Photo-Biomodulation as a Prevention Modality of Oral Mucositis in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Routine Oral Care
2.2. PBM Parameters
2.3. Assessment of OM
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hodgson, B.D.; Margolis, D.M.; Salzman, D.E.; Eastwood, D.; Tarima, S.; Williams, L.D.; Sande, J.E.; Vaughan, W.P.; Whelan, H.T. Amelioration of oral mucositis pain by NASA near-infrared light-emitting diodes in bone marrow transplant patients. Support Care Cancer 2012, 20, 1405–1415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellm, L.A.; Epstein, J.B.; Rose-ped, A.; Martin, P.; Fuchs, H.J. Patient reports of complications of bone marrow transplantation. Support Care Cancer 2000, 8, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Vagliano, L.; Feraut, C.; Gobetto, G.; Trunfio, A.; Errico, A.; Campani, V.; Costazza, G.; Mega, A.; Matozzo, V.; Berni, M.; et al. Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT—Results of a multicentre study. Bone Marrow Transplant. 2011, 46, 727–732. [Google Scholar] [CrossRef]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support Oncol. 2007, 5, 3–11. [Google Scholar]
- Vera-Llonch, M.; Oster, G.; Ford, C.M.; Lu, J.; Sonis, S. Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer 2007, 15, 491–496. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Sonis, S.T. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009, 45, 1015–1020. [Google Scholar] [CrossRef]
- De Felice, F.; de Vincentiis, M.; Valentini, V.; Musio, D.; Mezi, S.; Lo Mele, L.; Terenzi, V.; D’Aguanno, V.; Cassoni, A.; Di Brino, M.; et al. Follow-up program in head and neck cancer. Crit. Rev. Oncol. Hematol. 2017, 113, 151–155. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X.; Ma, Q.; Wang, J.; Jiang, P.; Teng, S.; Zhou, L.; Wu, D.; Wang, H. Oral cryotherapy for oral mucositis management in patients receiving allogeneic hematopoietic stem cell transplantation: A prospective randomized study. Support Care Cancer 2020, 28, 1747–1754. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 27, 3949–3967. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Al-Azri, A.R.; Bossi, P.; Stringer, A.M.; Joy, J.K.; Soga, Y.; Ranna, V.; Vaddi, A.; Raber-Durlacher, J.E.; et al. Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020, 28, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Kamsvåg, T.; Svanberg, A.; Legert, K.G.; Arvidson, J.; Von Essen, L.; Mellgren, K.; Toporski, J.; Winiarski, J.; Ljungman, G. Prevention of oral mucositis with cryotherapy in children undergoing hematopoietic stem cell transplantations-a feasibility study and randomized controlled trial. Support Care Cancer 2020, 28, 4869–4879. [Google Scholar] [CrossRef]
- Worthington, H.V.; Clarkson, J.E.; Bryan, G.; Furness, S.; Glenny, A.-M.; Littlewood, A.; McCabe, M.G.; Meyer, S.; Khalid, T. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst. Rev. 2011, 2011, CD000978. [Google Scholar] [CrossRef]
- Hensley, M.L.; Hagerty, K.L.; KewalRamani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Mitchell, R.B.; et al. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J Clin. Oncol. 2009, 27, 127–145. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, W.; Schubert, M.; Ang, K.-K.; Brizel, D.; Brown, E.; Eilers, J.G.; Elting, L.; Mittal, B.B.; Schattner, M.A.; Spielberger, R.; et al. NCCN Task Force Report. prevention and management of mucositis in cancer care. J. Natl. Compr. Canc. Netw. 2008, 6, S1–S21. [Google Scholar] [CrossRef]
- Peterson, D.E.; Bensadoun, R.J.; Roila, F. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol. 2011, 22. [Google Scholar] [CrossRef]
- Spanemberg, J.C.; Figueiredo, M.A.; Cherubini, K.; Salum, F.G. Low-level Laser Therapy: A Review of Its Applications in the Management of Oral Mucosal Disorders. Altern. Ther. Health Med. 2016, 22, 24–31. [Google Scholar]
- Romeo, U.; Palaia, G.; Tenore, G.; Del vecchio, A.; Nammour, S. Excision of oral mucocele by different wavelength lasers. Indian J Dent Res. 2013, 24, 211–215. [Google Scholar] [CrossRef]
- Romeo, U.; Palaia, G.; Nardo, A.; Tenore, G.; Telesca, V.; Kornblit, R.; Del Vecchio, A.; Frioni, A.; Valenti, P.; Berlutti, F. Effectiveness of KTP laser versus 980 nm diode laser to kill Enterococcus faecalis in biofilms developed in experimentally infected root canals. Aust. Endod. J. 2015, 41, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.; da Motta Silveira, F.M.; de Orange, F.A. Low-level laser therapy prevents severe oral mucositis in patients submitted to hematopoietic stem cell transplantation: A randomized clinical trial. Support Care Cancer 2016, 24, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.; Sacono, N.T.; Freire Mdo, C.; Costa, L.R.; Batista, A.C.; Silva, G.B. The Impact of Low-Level Laser Therapy on Oral Mucositis and Quality of Life in Patients Undergoing Hematopoietic Stem Cell Transplantation Using the Oral Health Impact Profile and the Functional Assessment of Cancer Therapy-Bone Marrow Transplantation Questionnaires. Photomed. Laser Surg. 2015, 33, 357–363. [Google Scholar] [PubMed]
- Silva, G.B.; Mendonça, E.F.; Bariani, C.; Antunes, H.S.; Silva, M.A. The prevention of induced oral mucositis with low-level laser therapy in bone marrow transplantation patients: A randomized clinical trial. Photomed. Laser Surg. 2011, 29, 27–31. [Google Scholar] [CrossRef]
- Zadik, Y.; Arany, P.R.; Fregnani, E.R.; Bossi, P.; Antunes, H.S.; Bensadoun, R.-J.; Gueiros, L.A.; Majorana, A.; Nair, R.G.; Ranna, V.; et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 27, 3969–3983. [Google Scholar] [CrossRef]
- Anschau, F.; Webster, J.; Capra, M.E.Z.; de Azeredo da Silva, A.L.F.; Stein, A.T. Efficacy of low-level laser for treatment of cancer oral mucositis: A systematic review and meta-analysis. Lasers Med. Sci. 2019, 34, 1053–1062. [Google Scholar] [CrossRef]
- Peng, J.; Shi, Y.; Wang, J.; Wang, F.; Dan, H.; Xu, H.; Zeng, X. Low-level laser therapy in the prevention and treatment of oral mucositis: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 387–397.e9. [Google Scholar] [CrossRef]
- De Lima, V.H.S.; de Oliveira-Neto, O.B.; da Hora Sales, P.H.; da Silva Torres, T.; de Lima, F.J.C. Effectiveness of low-level laser therapy for oral mucositis prevention in patients undergoing chemoradiotherapy for the treatment of head and neck cancer: A systematic review and meta-analysis. Oral Oncol. 2020, 102, 104524. [Google Scholar] [CrossRef]
- Oberoi, S.; Zamperlini-Netto, G.; Beyene, J.; Treister, N.S.; Sung, L. Effect of prophylactic low level laser therapy on oral mucositis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e107418. [Google Scholar] [CrossRef]
- Hamblin, M.R. How to Write a Good Photobiomodulation Article. Photobiomodul. Photomed. Laser Surg. 2019, 37, 325–326. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Silva, G.B.L.; Sacono, N.T.; Othon-Leite, A.F.; Mendonça, E.F.; Arantes, A.M.; Bariani, C.; Duarte, L.G.L.; Abreu, M.H.N.; Queiroz-Júnior, C.M.; Silva, T.A.; et al. Effect of low-level laser therapy on inflammatory mediator release during chemotherapy-induced oral mucositis: A randomized preliminary study. Lasers Med. Sci. 2015, 30, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Eduardo, F.P.; Guthrie, K.A.; Franquin, J.-C.; Bensadoun, R.-J.J.; Migliorati, C.A.; Lloid, C.M.E.; Eduardo, C.D.P.; Walter, N.-F.; Marques, M.M.; et al. A phase III randomized double-blind placebo-controlled clinical trial to determine the efficacy of low level laser therapy for the prevention of oral mucositis in patients undergoing hematopoietic cell transplantation. Support Care Cancer 2007, 15, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Rezk-Allah, S.S.; Abd Elshaf, H.M.; Farid, R.J.; Hassan, M.A.E.; Alsirafy, S.A. Effect of Low-Level Laser Therapy in Treatment of Chemotherapy Induced Oral Mucositis. J. Lasers Med. Sci. 2019, 10, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, J.M.; Johnson, M.I.; Iversen, V.; Aimbire, F.; Lopes-Martins, R.A. Low-level laser therapy in acute pain: A systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed. Laser Surg. 2006, 24, 158–168. [Google Scholar] [CrossRef]
- Cowen, D.; Tardieu, C.; Schubert, M.; Peterson, D.; Resbeut, M.; Faucher, C.; Franquin, J.-C. Low energy Helium-Neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: Results of a double blind randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 697–703. [Google Scholar] [CrossRef]
- Soto, M.; Lalla, R.V.; Gouveia, R.V.; Zecchin, V.G.; Seber, A.; Lopes, N.N. Pilot study on the efficacy of combined intraoral and extraoral low-level laser therapy for prevention of oral mucositis in pediatric patients undergoing hematopoietic stem cell transplantation. Photomed. Laser Surg. 2015, 33, 540–546. [Google Scholar] [CrossRef]
- Cidon, E.U. Chemotherapy induced oral mucositis: Prevention is possible. Chin. Clin. Oncol. 2018, 7, 6. [Google Scholar] [CrossRef]
- Wardley, A.M.; Jayson, G.C.; Swindell, R.; Morgenstern, G.R.; Chang, J.; Bloor, R.; Fraser, C.J.; Scarffe, J.H. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. Br. J. Haematol. 2000, 110, 292–299. [Google Scholar] [CrossRef]

| Characteristics | Preventive Group (PG) | Control Group (CG) |
|---|---|---|
| Mean age (range) | 46.5 (7–66) | 45.6 (19–65) |
| Gender | n (%) | n (%) |
| Male | 13 (65) | 10 (50) |
| Female | 7 (35) | 10 (50) |
| Type of aHSCT | ||
| Matched Unrelated Donor (MUD) | 14 (70) | 9 (45) |
| Matched Related Donor (MRD) | 6 (30) | 11 (55) |
| Underlying Pathology | ||
| Acute Lymphocytic Leukemia | 3 (15) | 4 (20) |
| Acute Myelogenous Leukemia (AML) | 3 (15) | 7 (35) |
| Myelofibrosis | 3 (15) | 3 (15) |
| Myelodisplastic Syndrome | 3 (15) | 1 (5) |
| Chronic Myelogenous Leukemia | 2 (10) | - |
| Non-Hodgkin’s Lymphoma | - | 2 (10) |
| Multiple Myeloma | 1 (5) | - |
| Myeloid Sarcoma (MS) | 1 (5) | - |
| Mixed Phenotype Acute Leukemia | 1 (5) | - |
| Chronic Lymphocytic Leukemia | - | 1 (5) |
| T-cell prolymphocytic leukemia | - | 1 (5) |
| Aplastic Anemia | - | 1 (5) |
| MS and AML | 1 (5) | - |
| Others | 2 (10) | - |
| aHSCT Conditioning Regimen | ||
| Thio, BUS, FLU, Meth | 14 (70) | 11 (55) |
| Thio, BUS, FLU, Cyclo | 4 (20) | 5 (25) |
| TBI, Thio, FLU, Meth | 1 (5) | - |
| Thio, FLU, and Meth | 1 (5) | - |
| Treo, FLU | - | 1 (5) |
| Rituximab, Cyclo, Thio, Cyclosporine | - | 1 (5) |
| Cyclo, Meth | - | 1 (5) |
| Cyclosporine, Thio, FLU, Cyclo | - | 1 (5) |
| Manufacturer | FISIOLINE |
| Model identifier | Lumix2® (Double diode laser) |
| Number and type of emitters | Two wavelengths, visible GaAs and infrared GaAs |
| Wavelength and bandwidth | 650 nm, and 904–910 nm |
| Pulse mode | For visible 650 nm: continuous mode and for 904–910 nm: 13 kHz |
| Beam spot size at target | ~0.5 cm2 |
| Exposure duration | 45 s per point |
| Number of points irradiated | 10 points intraorally including buccal mucosa, tip, ventral and marginal surface of the tongue, floor of the mouth, soft palate, and inner surfaces of the lip. |
| Area irradiated | ~5 cm2 |
| Application technique | Point by point in a defocused mode |
| Total irradiation energy per session | 40 J |
| Number and frequency of treatment sessions | 5 sessions per week (Monday to Friday) starting one day before the conditioning regimen and continued till the 10th day after transplantation (D+10) |
| Variables | Preventive Group (PG)Median ± SD | Control Group (CG)Median ± SD | p-Value |
|---|---|---|---|
| Age | 46.5 ± 14.43 | 45.55 ± 16.01 | 0.914 |
| Max grade of oral mucositis | 1.00 ± 0.92 | 2.25 ± 0.85 | <0.001 * |
| Duration of oral mucositis of: | |||
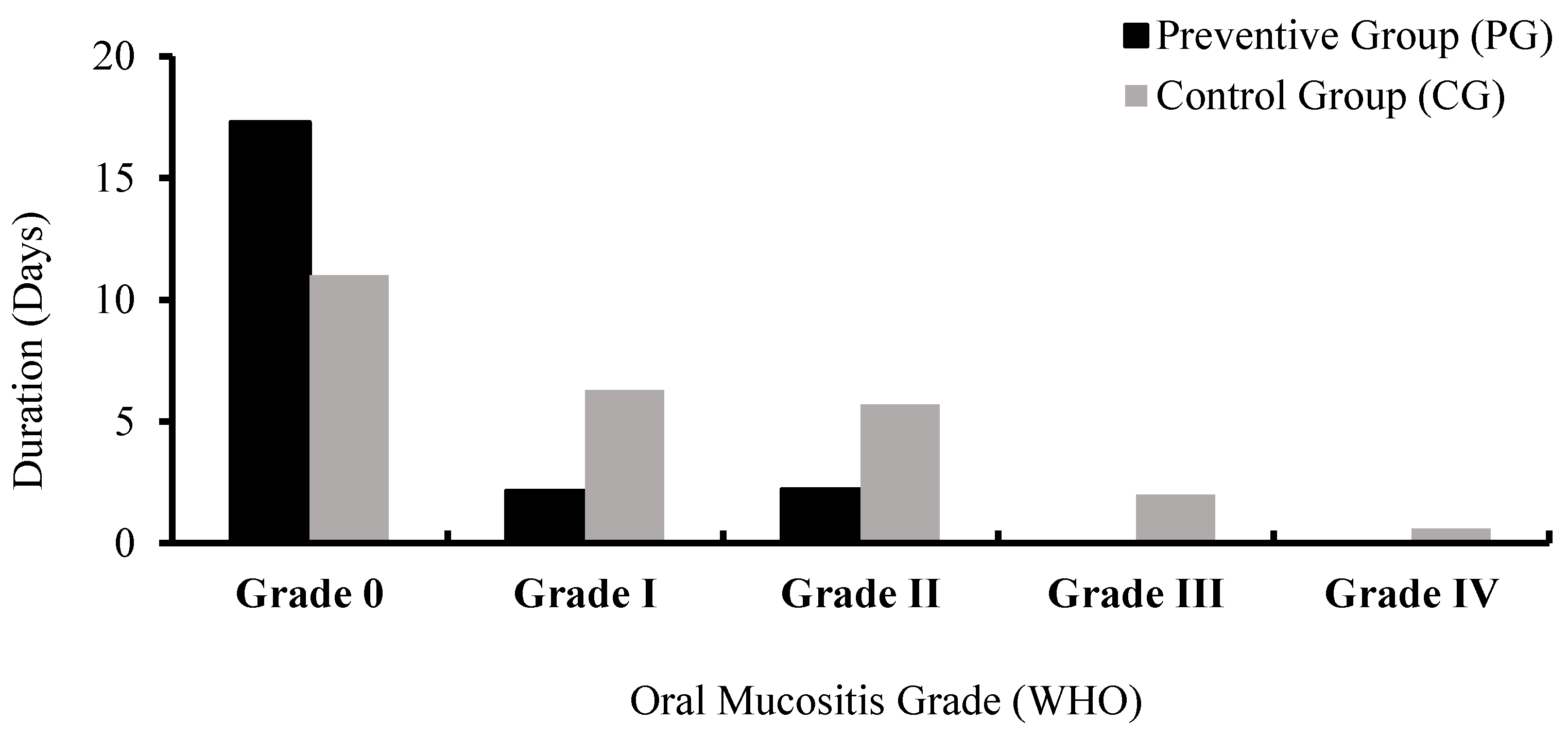
| Grade 0 | 17.30 ± 3.03 | 11.00 ± 5.20 | <0.001 * |
| Grade I | 2.15 ± 2.81 | 6.30 ± 3.42 | <0.001 * |
| Grade II | 2.20 ± 2.97 | 5.70 ± 6.16 | 0.025 * |
| Grade III | 0.00 ± 0.00 | 2.0 ± 4.36 | 0.009 * |
| Grade IV | 0.00 ± 0.00 | 0.55 ± 1.76 | 0.152 |
| Total duration of oral mucositis | 4.70 ± 5.05 | 15.00 ± 8.83 | <0.001 * |
| Mean oral mucositis score at Evaluation time points (E): | |||
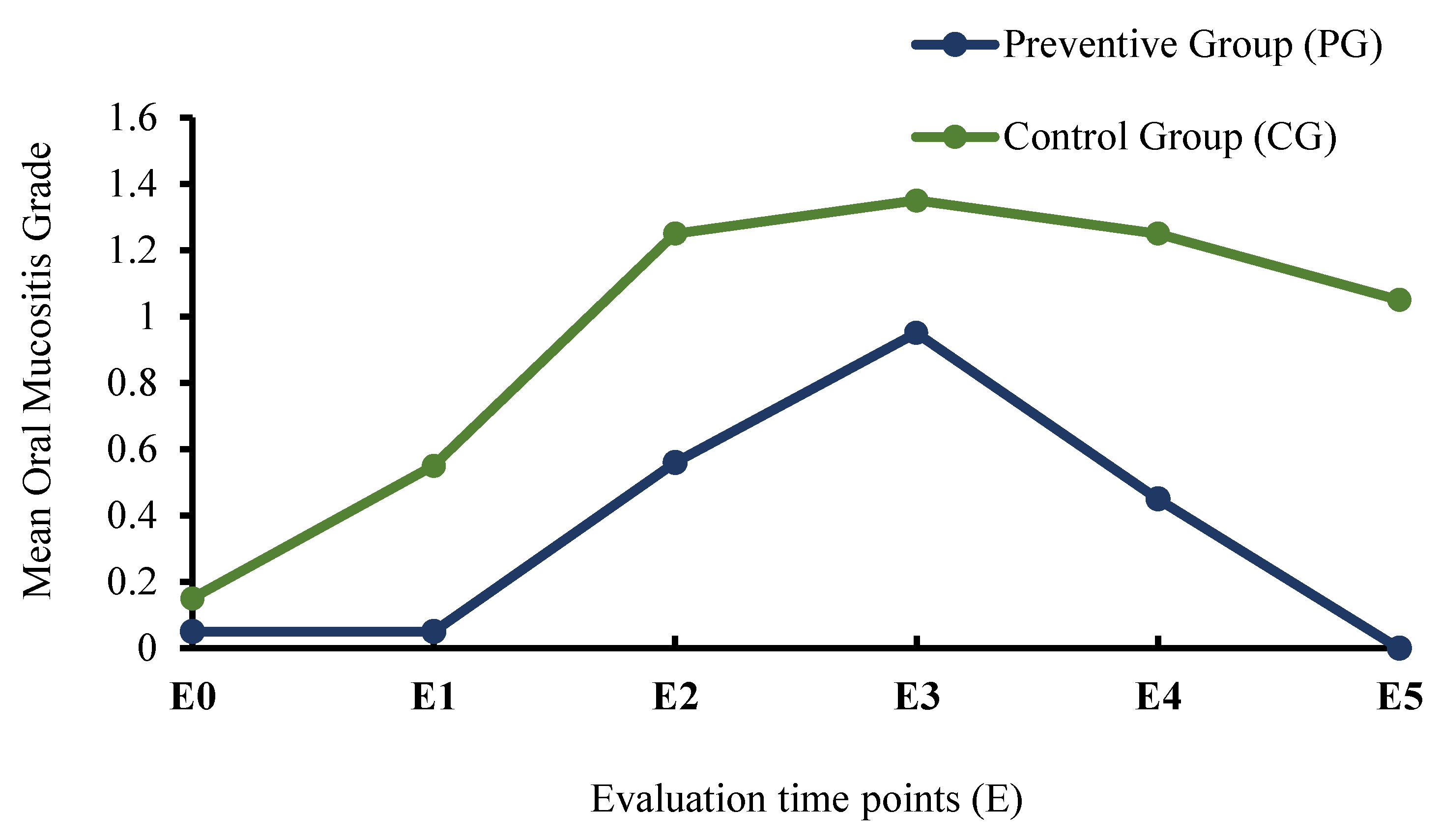
| E0 | 0.05 ± 0.22 | 0.15 ± 0.37 | 0.298 |
| E1 | 0.05 ± 0.22 | 0.55 ± 0.83 | 0.008 * |
| E2 | 0.56 ± 0.68 | 1.25 ± 1.12 | 0.005 * |
| E3 | 0.95 ± 0.94 | 1.35 ± 1.14 | 0.307 |
| E4 | 0.45 ± 0.69 | 1.25 ± 1.21 | 0.022 * |
| E5 | 0.00 ± 0.00 | 1.05 ± 0.94 | <0.001 * |
| Variables | Preventive Group (PG) | Control Group (CG) | χ2 | p-Value |
|---|---|---|---|---|
| Severity of Oral Mucositis (OM) | ||||
| Non-ulcerative OM (≤ Grade I) | 12 | 3 | 8424 | 0.003 * |
| Ulcerative OM (≥ Grade II) | 8 | 17 | ||
| Incidence of OM | ||||
| No OM (Grade 0) | 8 | 0 | 10,000 | 0.002 * |
| With OM (≥ Grade I) | 12 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsen, A.; Tenore, G.; Rocchetti, F.; Del Vecchio, A.; Ricci, R.; Barberi, W.; Cartoni, C.; Iori, A.P.; Pippi, R.; Polimeni, A.; et al. Photo-Biomodulation as a Prevention Modality of Oral Mucositis in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Appl. Sci. 2020, 10, 7479. https://doi.org/10.3390/app10217479
Mohsen A, Tenore G, Rocchetti F, Del Vecchio A, Ricci R, Barberi W, Cartoni C, Iori AP, Pippi R, Polimeni A, et al. Photo-Biomodulation as a Prevention Modality of Oral Mucositis in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Applied Sciences. 2020; 10(21):7479. https://doi.org/10.3390/app10217479
Chicago/Turabian StyleMohsen, Ahmed, Gianluca Tenore, Federica Rocchetti, Alessandro Del Vecchio, Roberto Ricci, Walter Barberi, Claudio Cartoni, Anna Paola Iori, Roberto Pippi, Antonella Polimeni, and et al. 2020. "Photo-Biomodulation as a Prevention Modality of Oral Mucositis in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation" Applied Sciences 10, no. 21: 7479. https://doi.org/10.3390/app10217479
APA StyleMohsen, A., Tenore, G., Rocchetti, F., Del Vecchio, A., Ricci, R., Barberi, W., Cartoni, C., Iori, A. P., Pippi, R., Polimeni, A., & Romeo, U. (2020). Photo-Biomodulation as a Prevention Modality of Oral Mucositis in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Applied Sciences, 10(21), 7479. https://doi.org/10.3390/app10217479











