Load Transfer during Magnetic Mucoperiosteal Distraction in Newborns with Complete Unilateral and Bilateral Orofacial Clefts: A Three-Dimensional Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Patient Data
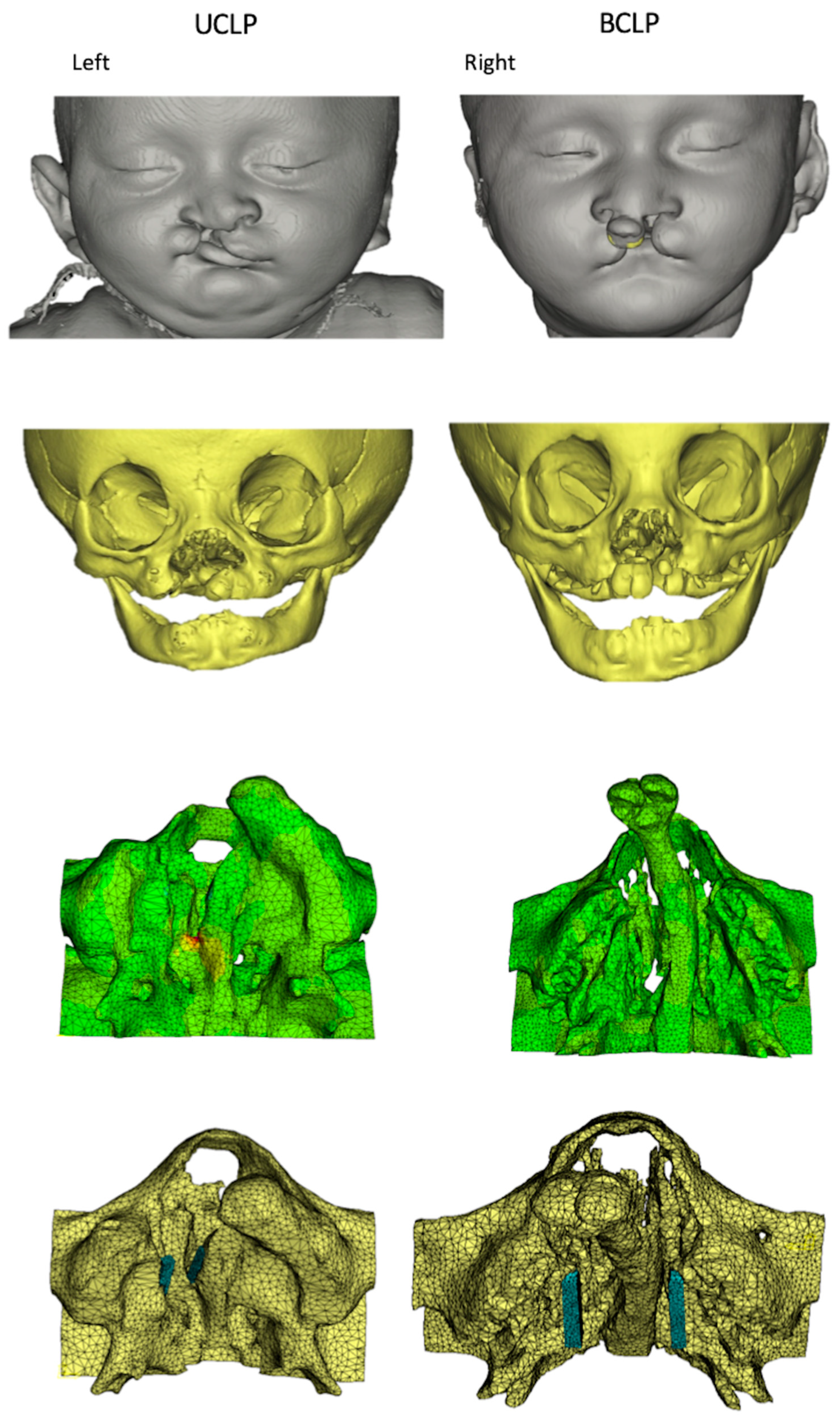
2.2. Model Construction
2.3. Magnet Implantation
2.4. FE Model Design
2.5. FE Analysis
3. Results
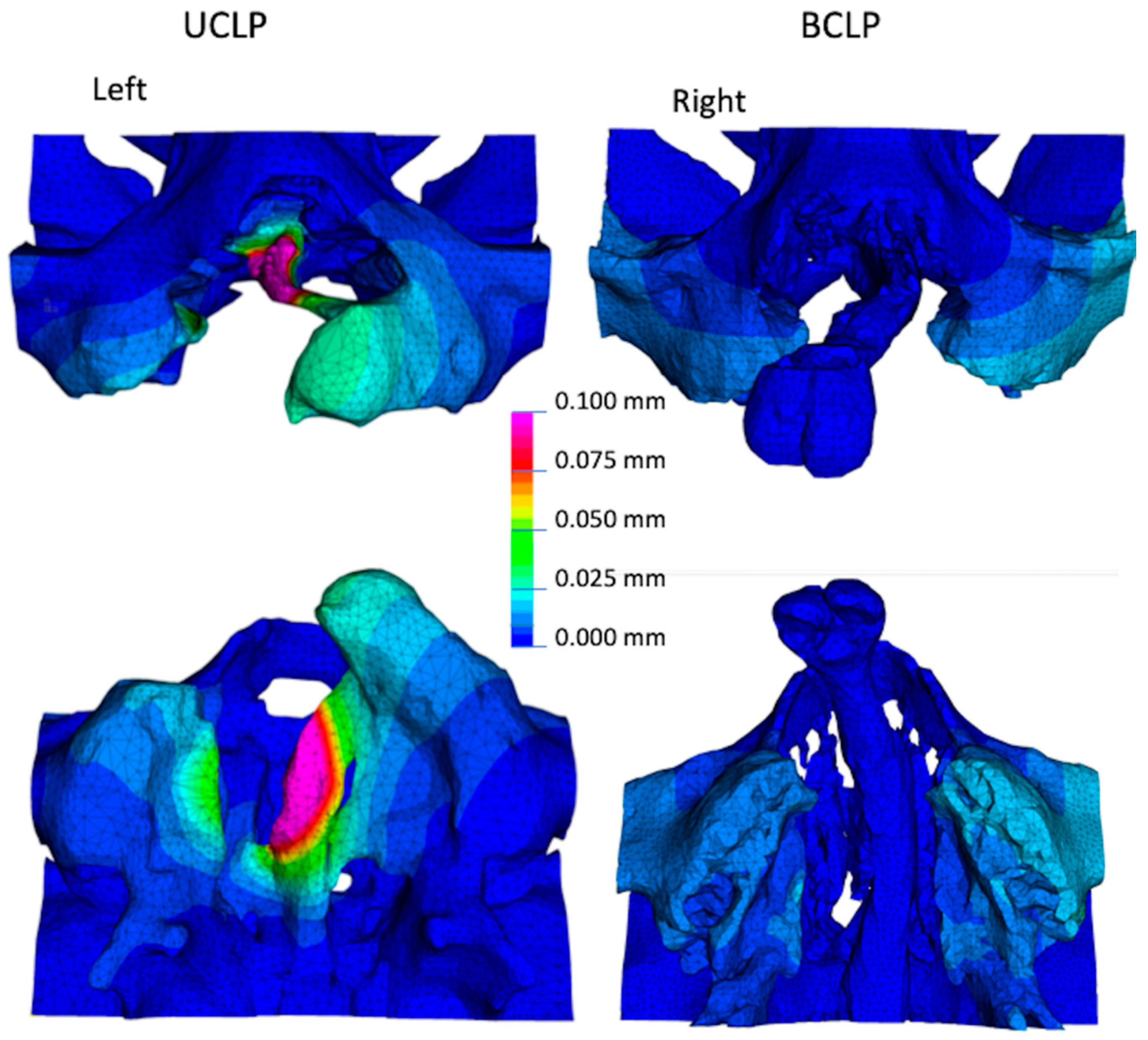
3.1. Overall Deformation of the Maxilla
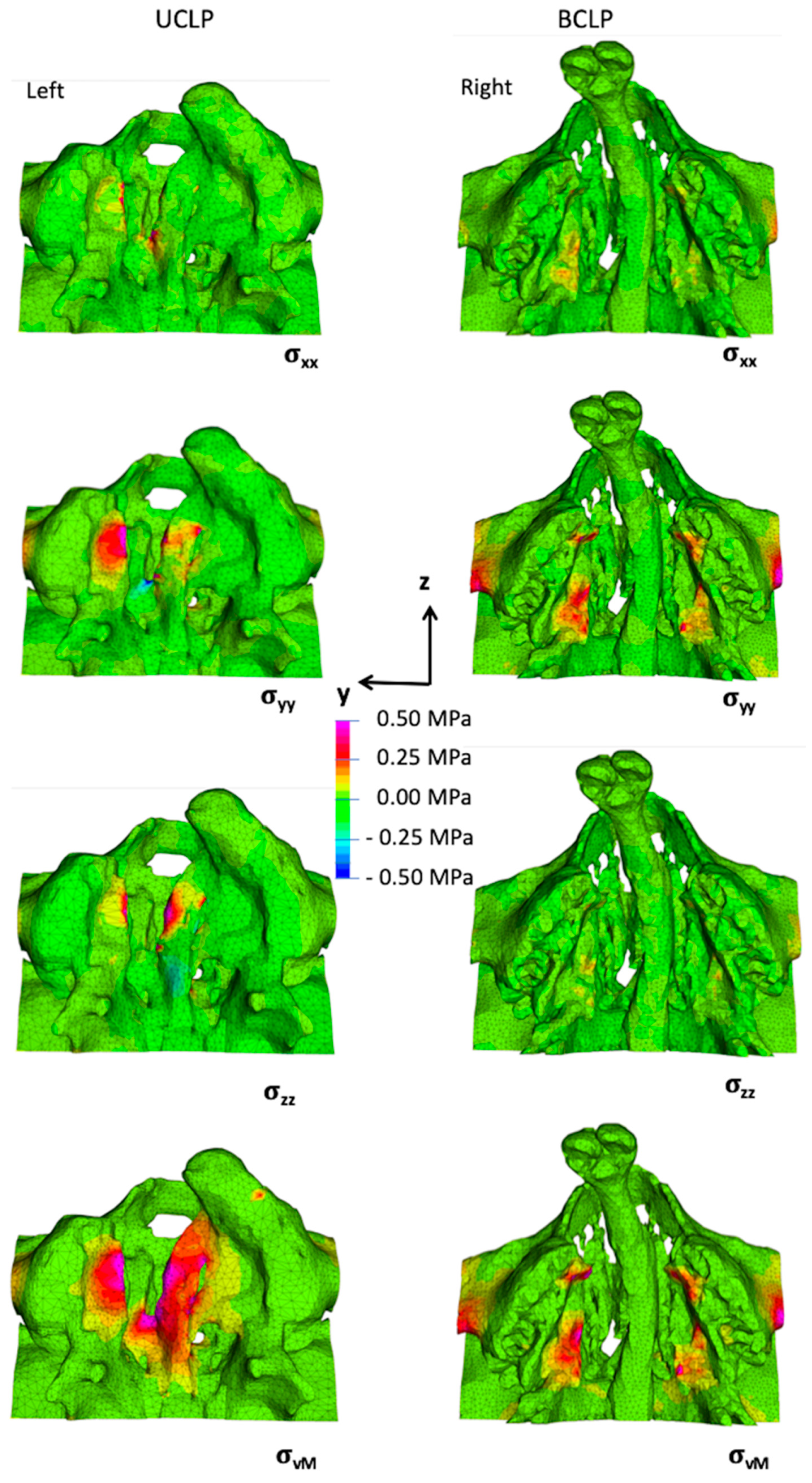
3.2. Stresses and Strains in the Maxilla
4. Discussion
5. Conclusions
- The present FE models have shown that the use of magnets as approved for dental applications can exert attraction with the potential to induce PDO around the greater and lesser segments of the palatal shelf ridge in cases of UCLP and BCLP.
- A magnetic attraction force of 5 N was found to be sufficient to reach strain values in excess of 1500 µstrain in the palatal bone in both UCLP and BCLP models, and thereby have the potential to initiate adaptive remodeling.
- The effects of magnetic load transfer were localized to their area of application, and did not spread out to distant structures.
- The anatomical variations both in external bone geometry and internal bone composition affect the load transfer across the palate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mossey, P. Global strategies to reduce the healthcare burden of craniofacial anomalies. Br. Dent. J. 2003, 195, 613. [Google Scholar] [CrossRef] [PubMed]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Schutte, B.C.; Murray, J.C. The many faces and factors of orofacial clefts. Hum. Mol. Genet. 1999, 8, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Mossey, P.A. Epidemiology of oral clefts: An international perspective. In Cleft Lip and Palate: From Origin to Treatment; Oxford University Press: Oxford, UK, 2002; ISBN 0195139062. [Google Scholar]
- Mossey, P. Epidemiology underpinning research in the aetiology of orofacial clefts. Orthod. Craniofac. Res. 2007, 10, 114–120. [Google Scholar] [CrossRef]
- Wong, F.K.; Hägg, U. An update on the aetiology of orofacial clefts. Hong Kong Med. J. 2004, 10, 331–336. [Google Scholar] [PubMed]
- Nanci, A.; Ten, C.A.R. Ten Cate’s Oral Histology Development, Structure, and Function; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef]
- Tarr, J.T.; Lambi, A.G.; Bradley, J.P.; Barbe, M.F.; Popoff, S.N. Development of normal and Cleft Palate: A central role for connective tissue growth factor (CTGF)/CCN2. J. Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef]
- Bührer, C.; Zimmermann, A. Cleft palate. In Neonatal Emergencies: A Practical Guide for Resuscitation, Transport and Critical Care of Newborn Infants; Cambridge University Press: Cambridge, UK, 2009; Volume 1, pp. 460–463. [Google Scholar] [CrossRef]
- Huddart, A.G. Arch Alignment and Presurgical Treatment—The West Midlands Approach. In Early Treatment of Cleft Lip and Palate; Hans Huber: Berne, Switzerland, 1986; ISBN 3-456-81406-2. [Google Scholar]
- Latief, B.S.; Lekkas, K.C.; Schols, J.G.J.H.; Fudalej, P.S.; Kuijpers, M.A.R. Width and elevation of the palatal shelves in unoperated unilateral and bilateral cleft lip and palate patients in the permanent dentition. J. Anat. 2012, 220, 263–270. [Google Scholar] [CrossRef]
- Atherton, J.D. A Descriptive Anatomy of the Face in Human Fetuses with Unilateral Cleft Lip and Palate. Cleft Palate J. 1967, 4, 104–114. [Google Scholar] [CrossRef]
- Diah, E.; Lo, L.J.; Huang, C.S.; Sudjatmiko, G.; Susanto, I.; Chen, Y.R. Maxillary growth of adult patients with unoperated cleft: Answers to the debates. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 407–413. [Google Scholar] [CrossRef]
- Berkowitz, S.; Duncan, R.; Evans, C.; Friede, H.; Kuijpers-Jagtman, A.M.; Prahl-Anderson, B.; Rosenstein, S. Timing of Cleft Palate Closure Should Be Based on the Ratio of the Area of the Cleft to That of the Palatal Segments and Not on Age Alone. Plast. Reconstr. Surg. 2005, 115, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers-Jagtman, A.M.; Long, R.E., Jr. The influence of surgery and orthopedic treatment on maxillofacial growth and maxillary arch development in patients treated for orofacial clefts. Cleft Palate-Craniofac. J. 2000, 37, 527. [Google Scholar] [CrossRef]
- Nalabothu, P.; Benitez, B.K.; Dalstra, M.; Verna, C.; Mueller, A.A. Three-Dimensional Morphological Changes of the True Cleft under Passive Presurgical Orthopaedics in Unilateral Cleft Lip and Palate: A Retrospective Cohort Study. J. Clin. Med. 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C.K. Oral and Facial Deformity; Pitman: London, UK, 1954. [Google Scholar]
- Fish, J. Growth of the palatal shelves of post-alveolar cleft palate infants. Effects of stimulation appliances. Br. Dent. J. 1972, 132, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Huddart, A.G. Presurgical changes in unilateral cleft palate subjects. Cleft Palate J. 1979, 16, 147–157. [Google Scholar]
- Prahl, C.; Kuijpers-Jagtmann, A.M.; Van’t Hof, M.A.; Prahl-Andersen, B. A randomised prospective clinical trial into the effect of infant orthopaedics on maxillary arch dimensions in unilateral cleft lip and palate (Dutchcleft). Eur. J. Oral Sci. 2001, 109, 297–305. [Google Scholar] [CrossRef]
- Shaw, W.C.; Semb, G.; Nelson, P.; Brattström, V.; Mølsted, K.; Prahl-Andersen, B.; Gundlach, K.K.H. The Eurocleft project 1996-2000: Overview. J. Cranio-Maxillofac. Surg. 2001, 29, 131–140. [Google Scholar] [CrossRef]
- Dorrance, G.M.; Shirazy, E. The Operative Story of Cleft Palate; W.B. Saunders Company: Philadelphia, PA, USA, 1933. [Google Scholar]
- Campbell, B.Y.A. The closure of congenital clefts of the hard palate. Br. J. Surg. 1926, 13, 715–719. [Google Scholar] [CrossRef]
- Pichler, H. Zur Operation der doppelten Lippen-Gaumenspalten. Dtsch. Z. Cir. 1926, 195, 104–107. [Google Scholar] [CrossRef]
- Bütow, K.W. Caudally-based single-layer septum-vomer flap for cleft palate closure. J. Cranio-Maxillofac. Surg. 1987, 15, 10–13. [Google Scholar] [CrossRef]
- Abyholm, F.E. Primary Closure of Cleft Lip and Palate. In Facial Clefts and Craniosynostosis: Principles and Management; Turvey, T.A., Vig, K.W.L., Fonseca, R.J., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1996. [Google Scholar]
- Langenbeck, B. Die Uranoplastik mittelst Ablösung des mucös-periostalen Gaumenüberzuges. Uranoplasty by means of raising mucoperiosteal flaps. Hirschwald: Berlin, 1861. Plast. Reconstr. Surg. 1972, 49, 326–330. [Google Scholar] [CrossRef]
- Veau, V.; Borel, S. Division palatine: Anatomie, Chirurgie Phonétique/Victor Veau, Avec la Collaboration de S. Borel; Masson: Paris, France, 1931. [Google Scholar]
- Bardach, J. Two-Flap palatoplasty: Bardach’s technique. Oper. Tech. Plast. Reconstr. Surg. 1995, 2, 211–214. [Google Scholar] [CrossRef]
- Millard, D.R., Jr. The island flap in cleft palate surgery. Surg. Gynecol. Obstet. 1963, 116, 297–300. [Google Scholar] [CrossRef]
- Mukherji, M.M. Cheek flap for short palates. Cleft Palate J. 1969, 6, 415–420. [Google Scholar] [PubMed]
- Stricker, M.; Chancholle, A.R.; Flot, F.; Malka, G.; Montoya, A. La greffe periostee dans la reparation de la fente totale du palais primaire [Periosteal graft in the repair of complete primary cleft palate]. Ann. Chir. Plast. 1977, 22, 117–125. [Google Scholar]
- Neiva, C.; Dakpe, S.; Gbaguidi, C.; Testelin, S.; Devauchelle, B. Calvarial periosteal graft for second-stage cleft palate surgery: A preliminary report. J. Cranio-Maxillofac. Surg. 2014, 42, e117–e124. [Google Scholar] [CrossRef]
- Shash, H.; Al-Halabi, B.; Jozaghi, Y.; Aldekhayel, S.; Gilardino, M.S. A review of tissue expansion-assisted techniques of cleft palate repair. J. Craniofac. Surg. 2016, 27, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Alkan, A.; Baş, B.; Özer, M.; Bayram, M. Closure of a large palatal fistula with maxillary segmental distraction osteogenesis in a cleft palate patient. Cleft Palate-Craniofac. J. 2007, 44, 112–115. [Google Scholar] [CrossRef]
- Martín-Del-Campo, M.; Rosales-Ibañez, R.; Rojo, L. Biomaterials for cleft lip and palate regeneration. Int. J. Mol. Sci. 2019, 20, 2176. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kung, L.; Jones, C.; Casap, N. Induced osteogenesis by periosteal distraction. J. Oral Maxillofac. Surg. 2002, 60, 1170–1175. [Google Scholar] [CrossRef]
- Sencimen, M.; Aydintug, Y.S.; Ortakoglu, K.; Karslioglu, Y.; Gunhan, O.; Gunaydin, Y. Histomorphometrical analysis of new bone obtained by distraction osteogenesis and osteogenesis by periosteal distraction in rabbits. Int. J. Oral Maxillofac. Surg. 2007, 36, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Haruyama, N.; Shimizu, Y.; Hara, J.; Kawamura, H. Osteogenesis by gradually expanding the interface between bone surface and periosteum enhanced by bone marrow stem cell administration in rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, M.A.; Darendeliler, A.; Mandurino, M. Clinical application of magnets in orthodontics and biological implications: A review. Eur. J. Orthod. 1997, 19, 431–442. [Google Scholar] [CrossRef]
- Brand, R.A.; Stanford, C.M.; Swan, C.C. How do tissues respond and adapt to stresses around a prosthesis? A primer on finite element stress analysis for orthopaedic surgeons. Iowa Orthop. J. 2003, 23, 13–22. [Google Scholar]
- Ghasemianpour, M.; Ehsani, S.; Tahmasbi, S.; Bayat, M.; Ghorbanpour, M.; Safavi, S.M.; Mirhashemi, F.S. Distraction osteogenesis for cleft palate closure: A finite element analysis. Dent. Res. J. 2014, 11, 92–99. [Google Scholar]
- Nalabothu, P.; Verna, C.; Steineck, M.; Mueller, A.A.; Dalstra, M. The biomechanical evaluation of magnetic forces to drive osteogenesis in newborn’s with cleft lip and palate. J. Mater. Sci. Mater. Med. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Gundlach, K.K.H.; Maus, C. Epidemiological studies on the frequency of clefts in Europe and world-wide. J. Cranio-Maxillofac. Surg. 2006, 34, 1–2. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, L.; Wang, C.; Qian, Y.; Pan, X. Biomechanical analysis of rapid maxillary expansion in the UCLP patient. Med. Eng. Phys. 2009, 31, 409–417. [Google Scholar] [CrossRef]
- McPherson, G.K.; Kriewall, T.J. The elastic modulus of fetal cranial bone: A first step towards an understanding of the biomechanics of fetal head molding. J. Biomech. 1980, 13, 9–16. [Google Scholar] [CrossRef]
- Bauer, F.X.; Heinrich, V.; Grill, F.D.; Wölfle, F.; Hedderich, D.M.; Rau, A.; Wolff, K.D.; Ritschl, L.M.; Loeffelbein, D.J. Establishment of a finite element model of a neonate’s skull to evaluate the stress pattern distribution resulting during nasoalveolar molding therapy of cleft lip and palate patients. J. Cranio-Maxillofac. Surg. 2018, 46, 660–667. [Google Scholar] [CrossRef]
- Pan, X.; Qian, Y.; Yu, J.; Wang, D. Biomechanical Effects of Rapid Palatal Expansion on the Craniofacial Skeleton with cleft palate: A three-dimensional finite element analysis. Cleft Palate-Craniofac. J. 2007, 44, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.R.; Schwab, G.H.; Spengler, D.M. Tensile fracture of cancellous bone. Acta Orthop. 1980, 51, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Ishikawa, H.; Chu, S.; Handa, A.; Iida, J.; Yoshida, S. Constriction of the maxillary dental arch by mucoperiosteal denudation of the palate. Cleft Palate-Craniofac. J. 2002, 39, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Shetye, P.R.; Evans, C.A. Midfacial morphology in adult unoperated complete unilateral cleft lip and palate patients. Angle Orthod. 2006, 76, 810–816. [Google Scholar] [CrossRef]
- Grayson, B.H.; Cutting, C.B. Presurgical nasoalveolar orthopedic molding in primary correction of the nose, lip, and alveolus of infants born with unilateral and bilateral clefts. Cleft Palate-Craniofac. J. 2001, 38, 193–198. [Google Scholar] [CrossRef]
- Cutting, C.B.; Ortolani, A.; Bianchi, M.; Mosca, M.; Caravelli, S.; Fuiano, M.; Marcacci, M.; Russo, A. The prospective opportunities offered by magnetic scaffolds for bone tissue engineering: A review. Joints 2017, 4, 228–235. [Google Scholar] [CrossRef]
- Ashley-Montagu, M.F. The form and dimensions of the palate in the newborn. Int. J. Orthod. Dent. Child. 1934, 20, 810–827. [Google Scholar] [CrossRef]
- Frost, H.M. The Utah paradigm of skeletal physiology: An overview of its insights for bone, cartilage and collagenous tissue organs. J. Bone Miner. Metab. 2000, 18, 305–316. [Google Scholar] [CrossRef]
- Stern, M.; Dodson, T.B.; Longaker, M.T.; Lorenz, H.P.; Harrison, M.R.; Kaban, L.B. Fetal cleft lip repair in lambs: Histologic characteristics of the healing wound. Int. J. Oral Maxillofac. Surg. 1993, 22, 371–374. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalabothu, P.; Verna, C.; Benitez, B.K.; Dalstra, M.; Mueller, A.A. Load Transfer during Magnetic Mucoperiosteal Distraction in Newborns with Complete Unilateral and Bilateral Orofacial Clefts: A Three-Dimensional Finite Element Analysis. Appl. Sci. 2020, 10, 7728. https://doi.org/10.3390/app10217728
Nalabothu P, Verna C, Benitez BK, Dalstra M, Mueller AA. Load Transfer during Magnetic Mucoperiosteal Distraction in Newborns with Complete Unilateral and Bilateral Orofacial Clefts: A Three-Dimensional Finite Element Analysis. Applied Sciences. 2020; 10(21):7728. https://doi.org/10.3390/app10217728
Chicago/Turabian StyleNalabothu, Prasad, Carlalberta Verna, Benito K. Benitez, Michel Dalstra, and Andreas A. Mueller. 2020. "Load Transfer during Magnetic Mucoperiosteal Distraction in Newborns with Complete Unilateral and Bilateral Orofacial Clefts: A Three-Dimensional Finite Element Analysis" Applied Sciences 10, no. 21: 7728. https://doi.org/10.3390/app10217728
APA StyleNalabothu, P., Verna, C., Benitez, B. K., Dalstra, M., & Mueller, A. A. (2020). Load Transfer during Magnetic Mucoperiosteal Distraction in Newborns with Complete Unilateral and Bilateral Orofacial Clefts: A Three-Dimensional Finite Element Analysis. Applied Sciences, 10(21), 7728. https://doi.org/10.3390/app10217728





