Insulin Secretion by β-Cell-Like Cells Derived from Pulp Stem Cells Depends on Augmented Cytosolic Zinc Levels than GABA Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Differentiation of SHED β-Cells
2.2. Isolation of Rodent-Islets
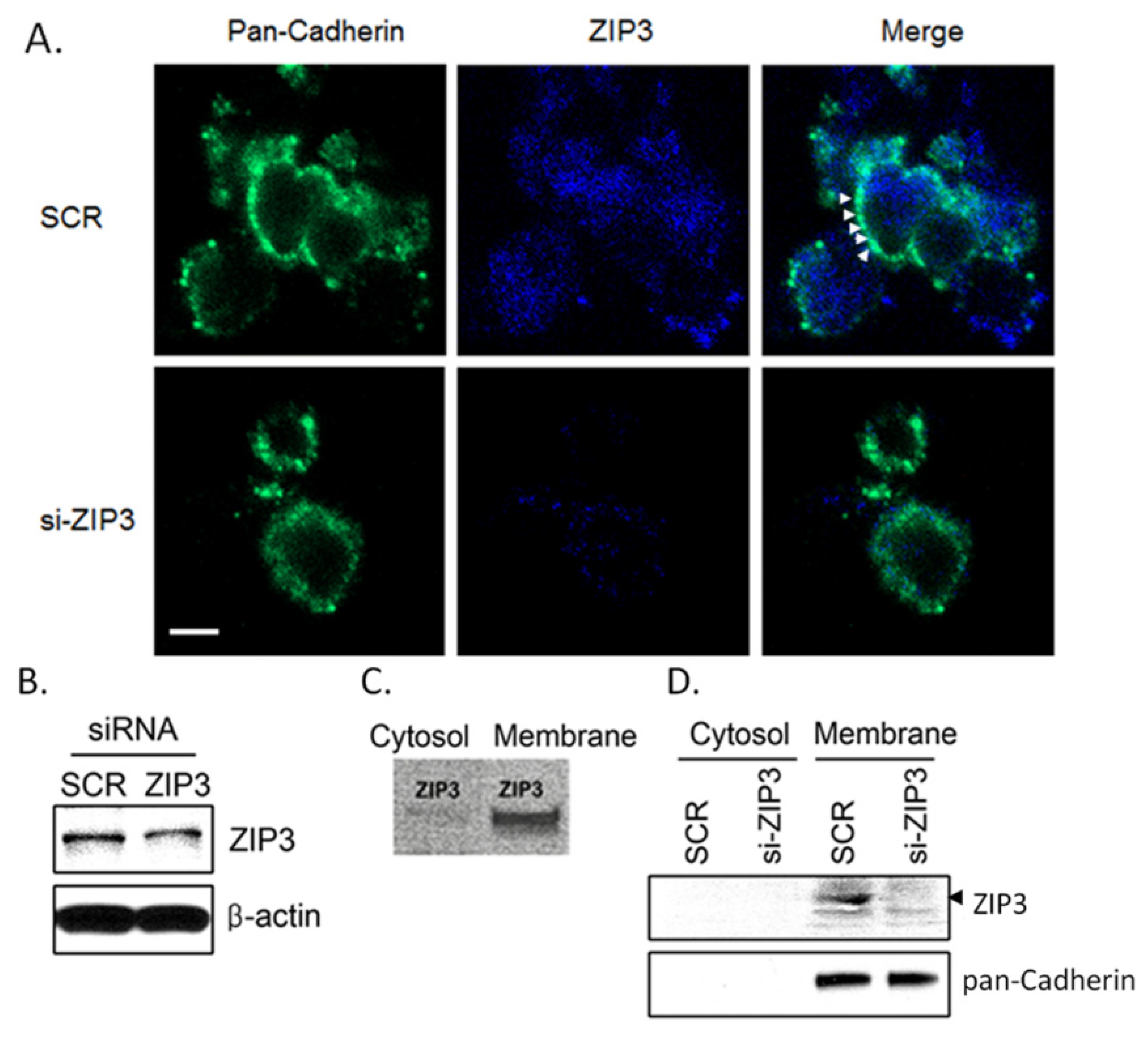
2.3. Immunofluorescence Assay
2.4. Western Blot Assays
2.5. RNA Interference
2.6. Real-Time qRT-PCR Assays
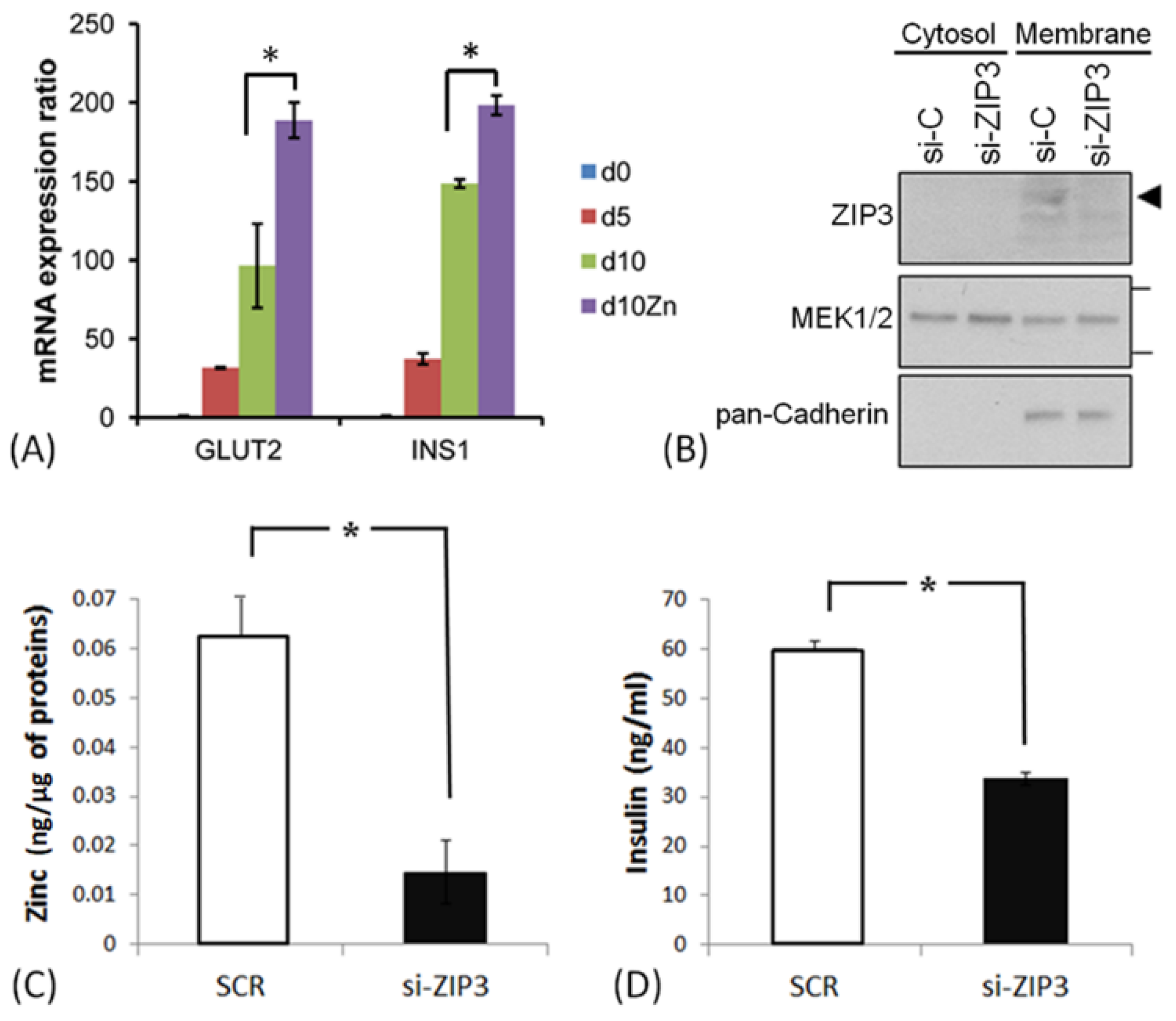
2.7. Zinc Assay
2.8. ELISA Assay
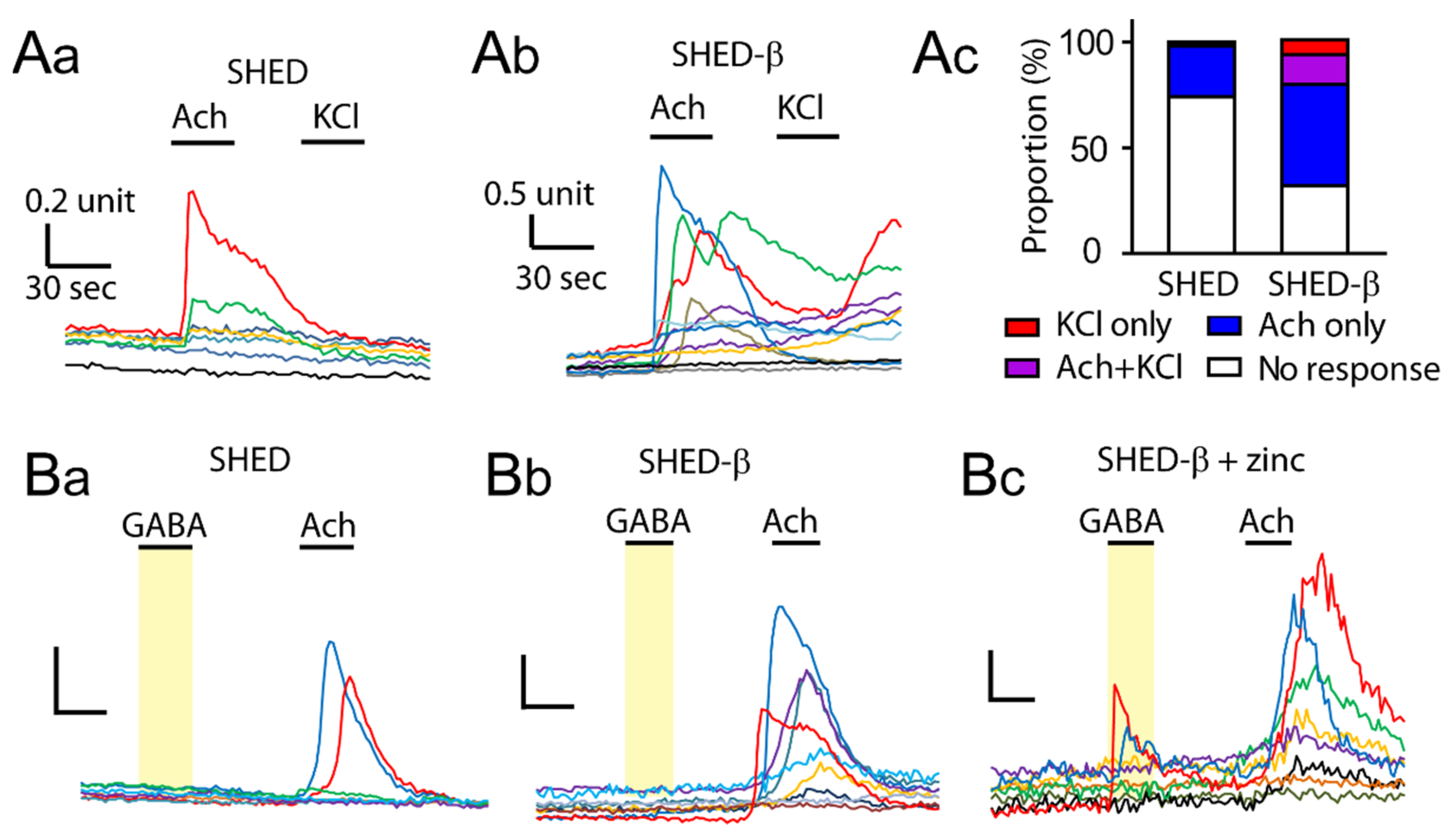
2.9. Ratiometric Calcium Imagimolecular Weight ng
2.10. Statistical Analysis
3. Results
3.1. Zip3 Zinc Uptake Transporters Reside in the Plasma Membrane of Rodent β-Cells
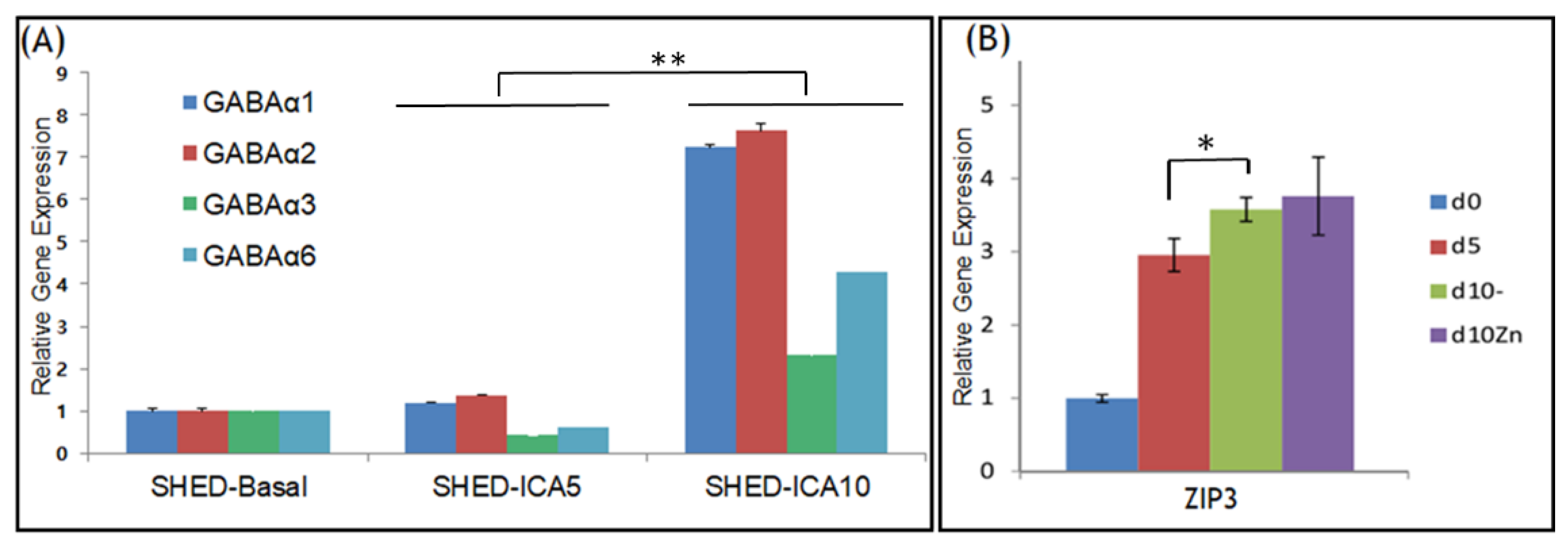
3.2. GABAA Receptors and ZIP3 Transporter mRNA Levels Increase during Differentiation of SHED into SHED β-Cells
3.3. ZIP3 Transporters in the Plasma Membrane of SHED β-Cells Are Functional
3.4. Supplementation of Zinc during Differentiation Affects GABA-Induced Ca2+ Responses in SHED β-Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rickels, M.R.; Robertson, R.P. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr. Rev. 2019, 40, 631–668. [Google Scholar] [CrossRef]
- Gamble, A.; Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. The journey of islet cell transplantation and future development. Islets 2018, 10, 80–94. [Google Scholar] [CrossRef]
- De Leon-Rodriguez, L.; Lubag, A.J., Jr.; Sherry, A.D. Imaging free zinc levels In Vivo—What can be learned? Inorg. Chim. Acta 2012, 393, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Foster, M.; Hancock, D.; Petocz, P.; Samman, S. Interrelationships among mediators of cellular zinc homeostasis in healthy and type 2 diabetes mellitus populations. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, N.; Chimienti, F.; Wheeler, M.B. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes. Metab. Suppl. 2009, 4, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Dufner-Beattie, J.; Huang, Z.L.; Geiser, J.; Xu, W.; Andrews, G.K. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis 2006, 44, 239–251. [Google Scholar] [CrossRef]
- Slepchenko, K.G.; Li, Y.V. Rising intracellular zinc by membrane depolarization and glucose in insulin-secreting clonal HIT-T15 beta cells. Exp. Diabetes Res. 2012, 190309. [Google Scholar] [CrossRef]
- Slepchenko, K.G.; Daniels, N.A.; Guo, A.; Li, Y.V. Autocrine effect of Zn2+ on the glucose-stimulated insulin secretion. Endocrine 2015, 50, 110–122. [Google Scholar] [CrossRef]
- Pae, E.K.; Kim, G. Insulin production hampered by intermittent hypoxia via impaired zinc homeostasis. PLoS ONE 2014, 9, e90192. [Google Scholar] [CrossRef]
- Kim, G.; Shin, K.H.; Pae, E.K. Zinc Up-Regulates Insulin Secretion from β Cell-Like Cells Derived from Stem Cells from Human Exfoliated Deciduous Tooth (SHED). Int. J. Mol. Sci. 2016, 17, 2092. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, J.M.; Min, B.H.; Chung, H.; Park, C.G. Absence of spontaneous regeneration of endogenous pancreatic β-cells after chemical-induced diabetes and no effect of GABA on α-to-β cell transdifferentiation in rhesus monkeys. Biochem. Biophys. Res. Commun. 2019, 508, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Waseem Ghani, M.; Ghani, H.; Jiang, W.; Waseem Birmani, M.; Ye, L.; Bin, L.; Cun, L.G.; Lilong, A.; Mei, X. Gimmicks of gamma-aminobutyric acid (GABA) in pancreatic β-cell regeneration through transdifferentiation of pancreatic α- to β-cells. Cell Biol. Int. 2020, 44, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Ramracheya, R.; Rorsman, P. Autocrine regulation of insulin secretion. Diabetes Obes. Metab. Suppl. 2012, 3, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, L.; Wan, Y.; Prud’homme, G.J. GABAergic regulation of pancreatic islet cells: Physiology and antidiabetic effects. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Untereiner, A.; Abdo, S.; Bhattacharjee, A.; Gohil, H.; Pourasgari, F.; Ibeh, N.; Lai, M.; Batchuluun, B.; Wong, A.; Khuu, N.; et al. GABA promotes β-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB J. 2019, 33, 3968–3984. [Google Scholar] [CrossRef]
- Bansal, P.; Wang, S.; Liu, S.; Xiang, Y.Y.; Lu, W.Y.; Wang, Q. GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 β-cells. PLoS ONE 2011, 6, e26225. [Google Scholar] [CrossRef]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Clark, A.; Walker, J.N.; Johnson, P.R.; Rorsman, P. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 2010, 59, 1694–1701. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, Z.; Liu, L.; Fan, Z. Decrease of γ-aminobutyric acid and zinc ions in the islet periportal circulation stimulates glucagon secretion during hypoglycemia. Exp. Ther. Med. 2018, 15, 2507–2511. [Google Scholar] [CrossRef]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef]
- Wijesekara, N.; Dai, F.F.; Hardy, A.B.; Giglou, P.R.; Bhattacharjee, A.; Koshkin, V.; Chimienti, F.; Gaisano, H.Y.; Rutter, G.A.; Wheeler, M.B. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010, 53, 1656–1668. [Google Scholar] [CrossRef]
- Govindasamy, V.; Ronald, V.S.; Abdullah, A.N.; Nathan, K.R.; Ab Aziz, Z.A.; Abdullah, M.; Musa, S.; Kasim, N.H.; Bhonde, R.R. Differentiation of dental pulp stem cells into islet-like aggregates. J. Dent. Res. 2011, 90, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Lee, H.; Caterina, M.J. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J. Biol. Chem. 2003, 22, 32037–32046. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Joseph, J.; Ro, J.Y.; Chung, M.K. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 2015, 156, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gyulkhandanyan, A.V.; Lee, S.C.; Bikopoulos, G.; Dai, F.; Wheeler, M.B. The Zn2+-transporting pathways in pancreatic beta-cells: A role for the L-type voltage-gated Ca2+ channel. J. Biol. Chem. 2006, 281, 9361–9372. [Google Scholar] [CrossRef]
- Henquin, J.C.; Dufrane, D.; Gmyr, V.; Kerr-Conte, J.; Nenquin, M. Pharmacological approach to understanding the control of insulin secretion in human islets. Diabetes Obes. Metab. 2017, 19, 1061–1070. [Google Scholar] [CrossRef]
- Taylor, J.T.; Huang, L.; Keyser, B.M.; Zhuang, H.; Clarkson, C.W.; Li, M. Role of high-voltage-activated calcium channels in glucose-regulated beta-cell calcium homeostasis and insulin release. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E900–E908. [Google Scholar] [CrossRef]
- Bloc, A.; Cens, T.; Cruz, H.; Dunant, Y. Zinc-induced changes in ionic currents of clonal rat pancreatic-cells: Activation of ATP-sensitive K+ channels. J. Physiol. 2000, 529, 723–734. [Google Scholar] [CrossRef]
- Capdor, J.; Foster, M.; Petocz, P.; Samman, S. Zinc and glycemic control: A meta-analysis of randomised placebo-controlled supplementation trials in humans. J. Trace Elem. Med. Biol. 2013, 27, 137–142. [Google Scholar] [CrossRef]
- Myers, S.A.; Nield, A.; Myers, M. Zinc transporters, mechanisms of action and therapeutic utility: Implications for type 2 diabetes mellitus. J. Nutr. Metab. 2012, 173712. [Google Scholar] [CrossRef]
| GABAA α1 | F: 5′GCC TCA GCT AAA AGC CCC CAC A; | R: 5′TGC TTT GCT CCA GAC TGC CAG A |
| GABAA α2 | F: 5′TGC AAC CAC GCC AGA ACC CAA; | R: 5′TGG CTG TTT TCG CAT GGA GTG CTT |
| GABAA α3 | F: 5′TGC ACT CTG CCC GCT GCA AAA; | R: 5′AGG TGG CAC GCA GGA TCA CA |
| GABAA α6 | F: 5′TTG AAT AGC TTG CGG CCA GGA CAA; | R: 5′TTT GCC CAC ACA TGC TTA CGG A |
| GLUT2 | F: 5′CCG CTG AGA AGA TTA GAC TTG G; | R: 5′GAC TAG CTC CTG CCT GTT TAT T |
| INS1 | F: 5′CTG GAG AAC TAC TGC AAC TAG AC; | R: 5′TGC TGG TTC AAG GGC TTT AT |
| ZIP3 | F: 5′CAG TCC CAT GTC ATG CAG AG | R: 5′GGA GCT CAA GGA ACA GGT CA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Chung, M.-K.; Pae, E.-K. Insulin Secretion by β-Cell-Like Cells Derived from Pulp Stem Cells Depends on Augmented Cytosolic Zinc Levels than GABA Levels. Appl. Sci. 2020, 10, 7476. https://doi.org/10.3390/app10217476
Kim G, Chung M-K, Pae E-K. Insulin Secretion by β-Cell-Like Cells Derived from Pulp Stem Cells Depends on Augmented Cytosolic Zinc Levels than GABA Levels. Applied Sciences. 2020; 10(21):7476. https://doi.org/10.3390/app10217476
Chicago/Turabian StyleKim, Gyuyoup, Man-Kyo Chung, and Eung-Kwon Pae. 2020. "Insulin Secretion by β-Cell-Like Cells Derived from Pulp Stem Cells Depends on Augmented Cytosolic Zinc Levels than GABA Levels" Applied Sciences 10, no. 21: 7476. https://doi.org/10.3390/app10217476
APA StyleKim, G., Chung, M.-K., & Pae, E.-K. (2020). Insulin Secretion by β-Cell-Like Cells Derived from Pulp Stem Cells Depends on Augmented Cytosolic Zinc Levels than GABA Levels. Applied Sciences, 10(21), 7476. https://doi.org/10.3390/app10217476







