Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy
Abstract
1. Introduction
2. Experiment
2.1. Experimental Instrument
2.2. Experimental Method and Theoretical Calculation
3. Results
4. Analysis and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gordy, W. Microwave spectroscopy. Rev. Mod. Phys. 1948, 20, 668–717. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Sugie, M.; Takeo, H.; Matsumura, C.; Kuchitsu, K. Rotational isomerism as studied by nuclear quadrupole coupling: Theoretical and experimental 14NX tensors for hydrazine, methylhydrazine, and 1,2-dimethylhydrazine. J. Mol. Struct. 1985, 126, 321–330. [Google Scholar] [CrossRef]
- Patterson, D.; Schnell, M.; Doyle, J.M. Enantiomer-specific detection of chiral molecules via microwave spectroscopy. Nat. Cell Biol. 2013, 497, 475–477. [Google Scholar] [CrossRef]
- Li, L.; Sun, M.; Li, X.-H.; Zhao, Z.-W.; Ma, H.-M.; Gan, H.-Y.; Lin, Z.-H.; Shi, S.-C.; Ziurys, L.M. Recent Advances on Rotational Spectroscopy and Microwave Spectroscopic Techniques. Chin. J. Anal. Chem. 2014, 42, 1369–1378. [Google Scholar] [CrossRef]
- Møllendal, H.; Samdal, S.; Guillemin, J.-C. Microwave and Quantum-Chemical Study of Conformational Properties and Intramolecular Hydrogen Bonding of 2-Hydroxy-3-Butynenitrile (HC≡CCH(OH)C≡N). J. Phys. Chem. A 2015, 119, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Kamaee, M.; Sun, M.; Luong, H.; Van Wijngaarden, J. Investigation of Structural Trends in Mono-, Di-, and Pentafluorobenzonitriles Using Fourier Transform Microwave Spectroscopy. J. Phys. Chem. A 2015, 119, 10279–102929. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kamaee, M.; Van Wijngaarden, J. Microwave Spectroscopic Investigation and Structural Determination of the Ar–Difluoropyridine van der Waals Complexes. J. Phys. Chem. A 2014, 118, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Balle, T.J.; Flygare, W.H. Fabry–Perot cavity pulsed Fourier transform microwave spectrometer with a pulsed nozzle particle source. Rev. Sci. Instrum. 1981, 52, 33–45. [Google Scholar] [CrossRef]
- Brown, G.G.; Dian, B.C.; Douglass, K.O.; Geyer, S.M.; Shipman, S.T.; Pate, B.H. A broadband Fourier transform microwave spectrometer based on chirped pulse excitation. Rev. Sci. Instrum. 2008, 79, 053103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Chan, E.H.W.; Wang, X.; Feng, X.; Guan, B.-O. Microwave Photonic Devices Based on Liquid Crystal on Silicon Technology. Appl. Sci. 2019, 9, 260. [Google Scholar] [CrossRef]
- El Mostrah, A.; Muller, A.; Favennec, J.-F.; Potelon, B.; Manchec, A.; Rius, E.; Quendo, C.; Clavet, Y.; Doukhan, F.; Le Nezet, J. An RF-MEMS-Based Digitally Tunable SIW Filter in X-Band for Communication Satellite Applications. Appl. Sci. 2019, 9, 1838. [Google Scholar] [CrossRef]
- Chen, B.; Yangyu, F.; Tian, Z.; Wang, W.; Kang, B.; Jiang, W.; Gao, Y. An Ultra-Wideband Microwave Photonic Channelized Receiver with Zero-IF Architecture. Appl. Sci. 2019, 10, 30. [Google Scholar] [CrossRef]
- Medcraft, C.; Mullaney, J.C.; Walker, N.R.; Legon, A.C. A complex Ar⋅⋅⋅Ag−I produced by laser ablation and characterised by rotational spectroscopy and ab initio calculations: Variation of properties along the series Ar⋅⋅⋅Ag−X (X = F, Cl, Br and I). J. Mol. Spectrosc. 2017, 335, 61–67. [Google Scholar] [CrossRef]
- Sun, M.; Halfen, D.T.; Min, J.; Harris, B.; Clouthier, D.J.; Ziurys, L.M. The rotational spectrum of CuCCH (1∑+): A Fourier transform microwave discharge assisted laser ablation spectroscopy and millimeter/submillimeter study. J. Chem. Phys. 2010, 133, 174301. [Google Scholar] [CrossRef]
- Hernandez-Castillo, A.O.; Abeysekera, C.; Hays, B.M.; Kleiner, I.; Nguyen, H.V.L.; Zwier, T.S. Conformational preferences and internal rotation of methyl butyrate by microwave spectroscopy. J. Mol. Spectrosc. 2017, 337, 51–58. [Google Scholar] [CrossRef]
- Thomas, J.; Sukhorukov, O.; Jager, W.; Xu, Y.J. Chirped-Pulse and Cavity-Based Fourier Transform Microwave Spectra of the Methyl Lactate…Ammonia Adduct. Angew. Chem. Int. Ed. 2013, 52, 4402–4405. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.D.; Seifert, N.A.; Heger, M.; Xie, F.; Thomas, J.; Xu, Y. Conformational dynamics of 1-phenyl-2,2,2-trifluoroethanol by rotational spectroscopy and ab initio calculations. J. Mol. Spectrosc. 2018, 351, 62–67. [Google Scholar] [CrossRef]
- Marshall, F.E.; Sedo, G.; West, C.; Pate, B.H.; Allpress, S.M.; Evans, C.; Godfrey, P.D.; McNaughton, D.; Grubbs, G. The rotational spectrum and complete heavy atom structure of the chiral molecule verbenone. J. Mol. Spectrosc. 2017, 342, 109–115. [Google Scholar] [CrossRef]
- Pate, B.H. Taking the Pulse of Molecular Rotational Spectroscopy. Science 2011, 333, 947–948. [Google Scholar] [CrossRef] [PubMed]
- Wehres, N.; Hermanns, M.; Wilkins, O.H.; Borisov, K.; Lewen, F.; Grabow, J.-U.; Schlemmer, S.; Müller, H. Rotational spectroscopy of the two conformers of 3-methylbutyronitrile (C4H9CN) between 2 and 400 GHz. Astron. Astrophys. 2018, 615, A140. [Google Scholar] [CrossRef]
- Kisiel, Z.; BialÃkowska-Jaworska, E.; Desyatnyk, O.; Pietrewicz, B.A.; Pszcz´olkowski, L. The Gas-Phase Electric Dipole Moments of the Symmetric Top Tertiary Butyl Molecules tBuX, X=F, Cl, Br, I, CN, and NC. J. Mol. Spectrosc. 2001, 208, 113–120. [Google Scholar] [CrossRef]
- Williams, J.Q.; Gordy, W. Microwave Spectra and Molecular Constants of Tertiary Butyl Chloride, Bromide, and Iodide. J. Chem. Phys. 1950, 18, 994. [Google Scholar] [CrossRef]
- Gierszal, S.; Miś-Kuźmińska, E.; Stankowski, J.; Galica, J. Stark effect in the J = 3–4 microwave line of tert-butyl chloride molecule. J. Mol. Struct. 1984, 114, 429–432. [Google Scholar] [CrossRef]
- Ellis, M.; Legon, A.; Rego, C.; Millen, D. Cl-nuclear quadrupole coupling in the microwave spectra of 1-chloroadamantane and t-butyl chloride. J. Mol. Struct. THEOCHEM 1989, 200, 353–359. [Google Scholar] [CrossRef]
- Kassi, S.; Petitprez, D.; Wlodarczak, G. Microwave Fourier transform spectroscopy of t -butylchloride and t -butylbromide isotopic species. J. Mol. Struct. 2000, 517–518, 375–386. [Google Scholar] [CrossRef]
- Jiao, C.; Duan, S.W.; Xu, K.Y.; Wu, Y.; Sun, M.; Li, L.; Gu, W.H.; Xian, L.L.; Zhang, Y.Z.; Chen, Q.; et al. Detection of a Chemical Reaction by a 1~18 GHz Chirped-Pulse Fourier Transform Microwave Spectrometer. Spectrosc. Sepct. Anal. 2020, 40, 991–996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B. Gaussian03, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2003. [Google Scholar]
- Thompson, H.B. Calculation of Cartesian Coordinates and Their Derivatives from Internal Molecular Coordinates. J. Chem. Phys. 1967, 47, 3407–3410. [Google Scholar] [CrossRef]
- Pickett, H.M. The fitting and prediction of vibration-rotation spectra with spin interactions. J. Mol. Spectrosc. 1991, 148, 371–377. [Google Scholar] [CrossRef]
- McMahon, T.J.; Bailey, J.R.; Bird, R.G. Structure and dynamics of succinic, methylsuccinic and itaconic anhydrides in the gas phase. J. Mol. Spectrosc. 2018, 347, 35–40. [Google Scholar] [CrossRef]
- Huff, A.K.; Smith, C.; Leopold, K.R. Microwave spectrum and structure of the lowest A—internal rotor state of Ar⋅⋅⋅CH3I. J. Mol. Spectrosc. 2018, 353, 6–10. [Google Scholar] [CrossRef]
- Ferres, L.; Stahl, W.; Kleiner, I.; Nguyen, H.V.L. The effect of internal rotation in p-methyl anisole studied by microwave spectroscopy. J. Mol. Spectrosc. 2018, 343, 44–49. [Google Scholar] [CrossRef]
- Wodyński, A.; Pecul, M. The influence of a presence of a heavy atom on the spin-spin coupling constants between two light nuclei in organometallic compounds and halogen derivatives. J. Chem. Phys. 2014, 140, 24319. [Google Scholar] [CrossRef]
- Anablea, J.P.; Hirda, D.E.; Stephensa, S.L.; Zaleskia, D.P.; Walkera, N.R.; Legonb, A.C. Characterisation of the weak halogen bond in N2⋯ICF3 by purerotational spectroscopy. Chem. Phys. Lett. 2015, 625, 179–185. [Google Scholar] [CrossRef]


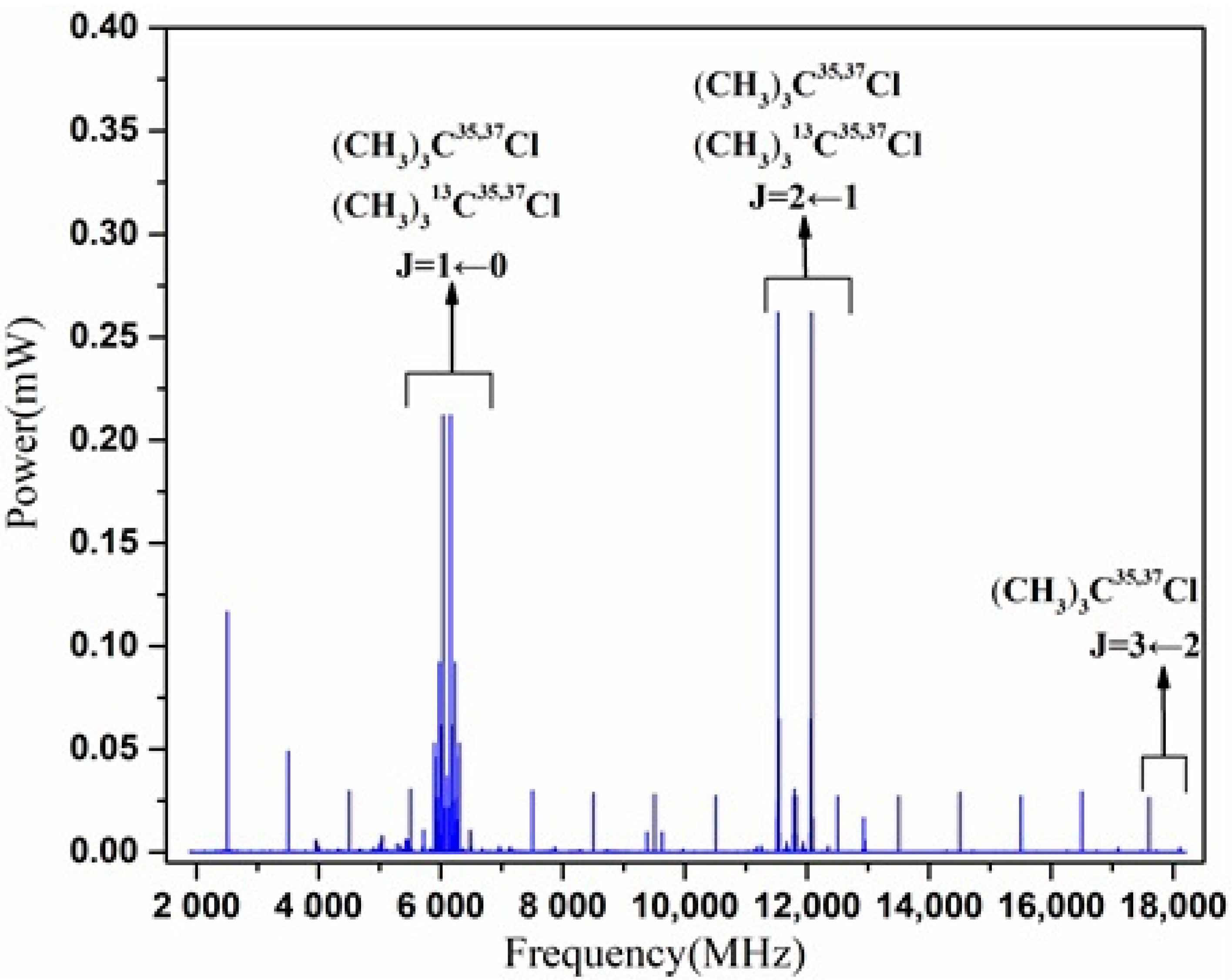
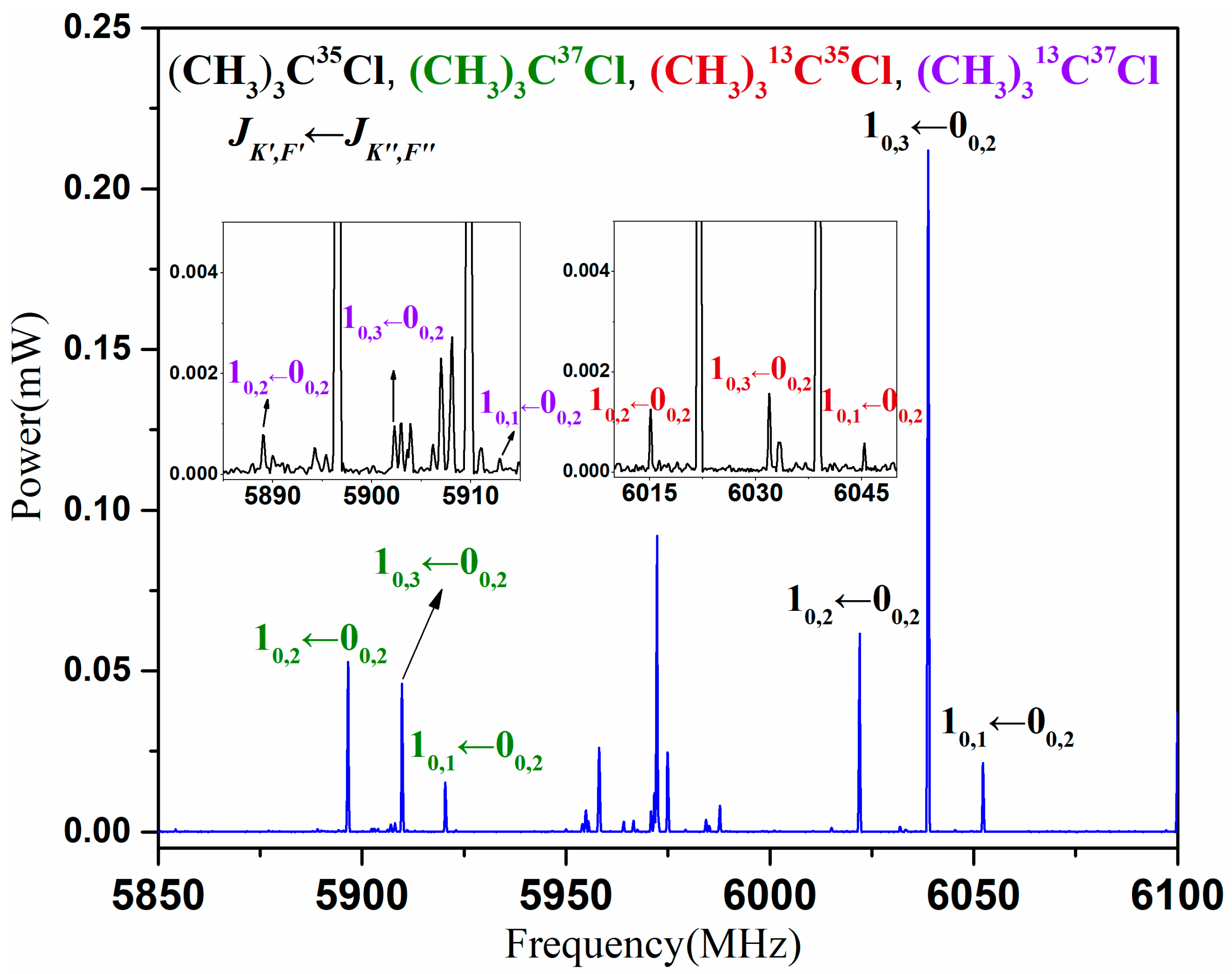
| Spectral Parameter | Tert-Butyl Chloride and Its Isotopologues | |||
|---|---|---|---|---|
| (CH3)3C35Cl d | (CH3)3C37Cl d | (CH3)313C35Cl | (CH3)313C37Cl | |
| A(MHz) a | 4557.36740 | 4557.36740 | 4571.70485 | 4571.70485 |
| B(MHz) a | 3035.35346 | 2970.78357 | 3025.45983 | 2960.51347 |
| C(MHz) a | 3035.35068 | 2970.78091 | 3025.45734 | 2960.51109 |
| I. a(amu·Å2) b | 110.892750 | 110.892750 | 110.544977 | 110.544977 |
| I. b(amu·Å2) b | 166.497580 | 170.116399 | 167.042048 | 170.706538 |
| I. c(amu·Å2) b | 166.497732 | 170.116552 | 167.042185 | 170.706575 |
| P. a(amu·Å2/s) c | 111.051277 | 114.670097 | 111.769630 | 115.434120 |
| P. b(amu·Å2/s) c | 55.446453 | 55.446453 | 55.272556 | 55.272556 |
| P. c(amu·Å2/s) c | 55.446301 | 55.446301 | 55.272419 | 55.272419 |
| Spectral Parameter | Tert-Butyl Chloride and Its Isotopologues | |||
|---|---|---|---|---|
| (CH3)3C35Cl | (CH3)3C37Cl | (CH3)313C35Cl | (CH3)313C37Cl | |
| B(MHz) | 3017.721862(66) | 2953.570814(67) | 3014.284148(62) | 2949.821282(65) |
| 3017.7177(9) g | 2953.5717(8) g | 3014.285415(40) f | 2949.8215(62) f | |
| DJ(kHz) b | 0.9675(50) | 0.3808(52) | −1.6551(42) | −0.4812(40) |
| 0.6(1) g | 0.6(l) g | 0.518(27) f | 0.511(35) f | |
| DJK(kHz) b | −0.2807(153) | −0.8866(162) | 0.01313(128) | −0.7978(100) |
| 1.2(3) g | 1.2(3) g | 1.158(73) f | 1.246(26) f | |
| eQq(MHz) c | −67.25407(104) | −53.10206(107) | −67.33081(93) | −53.07129(96) |
| −67.312(3) g | −53.053(3) g | −67.3266(41) f | −53.0694(64) f | |
| RMS(MHz) d | 0.0021 | 0.002369 | 0.001825 | 0.002044 |
| Ne | 24 | 22 | 30 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, C.; Duan, S.-w.; Wu, Y.; Sun, M.; Chen, Q.; Fang, P.-y.; Wang, D.-p. Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy. Appl. Sci. 2020, 10, 7650. https://doi.org/10.3390/app10217650
Jiao C, Duan S-w, Wu Y, Sun M, Chen Q, Fang P-y, Wang D-p. Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy. Applied Sciences. 2020; 10(21):7650. https://doi.org/10.3390/app10217650
Chicago/Turabian StyleJiao, Chao, Sheng-wen Duan, Yi Wu, Ming Sun, Qian Chen, Pei-yu Fang, and Da-peng Wang. 2020. "Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy" Applied Sciences 10, no. 21: 7650. https://doi.org/10.3390/app10217650
APA StyleJiao, C., Duan, S.-w., Wu, Y., Sun, M., Chen, Q., Fang, P.-y., & Wang, D.-p. (2020). Molecular Parameters of Tert-Butyl Chloride and Its Isotopologues Determined from High-Resolution Rotational Spectroscopy. Applied Sciences, 10(21), 7650. https://doi.org/10.3390/app10217650




