Microstructure and Magnetic Properties of La-Ca-Co Substituted M-Type Sr-Hexaferrites with Controlled Si Diffusion
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191. [Google Scholar] [CrossRef]
- Bai, J.; Liu, X.; Xie, T.; Wei, F.; Yang, Z. The effects of La–Zn substitution on the magnetic properties of Sr-magnetoplumbite ferrite nano-particles. Mater. Sci. Eng. 2000, 68, 182–185. [Google Scholar] [CrossRef]
- Ogata, Y.; Takami, T.; Kubota, Y. Development of La-Co Substituted Ferrite Magnets. J. Jpn. Soc. Powder Powder Metall. 2003, 50, 636–641. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hosokawa, S.; Oda, E.; Toyota, S. Magnetic properties and composition of Ca-La-Co M-type ferrites. J. Jpn. Soc. Powder Powder Metall. 2008, 55, 541–546. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Kwon, Y.-H.; Kim, M.-H.; Lee, D.-Y. Enhancement of magnetic properties in Mn–Zn substituted M-type Sr-hexaferrites. J. Magn. Magn. Mater. 2015, 382, 10–14. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Moon, K.-S. Magnetic properties of Ce–Mn substituted M-type Sr-hexaferrites. Ceram. Int. 2015, 41, 12828–12834. [Google Scholar] [CrossRef]
- Kang, Y.-M. High saturation magnetization in La–Ce–Zn–doped M-type Sr-hexaferrites. Ceram. Int. 2015, 41, 4354–4359. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nishiuchi, T.; Oda, E.; Nakagawa, T. Cation distribution analysis of Ca-La-Co M-type ferrites by neutron diffraction, extended X-ray absorption fine structure and X-ray magnetic circular dichroism. J. Jpn. Soc. Powder Powder Metall. 2016, 63, 101–108. [Google Scholar] [CrossRef][Green Version]
- Nishio, H.; Minachi, Y.; Yamamoto, H. Effect of Factors on Coercivity in Sr–La–Co Sintered Ferrite Magnets. IEEE Trans. Magn. 2009, 45, 5281–5288. [Google Scholar] [CrossRef]
- Sixtus, K.J.; Kronenberg, K.J.; Tenzer, R.K. Investigations on Barium Ferrite Magnets. J. Appl. Phys. 1956, 27, 1051–1057. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Lee, J.; Kang, Y.J.; Park, J.-B.; Kim, S.I.; Lee, S.M.; Ahn, K. Understanding on coercivity behavior of M-type strontium hexaferrite through thin-film experiment and micromagnetic modeling. Appl. Phys. Lett. 2013, 103, 122407. [Google Scholar] [CrossRef]
- Park, C.W.; Yoon, D.Y. Effects of SiO2, CaO2, and MgO additions on the grain growth of alumina. J. Am. Ceram. Soc. 2000, 83, 2605–2609. [Google Scholar] [CrossRef]
- Fisher, J.G.; Kim, M.S.; Lee, H.Y.; Kang, S.-J.L. Effect of Li2O and PbO additions on abnormal grain growth in the Pb(Mg1/3Nb2/3)O3-35 mol% PbTiO3 system. J. Am. Ceram. Soc. 2004, 87, 937–942. [Google Scholar] [CrossRef]
- Moon, K.-S.; Rout, D.; Lee, H.-Y.; Kang, S.-J.L. Effect of TiO2 addition on grain shape and grain coarsening behavior in 95Na1/2Bi1/2O3-BaTiO3. J. Eur. Ceram. Soc. 2011, 31, 1915–1920. [Google Scholar] [CrossRef]
- Cho, K.; Yoon, D.Y.; Kim, B.K. Surface roughening transition and coarsening of NbC grains in liquid cobalt-rich matrix. J. Am. Ceram. Soc. 2004, 87, 443–448. [Google Scholar] [CrossRef]
- Lee, B.K.; Chung, S.Y.; Kang, S.-J.L. Grain boundary faceting and abnormal grain growth in BaTiO3. Acta Mater. 2000, 48, 1575–1580. [Google Scholar] [CrossRef]
- Jung, Y.-I.; Choi, S.Y.; Kang, S.-J.L. Effect of oxygen partial pressure on grain boundary structure and grain growth behavior in BaTiO3. Acta Mater. 2006, 54, 2849–2855. [Google Scholar] [CrossRef]
- Fisher, J.G.; Rout, D.; Moon, K.-S.; Kang, S.-J.L. Structural changes in potassium sodium niobate ceramics sintered in different atmospheres. J. Alloy. Compd. 2009, 479, 467–472. [Google Scholar] [CrossRef]
- Boutz, M.M.R.; Winnubst, A.J.A.; Burggraaf, A.J. Yttria-ceria stabilized tetragonal zirconia polycrystals: Sintering, grain growth and grain boundary segregation. J. Eur. Ceram. Soc. 1994, 13, 89–102. [Google Scholar] [CrossRef]
- Kang, S.-J.L. Sintering: Densification, Grain Growth and Microstructure; Elsevier Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Howe, J.M. Interfaces in Materials, 1st ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Jung, Y.I.; Yoon, D.Y.; Kang, S.-J.L. Coarsening of polyhedral grains in a liquid matrix. J. Mater. Res. 2009, 24, 2949–2959. [Google Scholar] [CrossRef]
- Moon, K.-S.; Kang, Y.-M.; Han, I.; Lee, S.-E. Grain growth behavior of Ba1.5Sr1.5Co2Fe24O41 flakes in molten salt synthesis and the magnetic properties of flake/polymer composites. J. Appl. Phys. 2016, 120, 194102. [Google Scholar] [CrossRef]
- Moon, K.-S.; Kang, Y.-M. Structural and magnetic properties of Ca-Mn-Zn-substituted M-type Sr-hexaferrites. J. Eur. Ceram. Soc. 2016, 36, 3383–3389. [Google Scholar] [CrossRef]
- Fisher, J.G.; Sun, H.; Kook, Y.-G.; Kim, J.-S.; Le, P.G. Growth of single crystals of BaFe12O19 by solid state crystal growth. J. Magn. Magn. Mater. 2016, 416, 384–390. [Google Scholar] [CrossRef]
- Moon, K.-S.; Kang, Y.-M. Synthesis, structure, and magnetic properties of M-W hexaferrite composites. Ceram. Int. 2017, 43, 14309–14313. [Google Scholar] [CrossRef]
- Moon, K.-S.; Lim, E.-S.; Kang, Y.-M. Effect of Ca and La substitution on the structure and magnetic properties of M-type Sr-hexaferrites. J. Alloy. Compd. 2019, 771, 350–355. [Google Scholar] [CrossRef]
- Park, Y.J.; Hwang, N.M.; Yoon, D.Y. Abnormal growth of faceted (WC) grains in a (Co) liqiud matrix. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1996, 27, 2809–2819. [Google Scholar] [CrossRef]
- Rohrer, G.S.; Rohrer, C.L.; Mullins, W.W. Coarsening of faceted crystals. J. Am. Ceram. Soc. 2002, 85, 675–682. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kang, S.-J.L.; Chung, S.Y. Abnormal grain growth and intergranular amorphous film formation in BaTiO3. J. Am. Ceram. Soc. 2007, 90, 645–648. [Google Scholar] [CrossRef]
- Töpfer, J.; Schwarzer, S.; Senz, S.; Hesse, D. Influence of SiO2 and CaO additions on the microstructure and magnetic properties of sintered Sr-hexaferrite. J. Eur. Ceram. Soc. 2005, 25, 1681–1688. [Google Scholar] [CrossRef]
- Taguchi, H.; Hirata, F.; Takeishi, T. Effect of SiO2-SrCO3 Addition on Magnetic Properties of Anisotropic Ba-ferrite (Ferrite Magnets Having Equal Shrinkage Ratio in A-axis and C-axis). J. Jpn. Soc. Powder Powder Metall. 1996, 43, 19–24. [Google Scholar] [CrossRef][Green Version]
- Hussain, S.; Anis-ur-Rehman, M.; Maqsood, A.; Awan, M.S. The effect of SiO2 addition on structural, magnetic and electrical properties of strontium hexaferrites. J. Cryst. Growth 2006, 297, 403–410. [Google Scholar] [CrossRef]
- Huang, C.C.; Jiang, A.H.; Hung, Y.H.; Liou, C.H.; Wang, Y.C.; Lee, C.P.; Hung, T.Y.; Shaw, C.C.; Kuo, M.F.; Cheng, C.H. Influence of CaCO3 and SiO2 additives on magnetic properties of M-type Sr-ferrites. J. Magn. Magn. Mater. 2018, 451, 288–294. [Google Scholar] [CrossRef]
- Weast, R.C. (Ed.) CRC Handbook of Chemistry and Physics, 68th ed.; CRC Press: Boca Raton, FL, USA, 1987; p. F-159. [Google Scholar]
- Yoon, D.Y.; Park, C.W.; Koo, J.B. The Step Growth Hypothesis for Abnormal Grain Growth. In Ceramic Interfaces 2; Yoo, H.-I., Kang, S.-J.L., Eds.; Maney Publishing: London, UK, 2001; pp. 3–21. [Google Scholar]
- Fisher, J.G.; Choi, S.Y.; Kang, S.-J.L. Abnormal grain growth in barium titanate doped with alumina. J. Am. Ceram. Soc. 2006, 89, 2206–2212. [Google Scholar] [CrossRef]
- Kim, M.S.; Fisher, J.G.; Kang, S.-J.L.; Lee, H.Y. Grain growth control and solid-state crystal growth by Li2O/PbO addition and dislocation introduction in the PMN-35PT system. J. Am. Ceram. Soc. 2006, 89, 1237–1243. [Google Scholar] [CrossRef]
- Kang, S.-J.L.; Lee, M.G.; An, S.M. Microstructural evolution during sintering with control of the interface structure. J. Am. Ceram. Soc. 2009, 92, 1464–1471. [Google Scholar] [CrossRef]
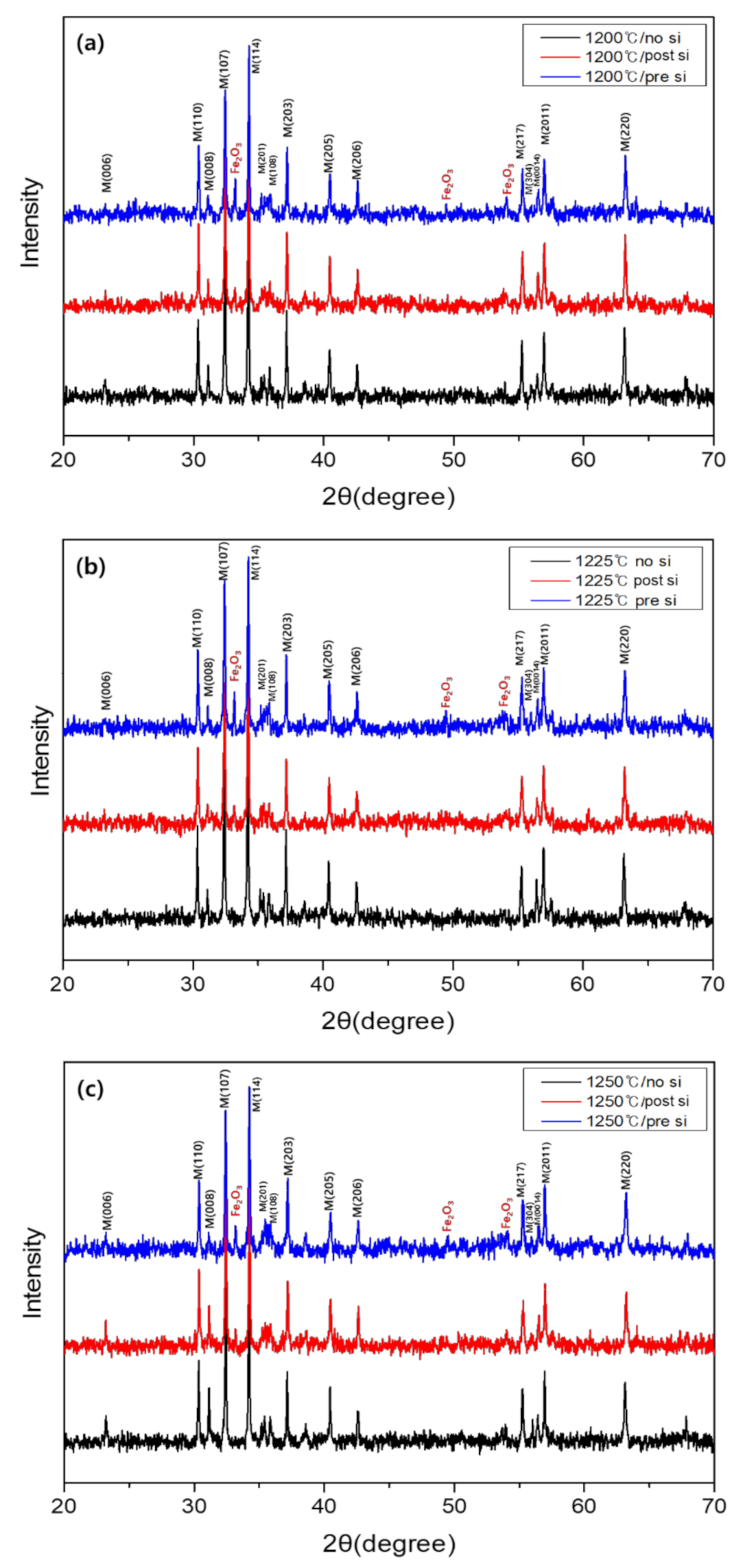
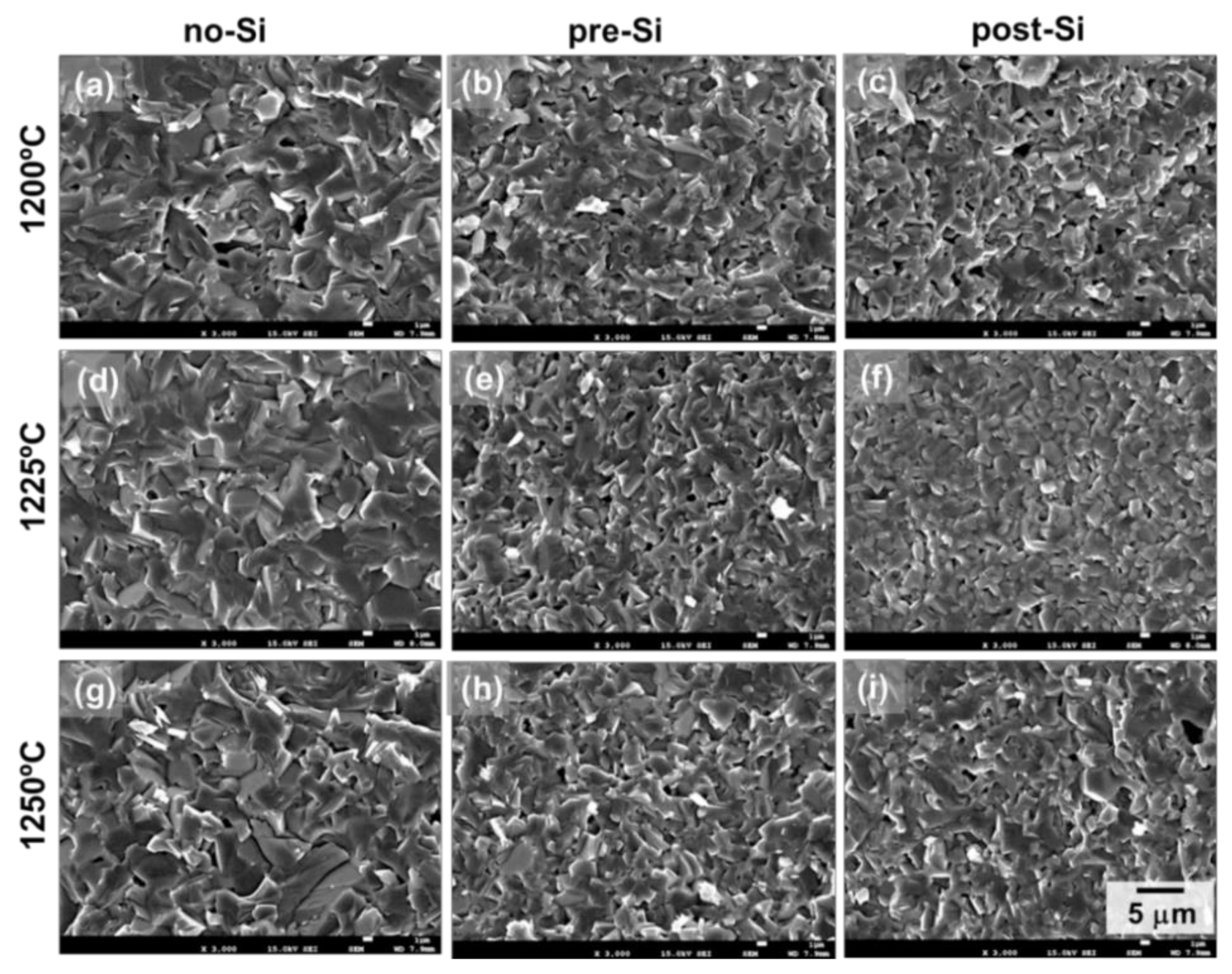

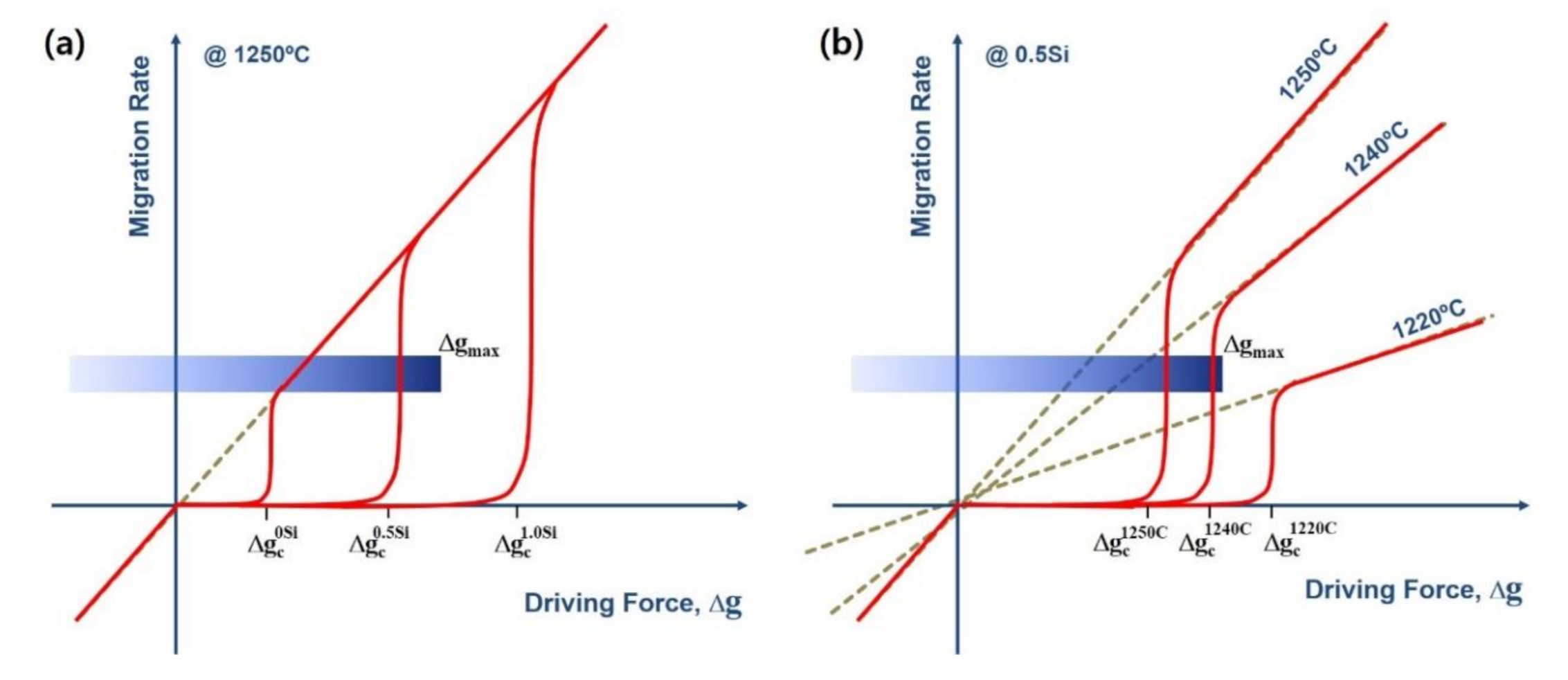
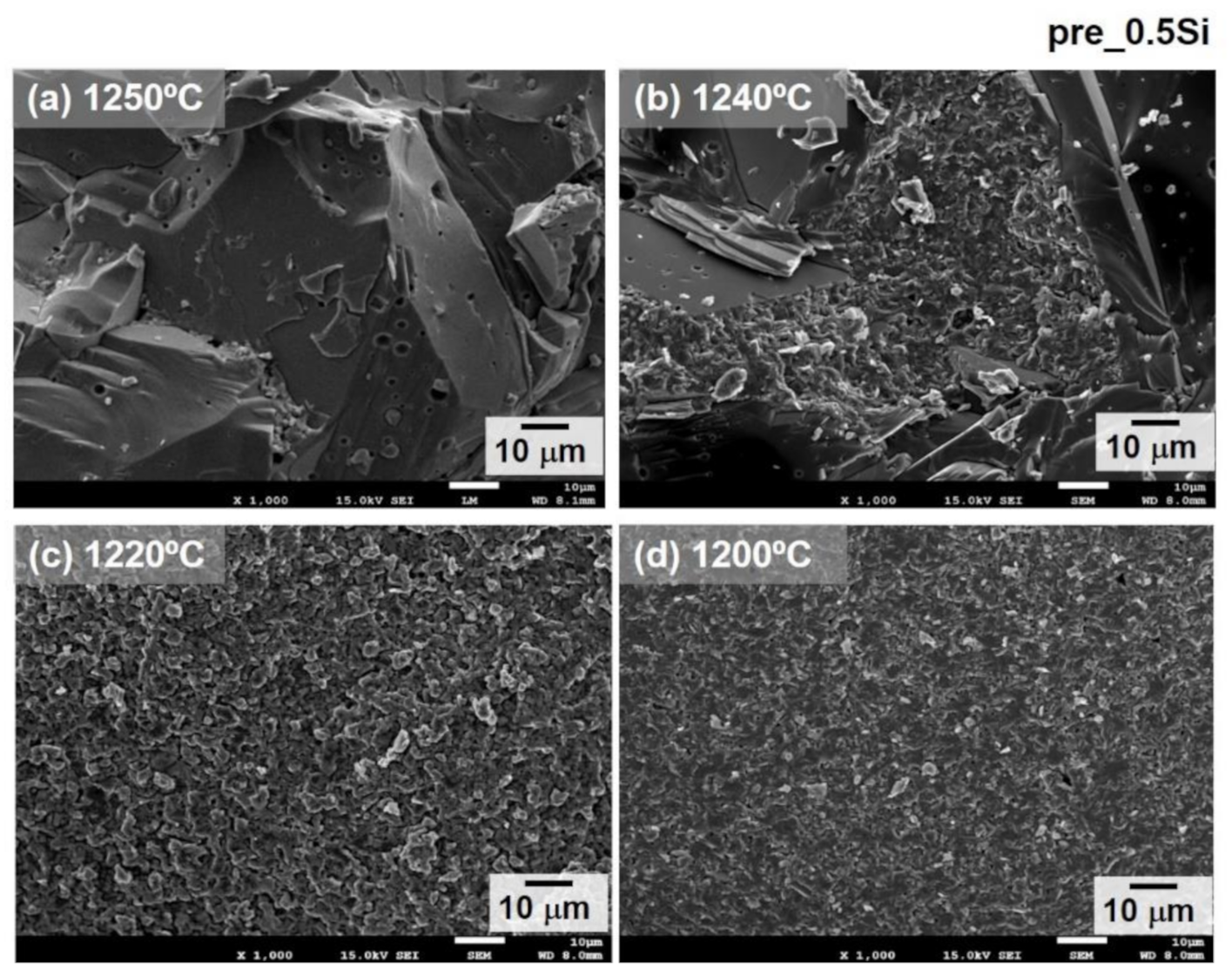
| Sintering Temperature | 1 wt% SiO2 Addition | Density, g/cm3 | Unit Cell Vol., Å3 |
|---|---|---|---|
| 1200 °C | no-Si | 4.91 | 688.481 |
| post-Si | 4.55 | 687.746 | |
| pre-Si | 4.78 | 687.518 | |
| 1225 °C | no-Si | 4.89 | 689.005 |
| post-Si | 4.72 | 688.073 | |
| pre-Si | 4.89 | 687.707 | |
| 1250 °C | no-Si | 4.95 | 689.025 |
| post-Si | 4.82 | 687.144 | |
| pre-Si | 4.93 | 686.250 |
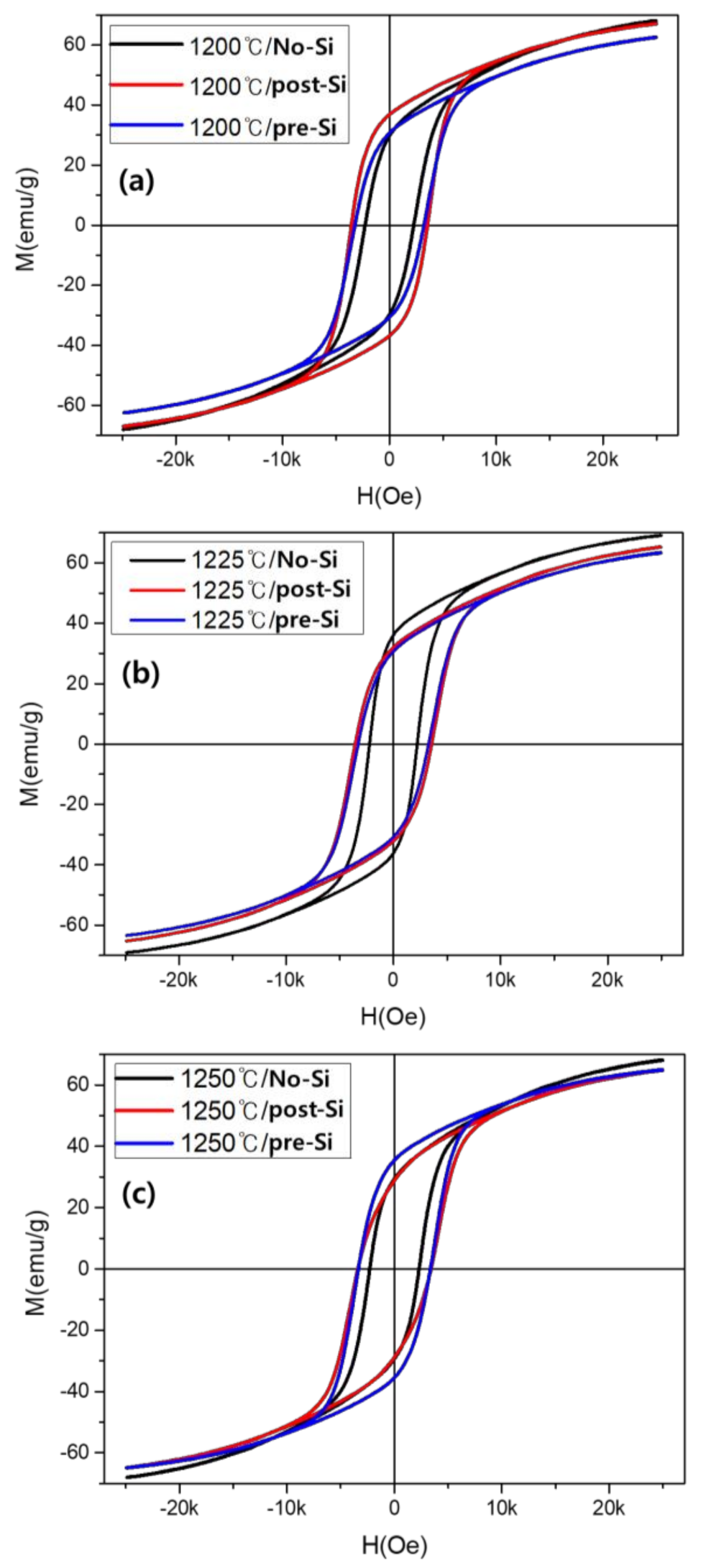
| Sintering Temperature | 1 wt% SiO2 Addition | Hc, Oe | Ms, emu/g |
|---|---|---|---|
| 1200 °C | no-Si | 2272 | 68.01 |
| post-Si | 3560 | 67.04 | |
| pre-Si | 3235 | 62.48 | |
| 1225 °C | no-Si | 2216 | 69.09 |
| post-Si | 3581 | 65.37 | |
| pre-Si | 3293 | 63.26 | |
| 1250 °C | no-Si | 2298 | 68.16 |
| post-Si | 3400 | 64.98 | |
| pre-Si | 3351 | 64.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, K.-S.; Yu, P.-y.; Kang, Y.-M. Microstructure and Magnetic Properties of La-Ca-Co Substituted M-Type Sr-Hexaferrites with Controlled Si Diffusion. Appl. Sci. 2020, 10, 7570. https://doi.org/10.3390/app10217570
Moon K-S, Yu P-y, Kang Y-M. Microstructure and Magnetic Properties of La-Ca-Co Substituted M-Type Sr-Hexaferrites with Controlled Si Diffusion. Applied Sciences. 2020; 10(21):7570. https://doi.org/10.3390/app10217570
Chicago/Turabian StyleMoon, Kyoung-Seok, Pyeong-yeol Yu, and Young-Min Kang. 2020. "Microstructure and Magnetic Properties of La-Ca-Co Substituted M-Type Sr-Hexaferrites with Controlled Si Diffusion" Applied Sciences 10, no. 21: 7570. https://doi.org/10.3390/app10217570
APA StyleMoon, K.-S., Yu, P.-y., & Kang, Y.-M. (2020). Microstructure and Magnetic Properties of La-Ca-Co Substituted M-Type Sr-Hexaferrites with Controlled Si Diffusion. Applied Sciences, 10(21), 7570. https://doi.org/10.3390/app10217570






