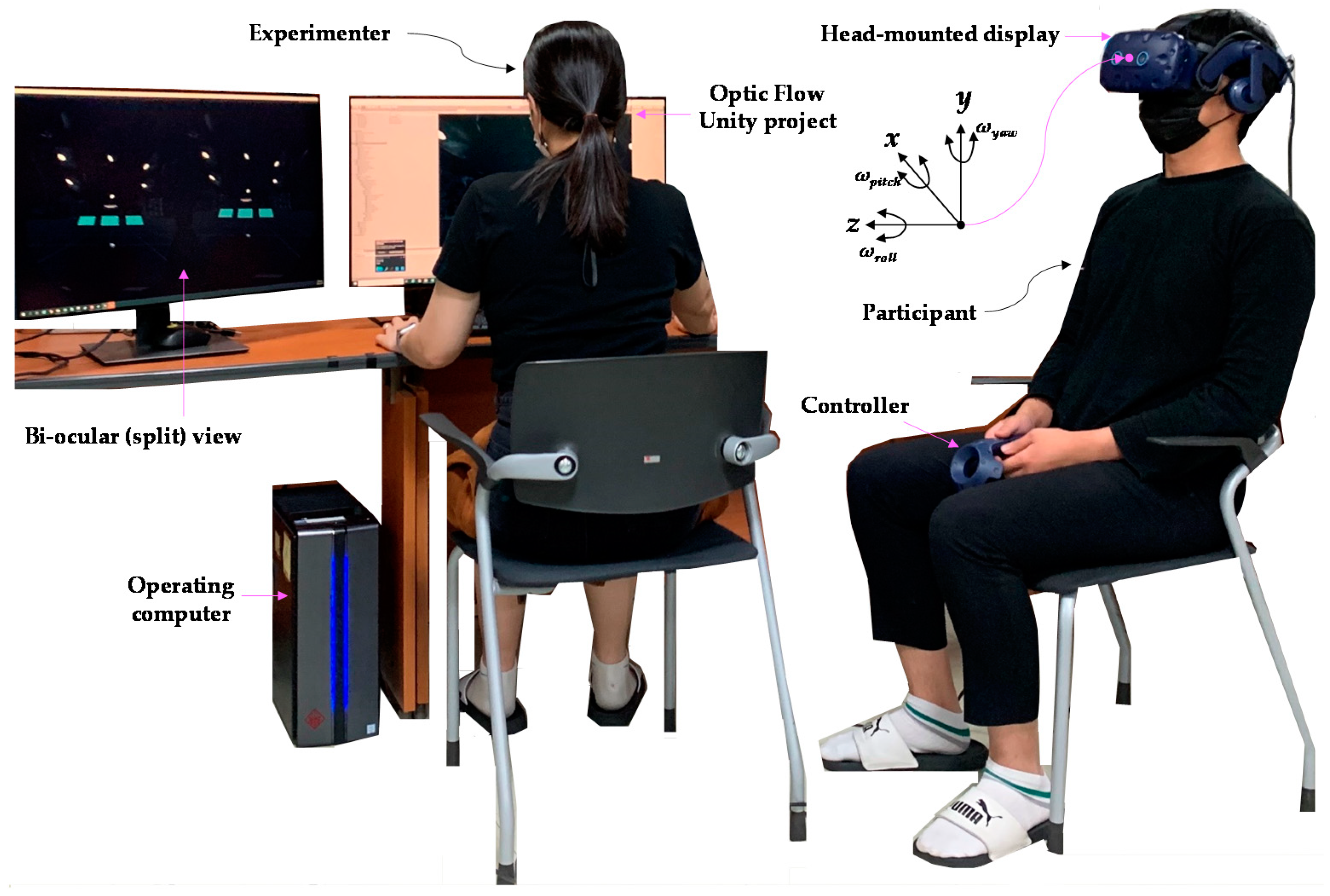
3.4. Virtual Environment and Apparatus
3.5. Experimental Conditions
3.6. Procedures
4. Results
4.1. Threshold
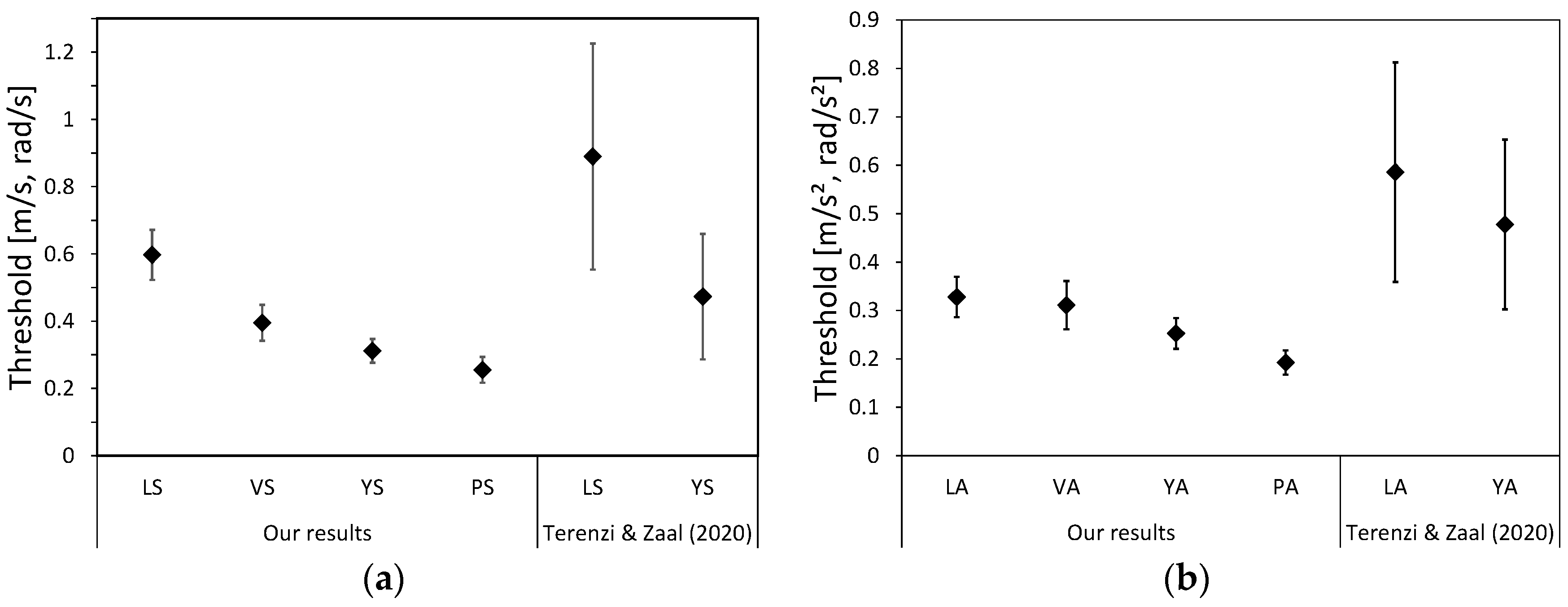
4.1.1. Detected Thresholds for Experimental Conditions
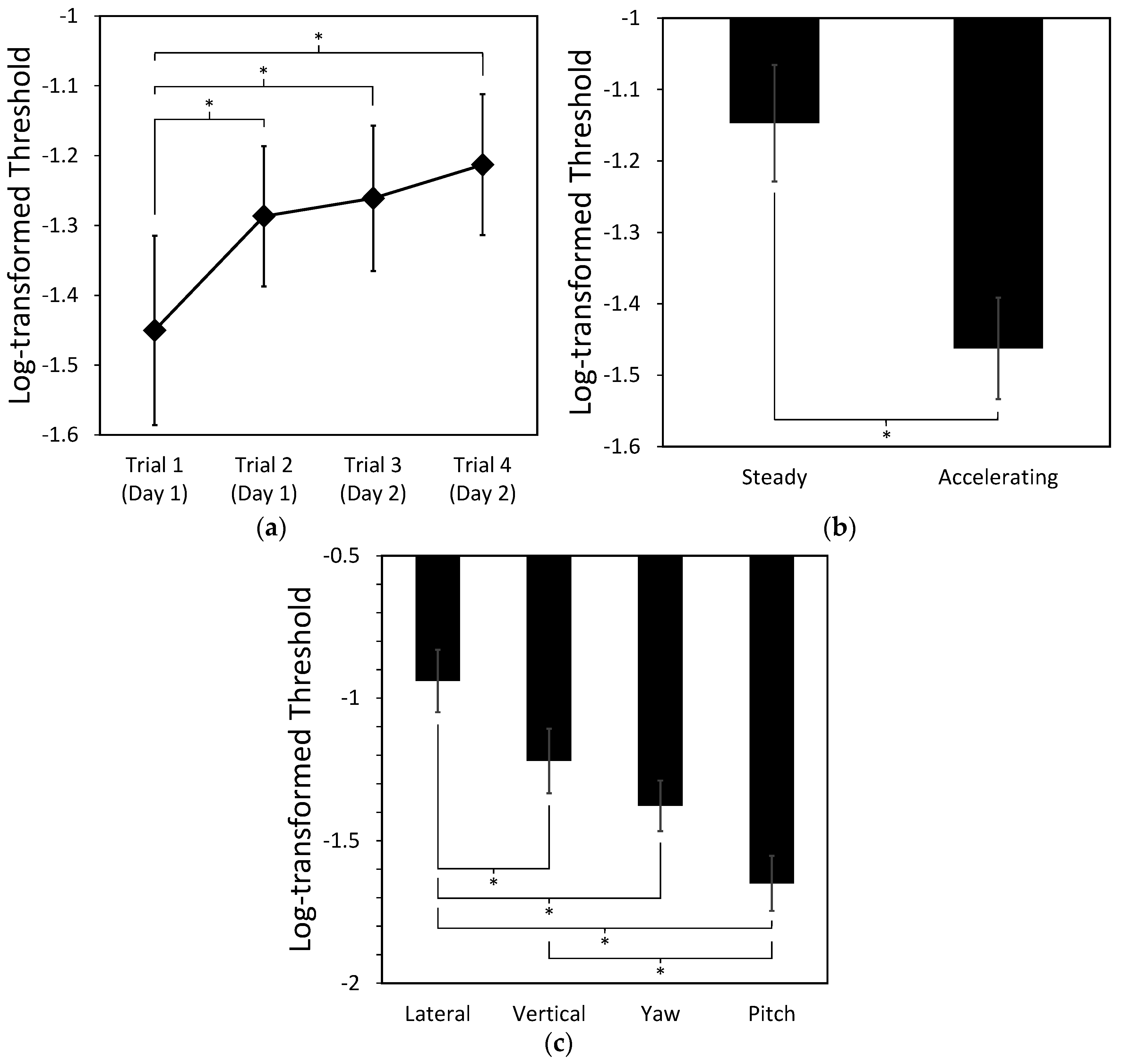
4.1.2. Effects of Repetition, Types of Motion, and Movement Direction on the Threshold
4.1.3. Correlation of Experimental Conditions
4.2. Severity Level of Cybersickness
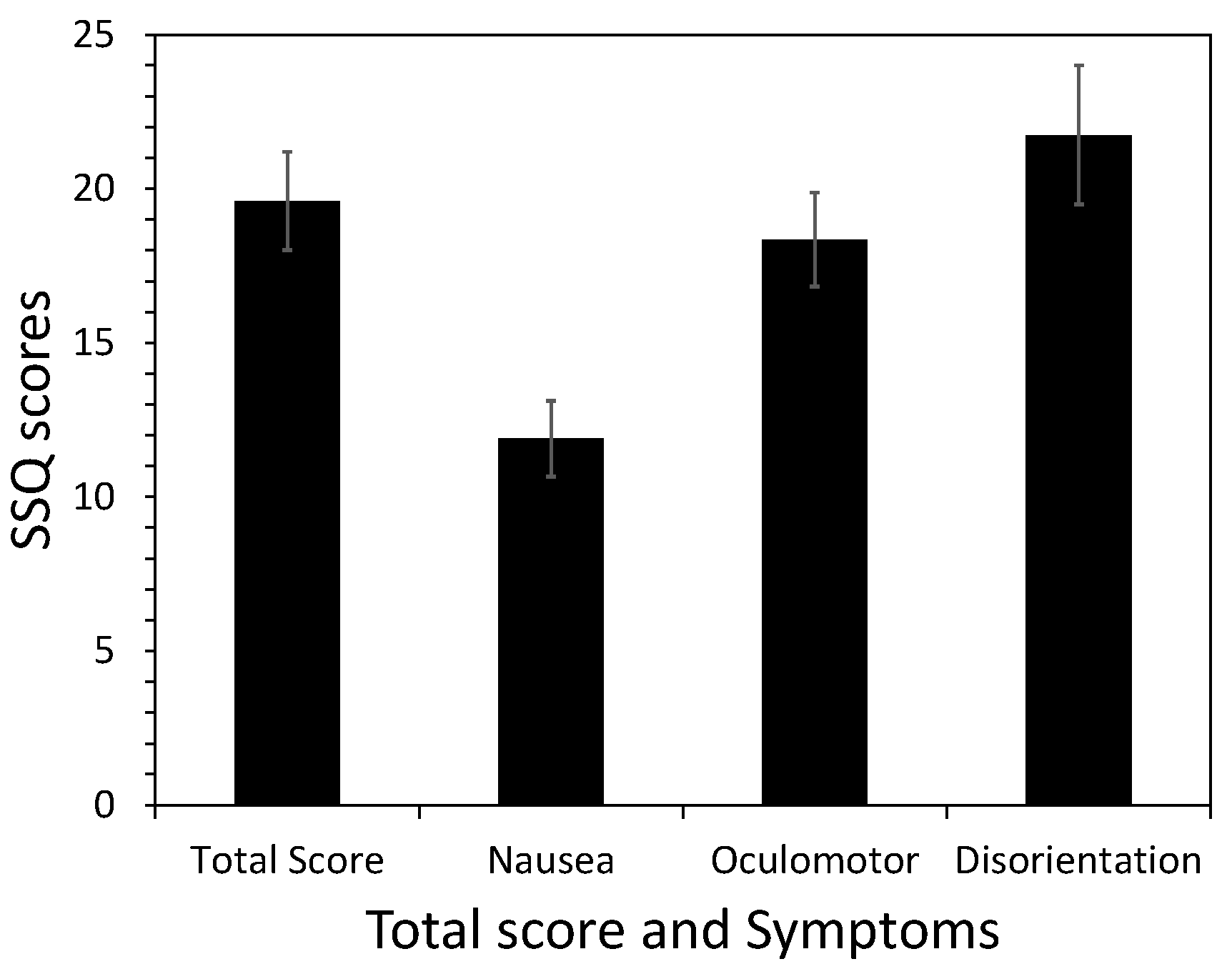
4.2.1. The Symptom Profile of SSQ Scores
4.2.2. Effects of Repetition, Types of Motion, and Movement Direction on Cybersickness
4.2.3. Correlation between the Detected Threshold and Severity Level of Cybersickness
5. Discussion
5.1. Dominant Axis Evoking Cybersickness
5.2. Implications for Reducing Cybersickness
5.3. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cobb, S.V.G.; Nichols, S.; Ramsey, A.; Wilson, J.R. Virtual Reality-Induced Symptoms and Effects (VRISE). Presence Teleoperators Virtual Environ. 1999, 8, 169–186. [Google Scholar] [CrossRef]
- McCauley, M.E.; Sharkey, T.J. Cybersickness: Perception of Self-Motion in Virtual Environments. Presence Teleoperators Virtual Environ. 1992, 1, 311–318. [Google Scholar] [CrossRef]
- So, R.H.Y.; Ho, A.; Lo, W.T. A Metric to Quantify Virtual Scene Movement for the Study of Cybersickness: Definition, Implementation, and Verification. Presence Teleoperators Virtual Environ. 2001, 10, 193–215. [Google Scholar] [CrossRef]
- Stanney, K.; Salvendy, G. Aftereffects and Sense of Presence in Virtual Environments: Formulation of a Research and Development Agenda. Int. J. Hum. Comput. Interact. 1998, 10, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.S.; Feiner, S.K. Combating VR sickness through subtle dynamic field-of-view modification. In Proceedings of the 2016 IEEE Symposium on 3D User Interfaces (3DUI), Greenville, SC, USA, 19–20 March 2016; pp. 201–210. [Google Scholar]
- Argelaguet, F. Adaptive navigation for virtual environments. In Proceedings of the 2014 IEEE Symposium on 3D User Interfaces (3DUI), Minneapolis, MN, USA, 29–30 March 2014; pp. 123–126. [Google Scholar]
- Argelaguet, F.; Maignant, M. GiAnt: Stereoscopic-compliant multi-scale navigation in VEs. In Proceedings of the 22nd ACM Conference on Virtual Reality Software and Technology, Munich, Germany, 2–4 November 2016; pp. 269–277. [Google Scholar]
- So, R.H.Y.; Lo, W.T.; Ho, A.T.K. Effects of Navigation Speed on Motion Sickness Caused by an Immersive Virtual Environment. Hum. Factors 2001, 43, 452–461. [Google Scholar] [CrossRef]
- Boletsis, C. The new era of virtual reality locomotion: A systematic literature review of techniques and a proposed typology. Multimodal Technol. Interact. 2017, 1, 24. [Google Scholar] [CrossRef]
- Davis, S.; Nesbitt, K.; Nalivaiko, E. Comparing the onset of cybersickness using the Oculus Rift and two virtual roller coasters. In Proceedings of the 11th Australasian Conference on Interactive Entertainment (IE 2015), Sydney, Australia, 27–30 January 2015; p. 30. [Google Scholar]
- Porcino, T.M.; Clua, E.; Trevisan, D.; Vasconcelos, C.N.; Valente, L. Minimizing cyber sickness in head mounted display systems: Design guidelines and applications. In Proceedings of the 2017 IEEE 5th International Conference on Serious Games and Applications for Health (SeGAH), Perth, Western Australia, 2–4 April 2017; pp. 1–6. [Google Scholar]
- Lo, W.T.; So, R.H.Y. Cybersickness in the presence of scene rotational movements along different axes. Appl. Ergon. 2001, 32, 1–14. [Google Scholar] [CrossRef]
- Chen, W. Effects of Navigation Velocities in Fore-And-Aft, Lateral, Yaw Axes on Cybersickness Caused by Exposure to a Virtual Environment. Master’s Thesis, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China, 2006. [Google Scholar]
- Rebenitsch, L.; Owen, C. Review on cybersickness in applications and visual displays. Virtual Real. 2016, 20, 101–125. [Google Scholar] [CrossRef]
- Joseph, J.A.; Griffin, M.J. Motion Sickness: Effect of the Magnitude of Roll and Pitch Oscillation. Aviat. SpaceEnviron. Med. 2008, 79, 390–396. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; So, R.H.Y. Visually induced motion sickness: Effects of translational visual motion along different axes. In Contemporary Ergonomics and Human Factors 2011, Proceedings of the International Conference on Ergonomics & Human Factors 2011, Stoke Rochford, Lincolnshire, 12–14 April 2011; Anderson, M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 281–287. [Google Scholar] [CrossRef]
- Bonato, F.; Bubka, A.; Palmisano, S. Combined Pitch and Roll and Cybersickness in a Virtual Environment. Aviat. Space Environ. Med. 2009, 80, 941–945. [Google Scholar] [CrossRef]
- Terenzi, L.; Zaal, P. Rotational and Translational Velocity and Acceleration Thresholds for the Onset of Cybersickness in Virtual Reality. In Proceedings of the AIAA Scitech 2020 Forum, Orlando, FL, USA, 6–10 January 2020. [Google Scholar] [CrossRef]
- Prothero, J.D. The Role of Rest Frames in Vection, Presence and Motion Sickness. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 1998. [Google Scholar]
- Bles, W. Coriolis effects and motion sickness modelling. Brain Res. Bull. 1998, 47, 543–549. [Google Scholar] [CrossRef]
- Bles, W.; Bos, J.E.; de Graaf, B.; Groen, E.; Wertheim, A.H. Motion sickness: Only one provocative conflict? Brain Res. Bull. 1998, 47, 481–487. [Google Scholar] [CrossRef]
- Bos, J.E.; Bles, W.; Groen, E.L. A theory on visually induced motion sickness. Displays 2008, 29, 47–57. [Google Scholar] [CrossRef]
- McCauley, M.E. Research Issues in Simulator Sickness: Proceedings of a Workshop; National Research Council Washington DC Committee on Human Factors: Washington, DC, USA, 1984.
- Kennedy, R.S.; Lane, N.E.; Berbaum, K.S.; Lilienthal, M.G. Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Bertolini, G.; Straumann, D. Moving in a Moving World: A Review on Vestibular Motion Sickness. Front. Neurol. 2016, 7. [Google Scholar] [CrossRef]
- Angelaki, D.E.; Cullen, K.E. Vestibular System: The Many Facets of a Multimodal Sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef]
- Leigh, R.J.; Zee, D.S. The Neurology of Eye Movements, 4th ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Ko, B.Y.; Kim, K.S.; Park, H.K. Understanding of VOR for the Beginner. Res. Vestib. Sci. 2012, 11, 73–79. [Google Scholar]
- Einstein, A. Über das Relativitätsprinzip und die aus Demselben Gezogenen Folgerungen; S. Hirzel: Leipzig, Germany, 1908. [Google Scholar]
- Fetsch, C.R.; Turner, A.H.; DeAngelis, G.C.; Angelaki, D.E. Dynamic Reweighting of Visual and Vestibular Cues during Self-Motion Perception. J. Neurosci. 2009, 29, 15601–15612. [Google Scholar] [CrossRef] [PubMed]
- Knill, D.C.; Pouget, A. The Bayesian brain: The role of uncertainty in neural coding and computation. Trends Neurosci. 2004, 27, 712–719. [Google Scholar] [CrossRef]
- Oman, C.M. Motion sickness: A synthesis and evaluation of the sensory conflict theory. Can. J. Physiol. Pharmacol. 1990, 68, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.S.; Howard, I.P.; Money, K.E. Visually-induced sickness in normal and bilaterally labyrinthine-defective subjects. Aviat. Space Environ. Med. 1991, 62, 527–531. [Google Scholar]
- Diels, C.; Howarth, P.A. Frequency Characteristics of Visually Induced Motion Sickness. Hum. Factors 2013, 55, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Reason, J.T.; Brand, J.J. Motion Sickness; Academic Press: Cambridge, MA, USA, 1975. [Google Scholar]
- Yang, T.; Pei, J. Motion sickness severity under interaction of vection and head movements. Aviat. Space Environ. Med. 1991, 62, 141–144. [Google Scholar]
- Horn, B.K.P.; Schunck, B.G. Determining optical flow. Artif. Intell. 1981, 17, 185–203. [Google Scholar] [CrossRef]
- Cornsweet, T.N. The Staircase-Method in Psychophysics. Am. J. Psychol. 1962, 75, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Levitt, H. Transformed Up-Down Methods in Psychoacoustics. J. Acoust. Soc. Am. 1971, 49, 467–477. [Google Scholar] [CrossRef]
- Golding, J.F. Predicting individual differences in motion sickness susceptibility by questionnaire. Personal. Individ. Differ. 2006, 41, 237–248. [Google Scholar] [CrossRef]
- Warren, W.H.; Hannon, D.J. Eye movements and optical flow. J. Opt. Soc. Am. A 1990, 7, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rokach, L.; Maimon, O. Clustering Methods. In Data Mining and Knowledge Discovery Handbook; Maimon, O., Rokach, L., Eds.; Springer: Boston, MA, USA, 2005; pp. 321–352. [Google Scholar] [CrossRef]
- Kennedy, R.S.; Stanney, K.M.; Dunlap, W.P. Duration and Exposure to Virtual Environments: Sickness Curves During and Across Sessions. Presence Teleoperators Virtual Environ. 2000, 9, 463–472. [Google Scholar] [CrossRef]
- Jarchow, T.; Young, L.R. Adaptation to head movements during short radius centrifugation. Acta Astronaut. 2007, 61, 881–888. [Google Scholar] [CrossRef]
- Moss, J.; Scisco, J.; Muth, E. Simulator Sickness during Head Mounted Display (HMD) of Real World Video Captured Scenes. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2008, 52, 1631–1634. [Google Scholar] [CrossRef]
- Singer, M.J.; Ehrlich, J.A.; Allen, R.C. Virtual Environment Sickness: Adaptation to and Recovery from a Search Task. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 1998, 42, 1506–1510. [Google Scholar] [CrossRef]
- Newman, M.C.; McCarthy, G.W.; Glaser, S.T.; Bonato, F.; Bubka, A. Motion Sickness Adaptation to Coriolis-Inducing Head Movements in a Sustained G Flight Simulator. Aviat. Space Environ. Med. 2013, 84, 104–109. [Google Scholar] [CrossRef]
- Keshavarz, B.; Riecke, B.E.; Hettinger, L.J.; Campos, J.L. Vection and visually induced motion sickness: How are they related? Front. Psychol. 2015, 6. [Google Scholar] [CrossRef]
- Koohestani, A.; Nahavandi, D.; Asadi, H.; Kebria, P.M.; Khosravi, A.; Alizadehsani, R.; Nahavandi, S. A Knowledge Discovery in Motion Sickness: A Comprehensive Literature Review. IEEE Access 2019, 7, 85755–85770. [Google Scholar] [CrossRef]


| Scene Movement | OFz | OFx | OFy | OFroll | OFpitch | OFyaw |
|---|---|---|---|---|---|---|
| Viewing Volume (Ii) | 0.503 | 0.640 | 0.640 | 0.878 | 1.042 | 1.042 |
| Scale factor (Iz/Ii) | 1.000 | 0.786 | 0.786 | 0.572 | 0.482 | 0.482 |
| Independent Variable | Condition Label | |||||||
|---|---|---|---|---|---|---|---|---|
| LS | LA | VS | VA | YS | YA | PS | PA | |
| Movement | Lateral | Lateral | Vertical | Vertical | Yaw | Yaw | Pitch | Pitch |
| Motion | Steady | Acc. | Steady | Acc. | Steady | Acc. | Steady | Acc. |
| Participant ID | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Number of Runs | 32 | 32 | 32 | 30 | 32 | 32 | 31 | 32 | 20 | 32 | 31 | 31 | 32 | 32 | 32 | 32 |
| Missed Scene | - | - | - | LA YA | - | - | VS | - | LS LA YA | - | VA | YA | - | - | - | - |
| Label | Motion | Threshold Mean | Threshold SD |
|---|---|---|---|
| LS | Steady | 0.597 | 0.297 |
| VS | Steady | 0.395 | 0.217 |
| YS | Steady | 0.312 | 0.144 |
| PS | Steady | 0.255 | 0.157 |
| LA | Accelerating | 0.328 | 0.164 |
| VA | Accelerating | 0.311 | 0.200 |
| YA | Accelerating | 0.253 | 0.123 |
| PA | Accelerating | 0.193 | 0.102 |
| Lateral | Vertical | Yaw | Pitch | ||
|---|---|---|---|---|---|
| Total score | Steady | 19.52 (18.21) | 22.21 (19.97) | 20.10 (19.77) | 24.43 (19.45) |
| Accelerating | 18.88 (19.21) | 14.90 (13.18) | 17.82 (20.21) | 18.93 (15.68) | |
| Nausea | Steady | 10.43 (13.78) | 14.16 (14.52) | 11.93 (15.30) | 17.44 (16.26) |
| Accelerating | 10.88 (15.75) | 7.45 (10.12) | 9.84 (13.49) | 12.97 (12.18) | |
| Oculomotor | Steady | 20.73 (17.93) | 20.25 (18.62) | 18.36 (17.60) | 20.37 (17.02) |
| Accelerating | 18.00 (18.84) | 15.87 (14.72) | 16.94 (19.65) | 16.23 (15.97) | |
| Disorientation | Steady | 19.45 (25.22) | 24.44 (30.76) | 23.49 (29.23) | 27.84 (28.28) |
| Accelerating | 21.71 (24.70) | 15.01 (17.42) | 20.45 (27.28) | 21.53 (21.61) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, T. The Onset Threshold of Cybersickness in Constant and Accelerating Optical Flow. Appl. Sci. 2020, 10, 7808. https://doi.org/10.3390/app10217808
Kim J, Park T. The Onset Threshold of Cybersickness in Constant and Accelerating Optical Flow. Applied Sciences. 2020; 10(21):7808. https://doi.org/10.3390/app10217808
Chicago/Turabian StyleKim, Jiwon, and Taezoon Park. 2020. "The Onset Threshold of Cybersickness in Constant and Accelerating Optical Flow" Applied Sciences 10, no. 21: 7808. https://doi.org/10.3390/app10217808
APA StyleKim, J., & Park, T. (2020). The Onset Threshold of Cybersickness in Constant and Accelerating Optical Flow. Applied Sciences, 10(21), 7808. https://doi.org/10.3390/app10217808





