Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Instrument
2.2. Experimental Method and Theoretical Calculation
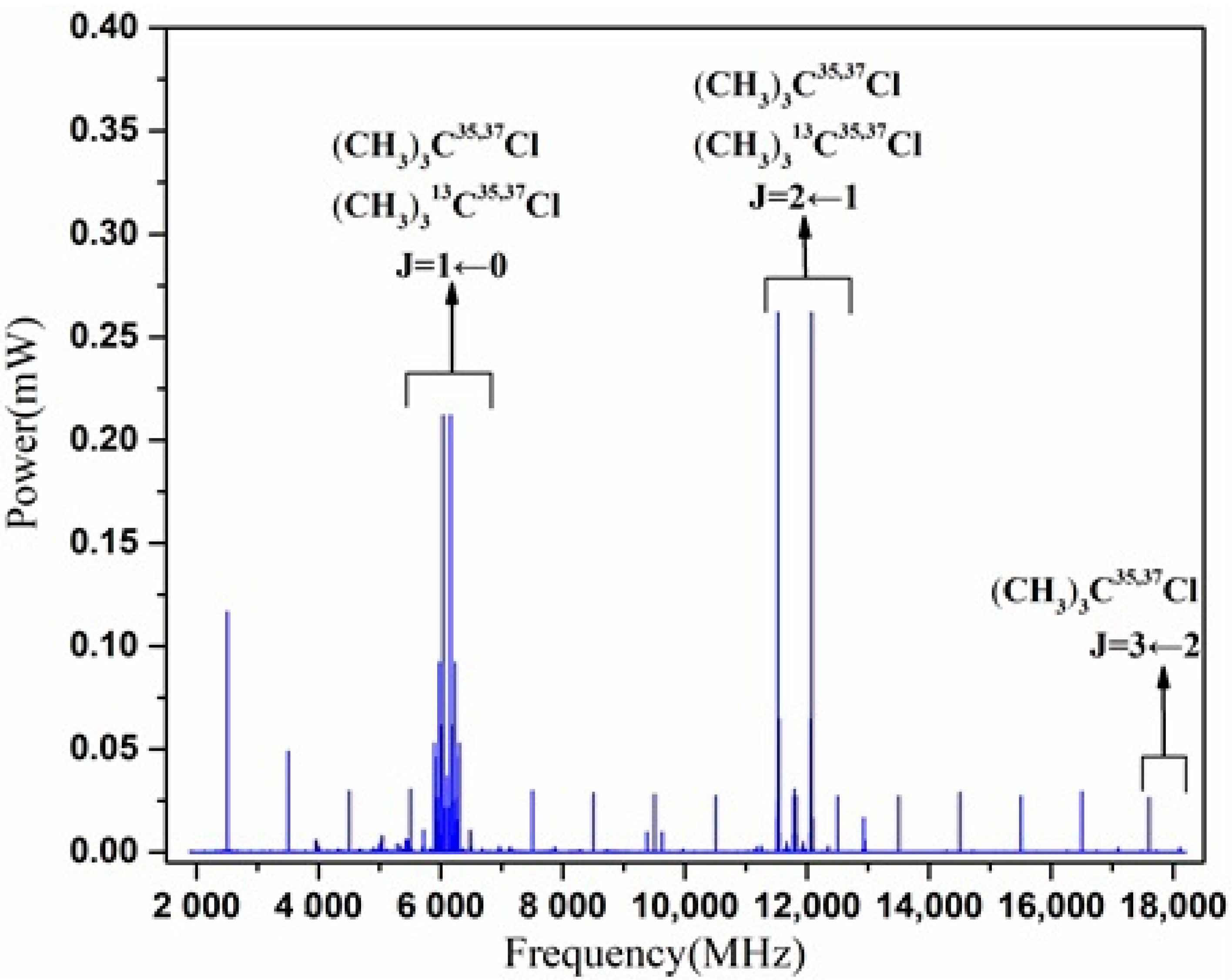
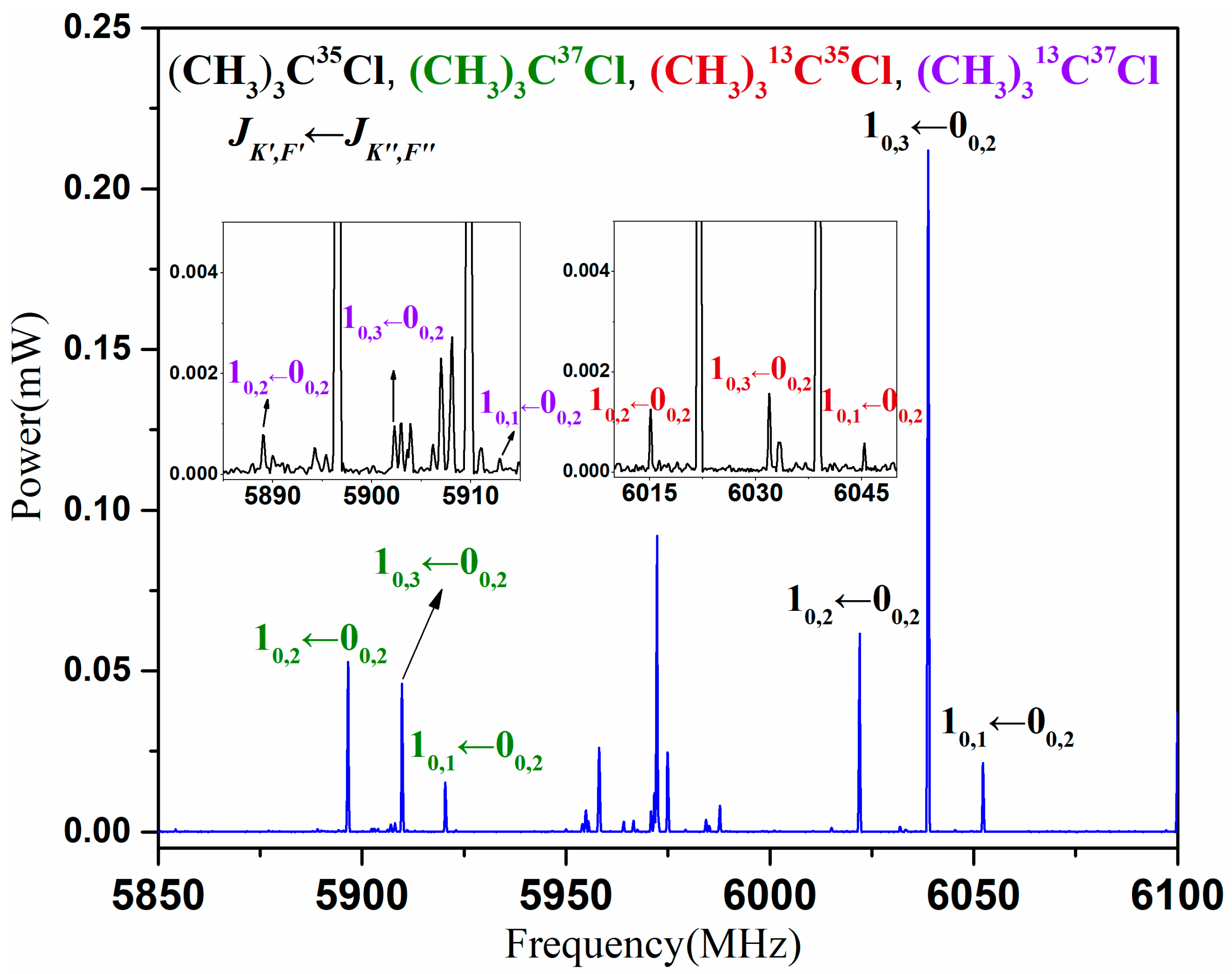
3. Results
4. Analysis and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gordy, W. Microwave spectroscopy. Rev. Mod. Phys. 1948, 20, 668–717. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Sugie, M.; Takeo, H.; Matsumura, C.; Kuchitsu, K. Rotational isomerism as studied by nuclear quadrupole coupling: Theoretical and experimental 14NX tensors for hydrazine, methylhydrazine, and 1,2-dimethylhydrazine. J. Mol. Struct. 1985, 126, 321–330. [Google Scholar] [CrossRef]
- Patterson, D.; Schnell, M.; Doyle, J.M. Enantiomer-specific detection of chiral molecules via microwave spectroscopy. Nat. Cell Biol. 2013, 497, 475–477. [Google Scholar] [CrossRef]
- Li, L.; Sun, M.; Li, X.-H.; Zhao, Z.-W.; Ma, H.-M.; Gan, H.-Y.; Lin, Z.-H.; Shi, S.-C.; Ziurys, L.M. Recent Advances on Rotational Spectroscopy and Microwave Spectroscopic Techniques. Chin. J. Anal. Chem. 2014, 42, 1369–1378. [Google Scholar] [CrossRef]
- Møllendal, H.; Samdal, S.; Guillemin, J.-C. Microwave and Quantum-Chemical Study of Conformational Properties and Intramolecular Hydrogen Bonding of 2-Hydroxy-3-Butynenitrile (HC≡CCH(OH)C≡N). J. Phys. Chem. A 2015, 119, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Kamaee, M.; Sun, M.; Luong, H.; Van Wijngaarden, J. Investigation of Structural Trends in Mono-, Di-, and Pentafluorobenzonitriles Using Fourier Transform Microwave Spectroscopy. J. Phys. Chem. A 2015, 119, 10279–102929. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kamaee, M.; Van Wijngaarden, J. Microwave Spectroscopic Investigation and Structural Determination of the Ar–Difluoropyridine van der Waals Complexes. J. Phys. Chem. A 2014, 118, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Balle, T.J.; Flygare, W.H. Fabry–Perot cavity pulsed Fourier transform microwave spectrometer with a pulsed nozzle particle source. Rev. Sci. Instrum. 1981, 52, 33–45. [Google Scholar] [CrossRef]
- Brown, G.G.; Dian, B.C.; Douglass, K.O.; Geyer, S.M.; Shipman, S.T.; Pate, B.H. A broadband Fourier transform microwave spectrometer based on chirped pulse excitation. Rev. Sci. Instrum. 2008, 79, 053103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Chan, E.H.W.; Wang, X.; Feng, X.; Guan, B.-O. Microwave Photonic Devices Based on Liquid Crystal on Silicon Technology. Appl. Sci. 2019, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- El Mostrah, A.; Muller, A.; Favennec, J.-F.; Potelon, B.; Manchec, A.; Rius, E.; Quendo, C.; Clavet, Y.; Doukhan, F.; Le Nezet, J. An RF-MEMS-Based Digitally Tunable SIW Filter in X-Band for Communication Satellite Applications. Appl. Sci. 2019, 9, 1838. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Yangyu, F.; Tian, Z.; Wang, W.; Kang, B.; Jiang, W.; Gao, Y. An Ultra-Wideband Microwave Photonic Channelized Receiver with Zero-IF Architecture. Appl. Sci. 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Medcraft, C.; Mullaney, J.C.; Walker, N.R.; Legon, A.C. A complex Ar⋅⋅⋅Ag−I produced by laser ablation and characterised by rotational spectroscopy and ab initio calculations: Variation of properties along the series Ar⋅⋅⋅Ag−X (X = F, Cl, Br and I). J. Mol. Spectrosc. 2017, 335, 61–67. [Google Scholar] [CrossRef]
- Sun, M.; Halfen, D.T.; Min, J.; Harris, B.; Clouthier, D.J.; Ziurys, L.M. The rotational spectrum of CuCCH (1∑+): A Fourier transform microwave discharge assisted laser ablation spectroscopy and millimeter/submillimeter study. J. Chem. Phys. 2010, 133, 174301. [Google Scholar] [CrossRef]
- Hernandez-Castillo, A.O.; Abeysekera, C.; Hays, B.M.; Kleiner, I.; Nguyen, H.V.L.; Zwier, T.S. Conformational preferences and internal rotation of methyl butyrate by microwave spectroscopy. J. Mol. Spectrosc. 2017, 337, 51–58. [Google Scholar] [CrossRef]
- Thomas, J.; Sukhorukov, O.; Jager, W.; Xu, Y.J. Chirped-Pulse and Cavity-Based Fourier Transform Microwave Spectra of the Methyl Lactate…Ammonia Adduct. Angew. Chem. Int. Ed. 2013, 52, 4402–4405. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.D.; Seifert, N.A.; Heger, M.; Xie, F.; Thomas, J.; Xu, Y. Conformational dynamics of 1-phenyl-2,2,2-trifluoroethanol by rotational spectroscopy and ab initio calculations. J. Mol. Spectrosc. 2018, 351, 62–67. [Google Scholar] [CrossRef]
- Marshall, F.E.; Sedo, G.; West, C.; Pate, B.H.; Allpress, S.M.; Evans, C.; Godfrey, P.D.; McNaughton, D.; Grubbs, G. The rotational spectrum and complete heavy atom structure of the chiral molecule verbenone. J. Mol. Spectrosc. 2017, 342, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Pate, B.H. Taking the Pulse of Molecular Rotational Spectroscopy. Science 2011, 333, 947–948. [Google Scholar] [CrossRef] [PubMed]
- Wehres, N.; Hermanns, M.; Wilkins, O.H.; Borisov, K.; Lewen, F.; Grabow, J.-U.; Schlemmer, S.; Müller, H. Rotational spectroscopy of the two conformers of 3-methylbutyronitrile (C4H9CN) between 2 and 400 GHz. Astron. Astrophys. 2018, 615, A140. [Google Scholar] [CrossRef] [Green Version]
- Kisiel, Z.; BialÃkowska-Jaworska, E.; Desyatnyk, O.; Pietrewicz, B.A.; Pszcz´olkowski, L. The Gas-Phase Electric Dipole Moments of the Symmetric Top Tertiary Butyl Molecules tBuX, X=F, Cl, Br, I, CN, and NC. J. Mol. Spectrosc. 2001, 208, 113–120. [Google Scholar] [CrossRef]
- Williams, J.Q.; Gordy, W. Microwave Spectra and Molecular Constants of Tertiary Butyl Chloride, Bromide, and Iodide. J. Chem. Phys. 1950, 18, 994. [Google Scholar] [CrossRef]
- Gierszal, S.; Miś-Kuźmińska, E.; Stankowski, J.; Galica, J. Stark effect in the J = 3–4 microwave line of tert-butyl chloride molecule. J. Mol. Struct. 1984, 114, 429–432. [Google Scholar] [CrossRef]
- Ellis, M.; Legon, A.; Rego, C.; Millen, D. Cl-nuclear quadrupole coupling in the microwave spectra of 1-chloroadamantane and t-butyl chloride. J. Mol. Struct. THEOCHEM 1989, 200, 353–359. [Google Scholar] [CrossRef]
- Kassi, S.; Petitprez, D.; Wlodarczak, G. Microwave Fourier transform spectroscopy of t -butylchloride and t -butylbromide isotopic species. J. Mol. Struct. 2000, 517–518, 375–386. [Google Scholar] [CrossRef]
- Jiao, C.; Duan, S.W.; Xu, K.Y.; Wu, Y.; Sun, M.; Li, L.; Gu, W.H.; Xian, L.L.; Zhang, Y.Z.; Chen, Q.; et al. Detection of a Chemical Reaction by a 1~18 GHz Chirped-Pulse Fourier Transform Microwave Spectrometer. Spectrosc. Sepct. Anal. 2020, 40, 991–996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B. Gaussian03, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2003. [Google Scholar]
- Thompson, H.B. Calculation of Cartesian Coordinates and Their Derivatives from Internal Molecular Coordinates. J. Chem. Phys. 1967, 47, 3407–3410. [Google Scholar] [CrossRef]
- Pickett, H.M. The fitting and prediction of vibration-rotation spectra with spin interactions. J. Mol. Spectrosc. 1991, 148, 371–377. [Google Scholar] [CrossRef]
- McMahon, T.J.; Bailey, J.R.; Bird, R.G. Structure and dynamics of succinic, methylsuccinic and itaconic anhydrides in the gas phase. J. Mol. Spectrosc. 2018, 347, 35–40. [Google Scholar] [CrossRef]
- Huff, A.K.; Smith, C.; Leopold, K.R. Microwave spectrum and structure of the lowest A—internal rotor state of Ar⋅⋅⋅CH3I. J. Mol. Spectrosc. 2018, 353, 6–10. [Google Scholar] [CrossRef]
- Ferres, L.; Stahl, W.; Kleiner, I.; Nguyen, H.V.L. The effect of internal rotation in p-methyl anisole studied by microwave spectroscopy. J. Mol. Spectrosc. 2018, 343, 44–49. [Google Scholar] [CrossRef]
- Wodyński, A.; Pecul, M. The influence of a presence of a heavy atom on the spin-spin coupling constants between two light nuclei in organometallic compounds and halogen derivatives. J. Chem. Phys. 2014, 140, 24319. [Google Scholar] [CrossRef]
- Anablea, J.P.; Hirda, D.E.; Stephensa, S.L.; Zaleskia, D.P.; Walkera, N.R.; Legonb, A.C. Characterisation of the weak halogen bond in N2⋯ICF3 by purerotational spectroscopy. Chem. Phys. Lett. 2015, 625, 179–185. [Google Scholar] [CrossRef] [Green Version]


| Spectral Parameter | Tert-Butyl Chloride and Its Isotopologues | |||
|---|---|---|---|---|
| (CH3)3C35Cl d | (CH3)3C37Cl d | (CH3)313C35Cl | (CH3)313C37Cl | |
| A(MHz) a | 4557.36740 | 4557.36740 | 4571.70485 | 4571.70485 |
| B(MHz) a | 3035.35346 | 2970.78357 | 3025.45983 | 2960.51347 |
| C(MHz) a | 3035.35068 | 2970.78091 | 3025.45734 | 2960.51109 |
| I. a(amu·Å2) b | 110.892750 | 110.892750 | 110.544977 | 110.544977 |
| I. b(amu·Å2) b | 166.497580 | 170.116399 | 167.042048 | 170.706538 |
| I. c(amu·Å2) b | 166.497732 | 170.116552 | 167.042185 | 170.706575 |
| P. a(amu·Å2/s) c | 111.051277 | 114.670097 | 111.769630 | 115.434120 |
| P. b(amu·Å2/s) c | 55.446453 | 55.446453 | 55.272556 | 55.272556 |
| P. c(amu·Å2/s) c | 55.446301 | 55.446301 | 55.272419 | 55.272419 |
| Spectral Parameter | Tert-Butyl Chloride and Its Isotopologues | |||
|---|---|---|---|---|
| (CH3)3C35Cl | (CH3)3C37Cl | (CH3)313C35Cl | (CH3)313C37Cl | |
| B(MHz) | 3017.721862(66) | 2953.570814(67) | 3014.284148(62) | 2949.821282(65) |
| 3017.7177(9) g | 2953.5717(8) g | 3014.285415(40) f | 2949.8215(62) f | |
| DJ(kHz) b | 0.9675(50) | 0.3808(52) | −1.6551(42) | −0.4812(40) |
| 0.6(1) g | 0.6(l) g | 0.518(27) f | 0.511(35) f | |
| DJK(kHz) b | −0.2807(153) | −0.8866(162) | 0.01313(128) | −0.7978(100) |
| 1.2(3) g | 1.2(3) g | 1.158(73) f | 1.246(26) f | |
| eQq(MHz) c | −67.25407(104) | −53.10206(107) | −67.33081(93) | −53.07129(96) |
| −67.312(3) g | −53.053(3) g | −67.3266(41) f | −53.0694(64) f | |
| RMS(MHz) d | 0.0021 | 0.002369 | 0.001825 | 0.002044 |
| Ne | 24 | 22 | 30 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, C.; Duan, S.-w.; Wu, Y.; Sun, M.; Chen, Q.; Fang, P.-y.; Wang, D.-p. Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy. Appl. Sci. 2020, 10, 7650. https://doi.org/10.3390/app10217650
Jiao C, Duan S-w, Wu Y, Sun M, Chen Q, Fang P-y, Wang D-p. Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy. Applied Sciences. 2020; 10(21):7650. https://doi.org/10.3390/app10217650
Chicago/Turabian StyleJiao, Chao, Sheng-wen Duan, Yi Wu, Ming Sun, Qian Chen, Pei-yu Fang, and Da-peng Wang. 2020. "Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy" Applied Sciences 10, no. 21: 7650. https://doi.org/10.3390/app10217650
APA StyleJiao, C., Duan, S.-w., Wu, Y., Sun, M., Chen, Q., Fang, P.-y., & Wang, D.-p. (2020). Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy. Applied Sciences, 10(21), 7650. https://doi.org/10.3390/app10217650




