Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism, Media and Culture Conditions
2.2. Analyses
3. Results and Discussion
3.1. Metabolic Characterization of Yarrowia lipolytica LMBF Y-46: Effect of Different Glycerol Sources under Nitrogen Limitation
3.2. Metabolic Characterization of Yarrowia lipolytica LMBF Y-46: Effect of Glycerol Concentration in Media with Constant Initial Nitrogen in Shake-Flask Trials
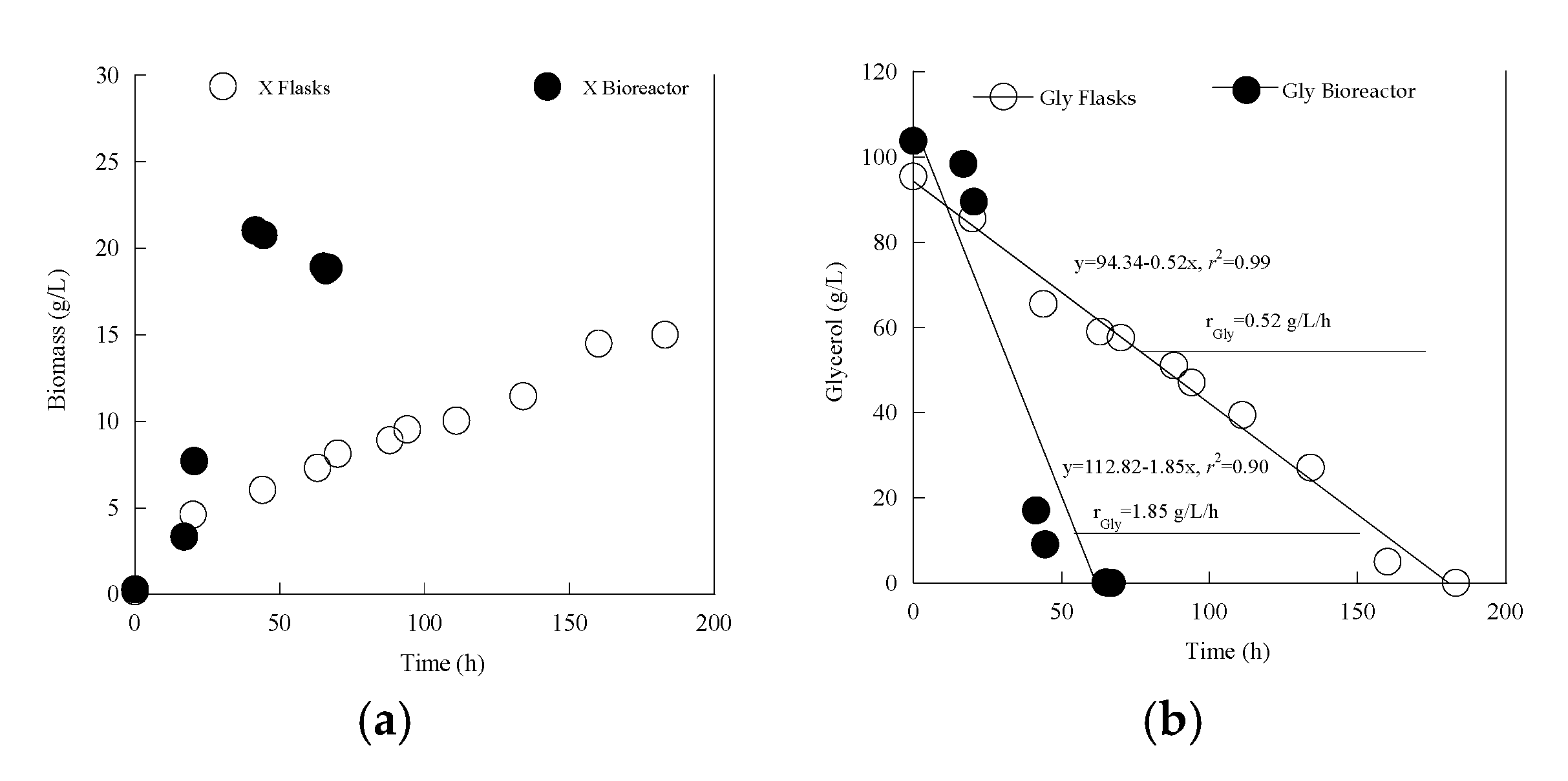
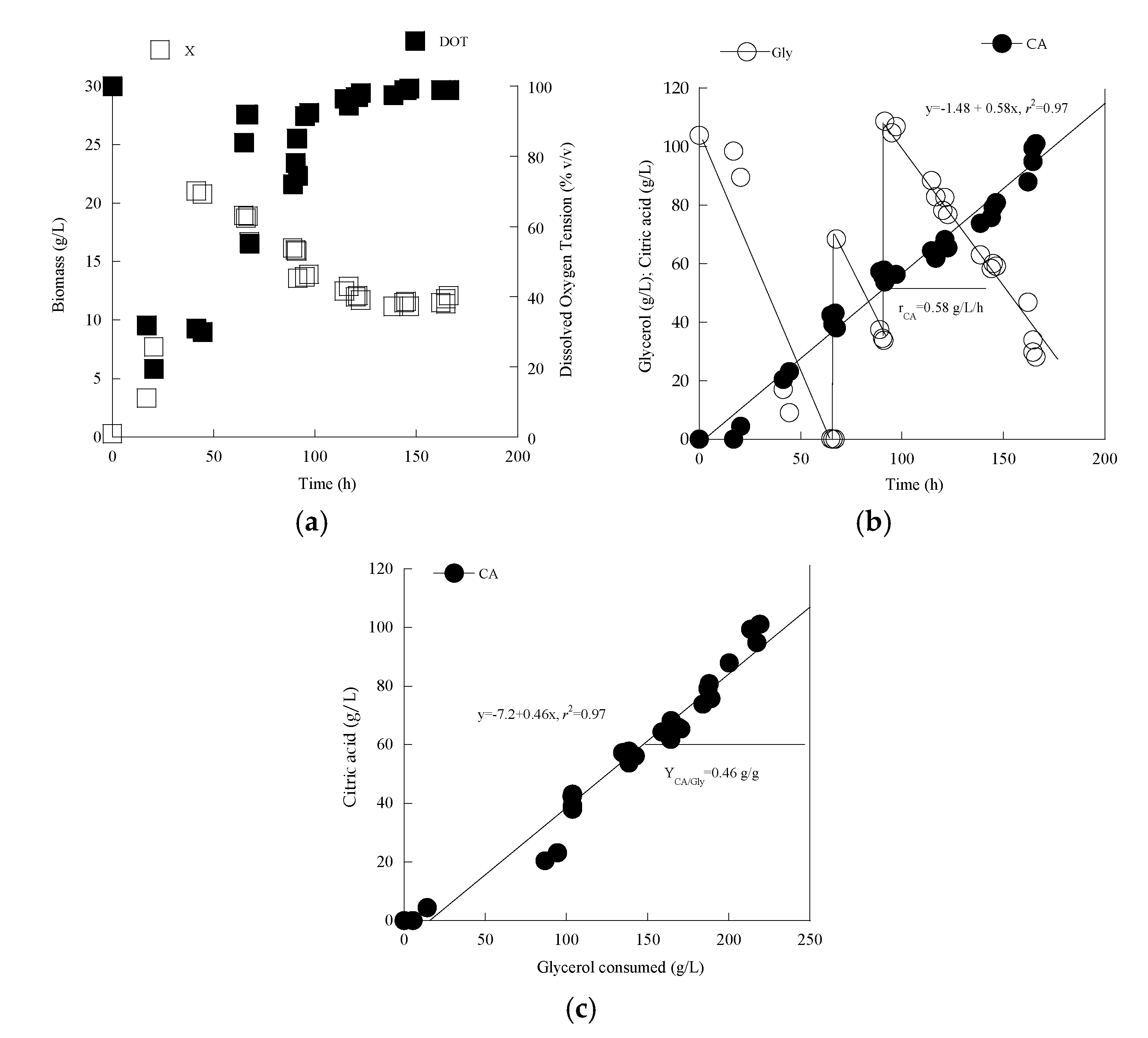
3.3. Metabolic Characterization of Yarrowia lipolytica LMBF Y-46: Scale-Up in Laboratory-Scale Bioreactor
3.4. Modeling the Production of Polyols by Yarrowia lipolytica LMBF Y-46 Growing on Glycerol in Shake-Flask Experiments
3.5. Cellular Lipids of Yarrowia lipolytica LMBF Y-46
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Chatzifragkou, A.; Papanikolaou, S. Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl. Microbiol. Biotechnol. 2012, 95, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Robak, M.; Lazar, Z.; Tomaszewska, L.; Rymowicz, W. Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenergy 2013, 48, 148–166. [Google Scholar] [CrossRef]
- Russmayer, H.; Egermeier, M.; Kalemasi, D.; Sauer, M. Spotlight on biodiversity of microbial cell factories for glycerol conversion. Biotechnol. Adv. 2019, 37, 107395. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trend Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl-esters) manufacturing process: Production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef]
- Fickers, P.; Cheng, H.; Lin, C.S.K. Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where are we? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef]
- Bankar, V.A.; Kumar, R.A.; Zinjarde, S.S. Environmental and industrial applications of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 84, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Zinjarde, S.S.; Apte, M.; Mohite, P. Yarrowia lipolytica and pollutants: Interactions and applications. Biotechnol. Adv. 2014, 32, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Carly, F.; Fickers, P. Erythritol production by yeasts: A snapshot of current knowledge. Yeast 2018, 35, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Timumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef]
- Egermeier, M.; Sauer, M.; Marx, H. Golden gate-based metabolic engineering strategy for wild-type strains of Yarrowia lipolytica. FEMS Microbiol. Lett. 2019, 366, fnz022. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef]
- Τryfinopoulou, P.; Tsakalidou, E.; Nychas, G.J.E. Characterization of Pseudomonas spp. associated with spoilage of gilt-head sea-bream stored under various conditions. Appl. Environ. Microbiol. 2002, 68, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Palaiogeorgou, A.M.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Kookos, I.K.; Koutinas, A.A. A newly isolated Enterobacter sp. strain produces 2,3-butanediol during its cultivation on low-cost carbohydrate-based substrates. FEMS Microbiol. Lett. 2019, 366, fny280. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef]
- Gardeli, C.; Athenaki, M.; Xenopoulos, E.; Mallouchos, A.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production and characterization by Mortierella (Umbelopsis) isabellina cultivated on lignocellulosic sugars. J. Appl. Microbiol. 2017, 123, 1461–1477. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Sarantou, S.; Komaitis, M.; Aggelis, G. Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J. Appl. Microbiol. 2004, 97, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Moustogianni, A.; Makri, A.; Aggelis, G. Lipids containing polyunsaturated fatty acids synthesized by Zygomycetes grown on glycerol. App. Biochem. Biotechnol. 2012, 166, 146–158. [Google Scholar] [CrossRef]
- Metsoviti, M.; Paraskevaidi, K.; Koutinas, A.; Zeng, A.P.; Papanikolaou, S. Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Proc. Biochem. 2012, 47, 1872–1882. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Dietz, D.; Komaitis, M.; Zeng, A.P.; Papanikolaou, S. Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnol. Bioeng. 2010, 107, 76–84. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef]
- Venkataramanan, K.P.; Boatman, J.J.; Kurniawan, Y.; Taconi, K.A.; Bothun, G.D.; Scholz, C. Impact of impurities in biodiesel-derived crude glycerol on the fermentation by Clostridium pasteurianum ATCC 6013. Appl. Microbiol. Biotechnol. 2012, 93, 1325–1335. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Furgala, I.; Rakicka, M.; Rymowicz, W. Enhanced production of erythritol by Yarrowia lipolytica on gycerol in repeated batch cultures. J. Ind. Microbiol. Biotechnol. 2014, 41, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Uprety, B.K.; Dalli, S.S.; Rakshit, S.K. Bioconversion of crude glycerol to microbial lipid using a robust oleaginous yeast Rhodosporidium toruloides ATCC 10788 capable of growing in the presence of impurities. Energy Conv. Manag. 2017, 135, 117–128. [Google Scholar] [CrossRef]
- Chebbi, H.; Leiva-Candia, D.; Carmona-Cabello, M.; Jaouani, A.; Dorado, M.P. Biodiesel production from microbial oil provided by oleaginous yeasts from olive oil mill wastewater growing on industrial glycerol. Ind. Crop. Prod. 2019, 139, 111535. [Google Scholar] [CrossRef]
- Carly, F.; Vandermies, M.; Telek, S.; Steels, S.; Thomas, S.; Nicaud, J.M.; Fickers, P. Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering. Metab. Eng. 2017, 42, 19–24. [Google Scholar] [CrossRef]
- Egermeier, M.; Russmayer, H.; Sauer, M.; Marx, H. Metabolic flexibility of Yarrowia lipolytica growing on glycerol. Front. Microbiol. 2017, 8, 49. [Google Scholar] [CrossRef]
- Filippoussi, R.; Antoniou, D.; Tryfinopoulou, P.; Nisiotou, A.A.; Nychas, G.J.; Koutinas, A.A.; Papanikolaou, S. Isolation, identification and screening of yeasts towards their ability to assimilate biodiesel-derived crude glycerol: Microbial production of polyols, endopolysaccharides and lipid. J. Appl. Microbiol. 2019, 127, 1080–1100. [Google Scholar] [CrossRef]
- André, A.; Chatzifragkou, A.; Diamantopoulou, P.; Sarris, D.; Philippoussis, A.; Galiotou-Panayotou, M.; Komaitis, M.; Papanikolaou, S. Biotechnological conversions of bio-diesel derived crude glycerol by Yarrowia lipolytica strains. Eng. Life Sci. 2009, 9, 468–478. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Beopoulos, A.; Koletti, A.; Thevenieau, F.; Koutinas, A.A.; Nicaud, J.M.; Aggelis, G. Importance of the methyl-citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica. J. Biotechnol. 2013, 168, 303–314. [Google Scholar] [CrossRef]
- Evans, C.T.; Ratledge, C. Phosphofructokinase and its regulation of the flux of carbon from glucose to lipid in the oleaginous yeast Rhodosporidium toruloides. J. Gen. Microbiol 1984, 130, 3251–3264. [Google Scholar]
- Park, W.S.; Murphy, P.A.; Glatz, B.A. Lipid metabolism and cell composition of the oleaginous yeast Apiotrichum curvatum grown at different carbon to nitrogen ratios. Can. J. Microbiol. 1990, 36, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, A.; Perdikouli, D.; Aggeli, D.; Aggelis, G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Zarowska, B.; Juszczyk, P. Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem. Pap. 2006, 60, 391–394. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Gladkowski, W. Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chem. Pap. 2008, 62, 239–246. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Marcinkiewicz, M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009, 31, 377–380. [Google Scholar] [CrossRef]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I.G. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Sampani, Z.; Rapti, A.; Papanikolaou, S. Valorization of crude glycerol, residue deriving from biodiesel-production process, with the use of wild-type new isolated Yarrowia lipolytica strains: Production of metabolites with pharmaceutical and biotechnological interest. Curr. Pharm. Biotechnol. 2019, 20, 881–894. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Dobrowolski, A.; Rakicka, M.; Rywińska, A.; Rymowicz, W. Newly isolated mutant of Yarrowia lipolytica MK1 as a proper hostfor efficient erythritol biosynthesis from glycerol. Proc. Biochem. 2015, 50, 61–68. [Google Scholar] [CrossRef]
- Rakicka, M.; Kieron, A.; Hapeta, P.; Neuvéglise, C.; Lazar, Z. Sweet and sour potential of yeast from the Yarrowia clade. Biomass Bioenergy 2016, 92, 48–54. [Google Scholar] [CrossRef]
- Rakicka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A.; Mirończuk, A.M. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of added-value chemical compounds through bioconversions of olive-mill wastewaters blended with crude glycerol by a Yarrowia lipolytica strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, X.; Wang, Z.; Xia, J.; Yan, Y.; Hu, L.; Wang, X.; Xu, J.; He, A.; Zhao, P. Enhanced erythritol production by a Snf1-deficient Yarrowia lipolytica strain under nitrogen-enrichedfermentation condition. Food Bioprod. Proc. 2020, 119, 306–316. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W.; Zarowska, B.; Skrzypiński, A. Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.; Aurich, A.; Perevoznikova, O.A.; Shishkanova, N.V.; Stottmeister, U.; Finogenova, T.V. Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol. Biotechnol. 2005, 43, 113–122. [Google Scholar]
- Förster, Α.; Aurich, Α.; Mauersberger, S.; Barth, G. Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 75, 1409–1417. [Google Scholar]
- Rywińska, A.; Rymowicz, W. Citric acid production from raw glycerol by Yarrowia lipolytica Wratislavia 1.31. In Microbial Conversions of Raw Glycerol; Aggelis, G., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 19–30. [Google Scholar]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Rymowicz, W. Chemostat study of citric acid production from glycerol by Yarrowia lipolytica. J. Biotechnol. 2011, 152, 54–57. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Fatykhova, A.R.; Dedyukhina, E.G.; Anastassiadis, S.G.; Golovchenko, N.P.; Morgunov, I.G. Citric acid production by yeast grown on glycerol-containing waste from biodiesel industry. Food Technol. Biotechnol. 2011, 49, 65–74. [Google Scholar]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Screening various Yarrowia lipolytica strains for citric acid production. Yeast 2019, 36, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 38, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen transfer rate and pH as major operating parameters of citric acid production from glycerol by Yarrowia lipolytica W29 and CBS 2073. Chem. Pap. 2016, 70, 869–876. [Google Scholar] [CrossRef]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen mass transfer impact on citric acid production by Yarrowia lipolytica from crude glycerol. Biochem. Eng. J. 2016, 110, 35–42. [Google Scholar] [CrossRef]
- Rywińska, A.; Musiał, I.; Rymowicz, W.; Żarowska, B.; Boruczkowski, T. Effect of agitation and aeration on the citric acid production by Yarrowia lipolytica grown on glycerol. Prep. Biochem. Biotechnol. 2012, 42, 279–291. [Google Scholar] [CrossRef]
- Tan, M.-J.; Chen, X.; Wang, Y.-K.; Liu, G.-L.; Chi, Z.-M. Enhanced citric acid production by a yeast Yarrowia lipolytica over-expressing a pyruvate carboxylase gene. Bioprocess. Biosyst. Eng. 2016, 39, 1289–1296. [Google Scholar] [CrossRef]
- Moresi, C. Effect of glucose concentration on citric acid production by Yarrowia lipolytica—Kinetics of the trophophase, citrate lag phase and idiophase. J. Chem. Technol. Biotechnol. 1994, 60, 387–395. [Google Scholar] [CrossRef]
- Klasson, T.K.; Clausen, E.C.; Gaddy, J.L. Continuous fermentation for the production of citric acid from glucose. Appl. Biochem. Biotechnol. 1989, 21, 491–509. [Google Scholar] [CrossRef]
- Rane, K.; Sims, K. Production of citric acid by Candida lipolytica Y 1095: Effect of glucose concentration on yield and productivity. Enz. Microb. Technol. 1993, 15, 646–651. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shishkanova, N.; Morgunov, I.G.; Finogenova, T.V. Oxygen requirements for growth and citric acid production of Yarrowia lipolytica. FEMS Yeast Res. 2003, 3, 217–222. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W.; Marcinkiewicz, M. Valorization of raw glycerol for citric acid production by Yarrowia lipolytica yeast. Electron. J. Biotechnol. 2010, 13, 4. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W. High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Lunina, J.N.; Morgunov, I.G. Biochemistry of citric acid production from rapeseed oil by Yarrowia lipolytica yeast. J. Am. Oil Chem. Soc. 2011, 88, 1965–1976. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chi, Z.; Liu, G.L.; Madzak, C.; Chi, Z.M. Both decrease in ACL1 gene expression and increase in ICL1 gene expression in marine-derived yeast Yarrowia lipolytica expressing INU1 gene enhance citric acid production from inulin. Mar. Biotechnol. 2013, 15, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric acid production by Yarrowia lipolytica yeast on different renewable raw materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Stoforos, N.G.; Xenopoulos, E.; Sarris, D.; Psarianos, D.; Philippoussis, A.; Papanikolaou, S. Lipid production by Cryptococcus curvatus growing on commercial xylose and subsequent valorization of fermentation waste-waters for the production of edible and medicinal mushrooms. Biochem. Eng. J. 2020, 162, 107706. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef]
- Aggelis, G.; Sourdis, J. Prediction of lipid accumulation-degradation in oleaginous micro-organisms growing on vegetable oils. Antonie Leeuwenhoek 1997, 72, 159–165. [Google Scholar] [CrossRef]
- Galiotou-Panayotou, M.; Kalantzi, O.; Aggelis, G. Modelling of simultaneous production of polygalacturonase and exopolysaccharide by Aureobasidium pullulans ATHUM 2915. Antonie Leeuwenhoek 1998, 73, 155–162. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Modelling aspects of the biotechnological valorization of raw glycerol: Production of citric acid by Yarrowia lipolytica and 1,3-propanediol by Clostridium butyricum. J. Chem. Technol. Biotechnol. 2003, 78, 542–547. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef]
- Ochoa-Estopier, A.; Guillouet, S.E. D-stat culture for studying the metabolic shifts from oxidative metabolism to lipid accumulation and citric acid production in Yarrowia lipolytica. J. Biotechnol. 2014, 170, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Triantaphyllidou, I.E.; Mizerakis, P.; Aggelis, G. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J. Biotechnol. 2016, 234, 116–126. [Google Scholar] [CrossRef]
- Beopoulos, A.; Haddouche, R.; Kabran, P.; Dulermo, T.; Chardot, T.; Nicaud, J.M. Identification and characterization of DGA2, an acyltransferase of the DGAT1 acyl-CoA:diacylglycerol acyltransferase family in the oleaginous yeast Yarrowia lipolytica. New insights into the storage lipid metabolism of oleaginous yeasts. Appl. Microbiol. Biotechnol. 2012, 93, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Imam Abidi, S.H.; Liu, H.; Zhang, H.; Chakraborty, S.; Watson, N.; Ajikumar, P.K.; Stephanopoulos, G. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica. Metab. Eng. 2015, 29, 56–65. [Google Scholar] [CrossRef]
- Davies, R.J.; Holdsworth, J.E.; Reader, S.L. The effect of low oxygen uptake rate on the fatty acid profile of the oleaginous yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1990, 33, 569–573. [Google Scholar] [CrossRef]
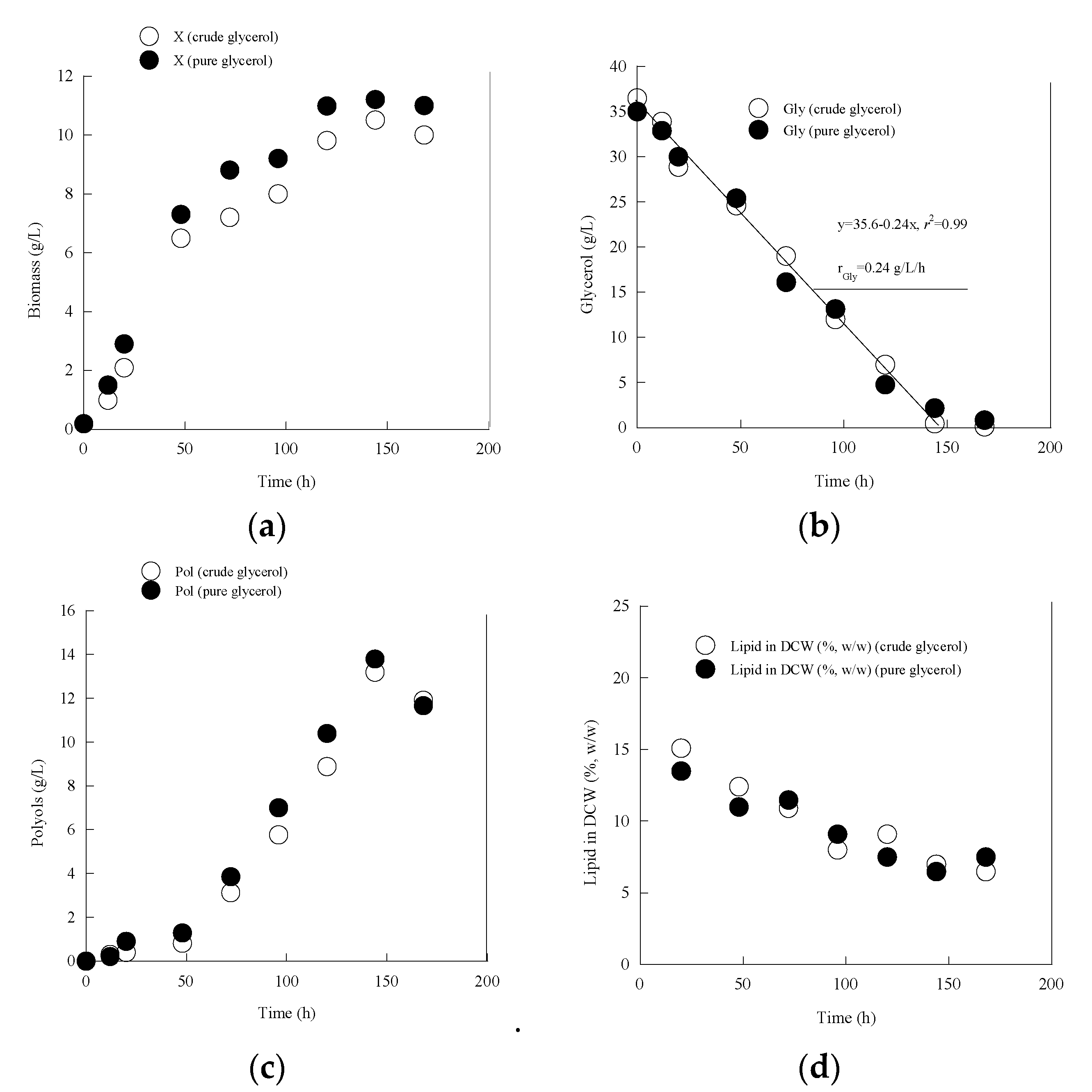
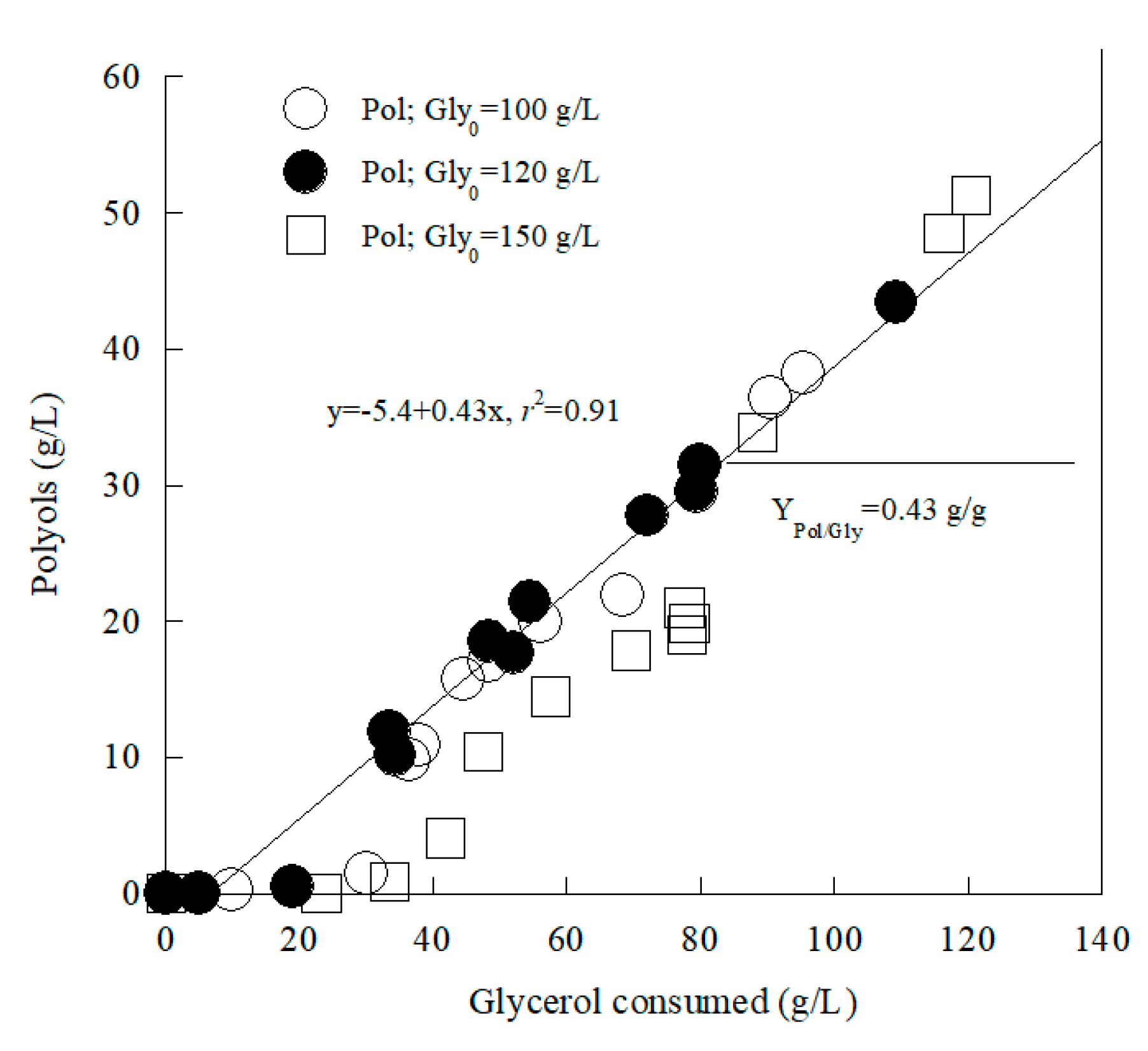

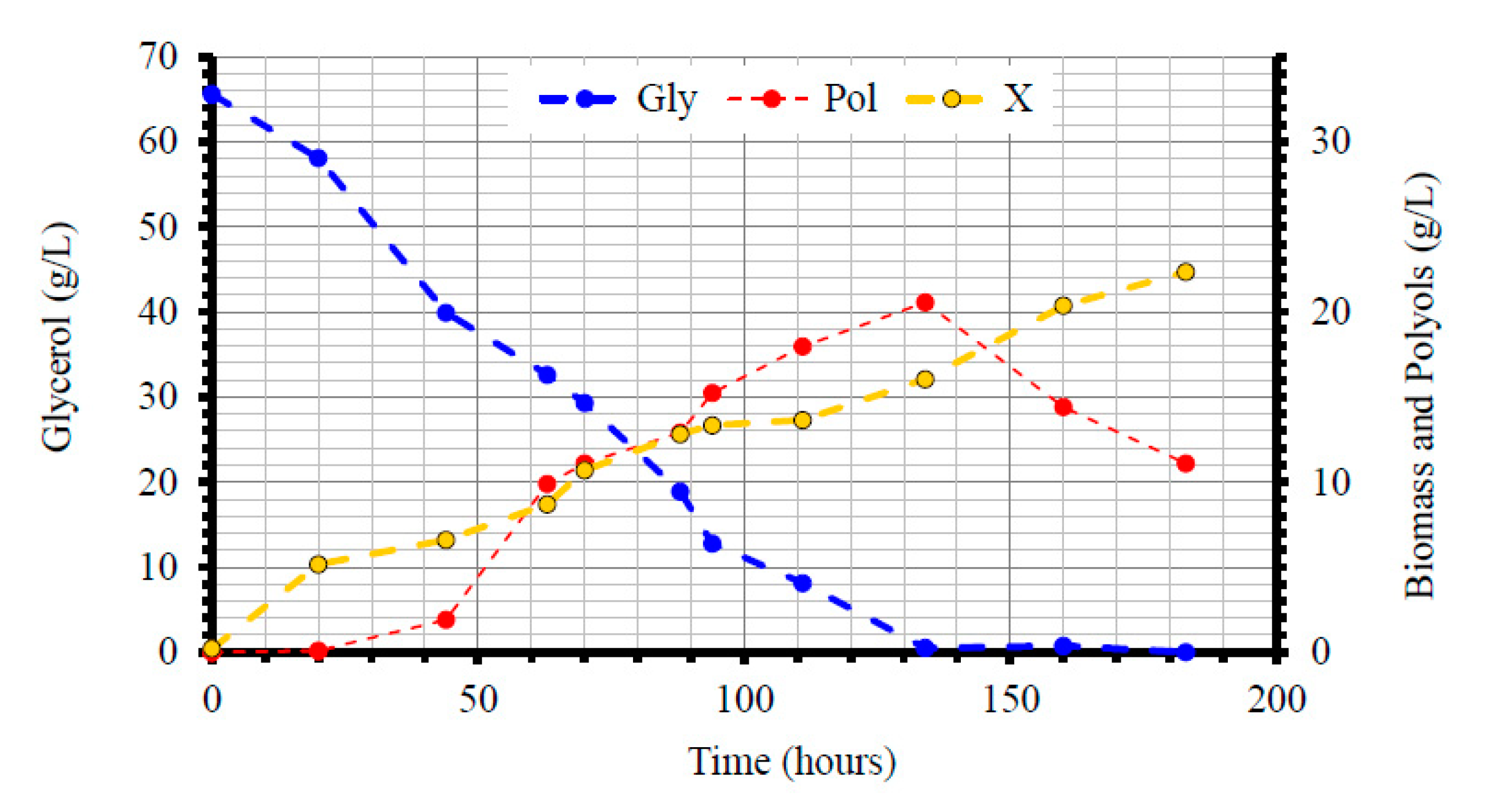
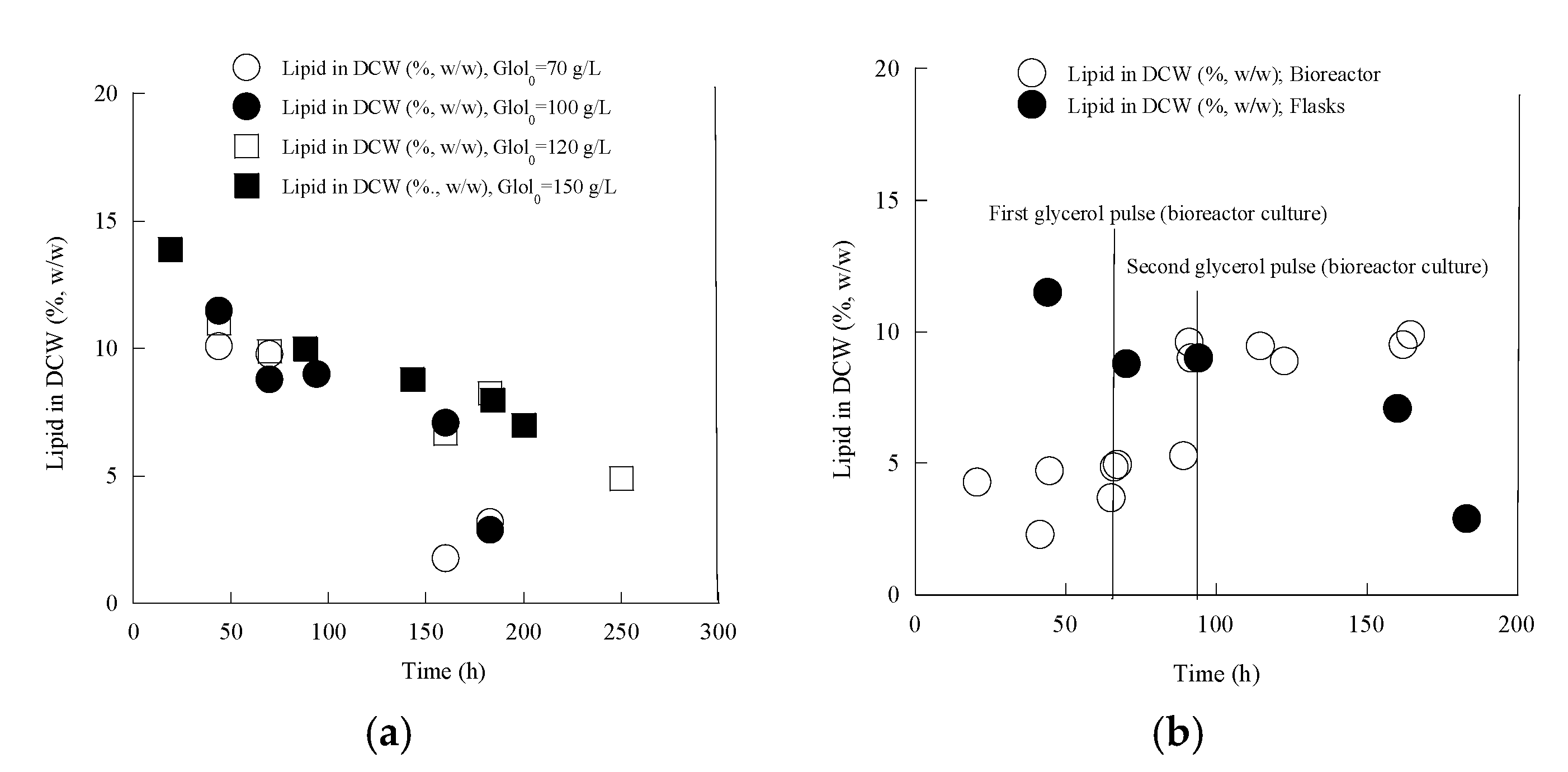
| Gly0 (g/L) | Time (h) | Glycons (g/L) | rGly (g/L/h) | X (g/L) | Ml (g/L) | Ery (g/L) | Ara (g/L) | Pol (g/L) | YPol/Gly (g/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| ≈70 | a, b, c | 134 | 65.0 ± 4.5 | 0.54 | 16.1 ± 1.5 | 9.2 ± 1.4 | 7.3 ± 1.2 | 4.1 ± 0.7 | 20.6 ± 3.3 | 0.32 |
| d | 183 | 65.6 ± 3.9 | 22.4 ± 2.0 | 6.1 ± 0.8 | 3.1 ± 0.6 | 1.9 ± 0.4 | 11.1 ± 1.8 | 0.17 | ||
| ≈100 | a, b, c, d | 183 | 95.4 ± 7.1 | 0.52 | 15.0 ± 1.9 | 13.8 ± 2.2 | 17.1 ± 2.5 | 7.3 ± 1.2 | 38.2 ± 5.9 | 0.40 |
| ≈120 | a, b, c, d | 250 | 109.2 ± 9.0 | 0.45 | 13.5 ± 2.1 | 15.7 ± 2.4 | 20.6 ± 2.8 | 7.1 ± 1.1 | 43.4 ± 6.3 | 0.40 |
| ≈150 | a, b, c, d | 280 | 120.5 ± 12.8 | 0.37 | 11.1 ± 1.8 | 13.1 ± 2.0 | 24.1 ± 4.0 | 14.1 ± 2.1 | 51.3 ± 8.1 | 0.43 |
| Strain | Ery (g/L) | Ml (g/L) | Ara (g/L) | Pol (g/L) | YPol/Gly (g/g) | Cultivation Type | Reference |
|---|---|---|---|---|---|---|---|
| 1.22 & | 93.5 | 34.0 | - | 127.5 | 0.43 | Fed-batch reactor | Rymowicz et al. [46] |
| Wratislavia 1.31 * | 132.0 | 23.0 | - | 155.0 | 0.52 | Fed-batch reactor | Rymowicz et al. [46] |
| Wratislavia K1 * | 170.0 | 12.0 | - | 182.0 | 0.60 | Fed-batch reactor | Rymowicz et al. [46] |
| CCY-29–26-3 & | 23.0 | 2.6 | 2.3 | 27.9 | 0.40 | Batch flasks | Tomaszewska et al. [29] |
| CCY-29–26-4 & | 26.7 | 1.0 | 2.2 | 29.9 | 0.40 | Batch flasks | Tomaszewska et al. [29] |
| A-15 & | 71.0 | 8.0 | 1.8 | 80.8 | 0.50 | Batch reactor | Tomaszewska et al. [29] |
| A UV’1 * | 63.0 | 8.8 | 9.2 | 81.0 | 0.50 | Batch reactor | Tomaszewska et al. [29]) |
| Wratislavia K1 * | 80.0 | 2.6 | 0.3 | 82.9 | 0.51 | Batch reactor | Tomaszewska et al. [29] |
| Wratislavia K1 * | 135.5 | 3.9 | 0.1 | 139.5 | 0.58 | Repeated-batch reactor | Mirończuk et al. [31] |
| Wratislavia K1 * | 208.0 | 0.2 | - | 208.2 | 0.41 | Repeated-batch reactor | Mirończuk et al. [31] |
| Wratislavia 1.31 * | 26.2 | 16.8 | 3.7 | 46.7 | 0.36 | Batch reactor | Tomaszewska et al. [48] |
| Wratislavia AWG7 * | 25.7 | 17.1 | 2.7 | 45.5 | 0.30 | Batch reactor | Tomaszewska et al. [48] |
| Wratislavia K1 * | 40.7 | 15.1 | 2.9 | 58.7 | 0.40 | Batch reactor | Tomaszewska et al. [48] |
| MK1 * | 79.5 | 2.7 | 0.4 | 82.6 | 0.55 | Batch reactor | Mirończuk et al. [50] |
| MK1 * | 138.8 | 3.3 | 0.3 | 142.4 | 0.69 | Repeated-batch reactor | Mirończuk et al. [50] |
| MK1 * | 177.3 | 2.2 | - | 179.5 | 0.67 | Repeated-batch reactor | Mirończuk et al. [50] |
| CBS10146 ╬ | 44.6 | 5.2 | 10.5 | 60.3 | 0.53 | Batch reactor | Rakicka et al. [51] |
| CBS4855 ╬╬ | 33.4 | 7.6 | 6.8 | 47.8 | 0.43 | Batch reactor | Rakicka et al. [51] |
| CBS11013 ╬╬╬ | 35.4 | 0.6 | 8.9 | 44.9 | 0.41 | Batch reactor | Rakicka et al. [51] |
| FCY 214 * | 79.4 | n.i. | n.i. | 79.4 | 0.48 | Batch reactor | Carly et al. [35] |
| FCY 218 * | 80.6 | n.i. | n.i. | 80.6 | 0.53 | Batch reactor | Carly et al. [35] |
| HA 829 &$ | ≈4 | ≈28 | ≈6 | ≈38 | n.i. | Batch reactor | Egermeier et al. [36] |
| HA 1251 &$ | ≈4 | ≈32 | ≈5 | ≈41 | n.i. | Batch reactor | Egermeier et al. [36] |
| ACA YC 5029 &$ | 33.6 | 28.9 | - | 62.5 | 0.45 | Batch flasks | Papanikolaou et al. [18] |
| ACA YC 5030 &$ | 35.5 | 32.1 | - | 67.6 | 0.49 | Batch flasks | Papanikolaou et al. [18] |
| AIB & | 56.7 | 12.6 | 6.0 | 75.3 | 0.49 | Fed-batch reactor | Rakicka et al. [52] |
| AIB pADUTGUT1 * | 82.2 | 11.0 | 7.5 | 100.7 | 0.67 | Fed-batch reactor | Rakicka et al. [52] |
| ACA-DC 5029 &$ | 65.8 | 6.5 | 3.4 | 75.7 | 0.44 | Batch flasks | Sarris et al. [53] |
| ACA-DC 5029 &$ | 15.6 | 10.5 | 3.4 | 29.5 | 0.39 | Batch flasks | Sarris et al. [49] |
| M53-S * | 69.9 | 10.1 | - | 80.0 | 0.80 | Batch flasks | Liu et al. [54] |
| M53-S * | 72.5 | 10.0 | - | 82.5 | 0.82 | Batch reactor | Liu et al. [54] |
| LMBF Y-46 | 24.1 | 13.1 | 14.1 | 51.3 | 0.43 | Batch flasks | Present study |
| Gly0 (g/L) | Time (h) | Glycons (g/L) | X (g/L) | Ml (g/L) | Ery (g/L) | Ara (g/L) | CA (g/L) | Pol (g/L) | YCA/Gly (g/g) | YPol/Gly (g/g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≈100 Flasks | a, c | 183 | 95.4 ± 7.1 | 15.0 ± 1.9 | 13.8 ± 2.2 | 17.1 ± 2.5 | 7.3 ± 1.2 | - | 38.2 ± 5.9 | - | 0.40 |
| ≈100 | a, c | 41.5 | 86.8 ± 6.1 | 21.0 ± 3.1 | 3.0 ± 0.5 | - | - | 20.4 ± 3.3 | 3.0 ± 0.5 | 0.24 | 0.03 |
| Bioreactor | b | 65 | 103.5 ± 9.9 | 19.0 ± 3.4 | 0.5 ± 0.1 | - | - | 42.4 ± 5.5 | 0.5 ± 0.1 | 0.41 | 0.004 |
| Strain | Citric Acid (g/L) | Substrate | Yield (g/g) | Bioreactor Configuration | Reference |
|---|---|---|---|---|---|
| NRRL Y-7576 $ | 51.5 | Glucose | 0.71 | Fed-batch | Klasson et al. [69] |
| Y-1095 $ | 78.5 | Glucose | 0.79 | Fed-batch | Rane and Sims [70] |
| ATCC 20346 | 69.0 | Glucose | 0.52 | Fed-batch | Moresi [68] |
| N1 * | 120.0 | Ethanol | 0.85 | Fed-batch | Kamzolova et al. [71] |
| 187/1 $ | 135.1 | Rapeseed oil | 1.55 | Fed-batch | Kamzolova et al. [56] |
| Wratislavia AWG7 * | 88.1 | Crude glycerol | 0.46 | Batch | Rymowicz et al. [44] |
| Wratislavia K1 * | 75.7 | Crude glycerol | 0.40 | Batch | Rymowicz et al. [44] |
| H222-S4(p67ICL1)T5 * | 91.0 & | Sucrose | 0.53 | Fed-batch | Förster et al. [57] |
| H222-S4(p67ICL1)T5 * | 133.0 & | Sucrose | 0.78 | Fed-batch | Förster et al. [57] |
| A-101–1.22 * | 119.1 & | Crude glycerol | 0.64 | Fed-batch | Rymowicz et al. [47] |
| A-101–1.22 * | 115.6 & | Crude glycerol | 0.68 | Repeated batch | Rymowicz et al. [47] |
| A-101 $ | 69.3 | Glucose | 0.45 | Batch | Rywińska et al. [55] |
| A-101 $ | 66.5 | Pure glycerol | 0.44 | Batch | Rywińska et al. [55] |
| A-101 $ | 66.8 | Crude glycerol | 0.43 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 76.4 | Glucose | 0.52 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 63.9 | Pure glycerol | 0.40 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 82.0 | Crude glycerol | 0.53 | Batch | Rywińska et al. [55] |
| Wratislavia 1.31 * | 126.0 & | Crude glycerol | 0.63 | Fed-batch | Rywińska et al. [72] |
| Wratislavia AWG7 * | 157.5 & | Crude glycerol | 0.58 | Fed-batch | Rywińska et al. [72] |
| Wratislavia AWG7 * | 160.5 & | Crude glycerol | 0.81 | Repeated batch | Rywińska and Rymowicz [73] |
| Wratislavia 1.31 * | 124.9 & | Crude glycerol | 0.59 | Repeated batch | Rywińska and Rymowicz [73] |
| N15$ | 98.0 | Pure glycerol | 0.70 | Fed-batch | Kamzolova et al. [61] |
| NG40/UV7 * | 175.0 | Rapeseed oil | 1.50 | Fed-batch | Kamzolova et al. [74] |
| Wratislavia AWG7 * | 63.3 | Pure glycerol | 0.67 | Continuous | Rywińska et al. [60] |
| Wratislavia 1.31 * | 92.8 | Pure glycerol | 0.63 | Batch | Rywińska et al. [66] |
| SWJ-1b * | 84.0 | Inulin | 0.89 | Batch | Liu et al. [75] |
| NG40/UV7 * | 115.0 | Pure glycerol | 0.64 | Fed-batch | Morgunov et al. [59] |
| NG40/UV7 * | 112.0 | Crude glycerol | 0.90 | Fed-batch | Morgunov et al. [59] |
| Wratislavia AWG7 * | 85.7 | Crude glycerol | 0.52 | Batch | Tomaszewska et al. [48] |
| Wratislavia K1 * | 65.0 | Crude glycerol | 0.43 | Batch | Tomaszewska et al. [48] |
| SWJ-1b * | 101.6 | Glucose | 0.89 | Fed-batch | Tan et al. [67] |
| CBS 6114 $ | ≈55 & | Pure glycerol | n.i. | Batch | Egermeier et al. [36] |
| H222 $ | ≈50 & | Pure glycerol | n.i. | Batch | Egermeier et al. [36] |
| DSM 1345 $ | ≈52 & | Pure glycerol | n.i. | Batch | Egermeier et al. [36] |
| VKM Y-2373 $ | 80–85 | Glucose | 0.70–0.75 | Batch | Kamzolova and Morgunov [76] |
| ACA YC 5029 $ | 39.0 | Crude glycerol | 0.42 | Batch | Papanikolaou et al. [18] |
| NG40/UV5 * | 140.0 | Rapeseed oil | 1.50 | Fed-batch | Morgunov et al. [77] |
| NG40/UV5 * | 108.8 | Glucose | 0.80 | Fed-batch | Morgunov et al. [77] |
| NG40/UV5 * | 87.0 | Crude glycerol | 0.64 | Fed-batch | Morgunov et al. [77] |
| K57 $ | 72.1 | Glucose | 0.77 | Batch | Carsanba et al. [62] |
| AJD pADUTGut 1/2 * | 75.9 && | Crude glycerol | 0.51 | Batch | Rzechonek et al. [63] |
| LMBF Y-46 $ | 42.4 & | Pure glycerol | 0.41 | Batch | Present study |
| LMBF Y-46 $ | 101.3 & | Pure glycerol | 0.46 | Fed-batch | Present study |
| Equation | Number of Data Points | Glycerol Concentration (g/L) | |||
|---|---|---|---|---|---|
| 100 | 120 | 150 | |||
| 11 | 12 | 13 | |||
| 1 | μmax (h−1) | 0.0296 | 0.0252 | 0.0350 | |
| Xmax (gX/L) | 20.63 | 16.94 | 10.62 | ||
| 2 | X at t = 0 (gX/L) | 2.26 | 2.22 | 2.23 | |
| SSE | 13.09 | 9.48 | 8.03 | ||
| R2 | 0.942 | 0.937 | 0.935 | ||
| 3 | qPolmax (gPol/(gXh)) | 0.0415 | 0.0420 | 0.0251 | |
| Polmax (gPol/L) | 40.14 | 48.86 | 162.87 | ||
| 4 | SSE | 127.02 | 59.43 | 68.33 | |
| R2 | 0.951 | 0.974 | 0.981 | ||
| 5 | YX/Gly (gX/gGlyc) | 0.2160 | 0.2749 | 0.1210 | |
| YPol/Gly (gPol/gGlyc) | 0.9997 | 0.6575 | 1.0586 | ||
| SSE | 445.2 | 187.1 | 1027.8 | ||
| R2 | 0.954 | 0.984 | 0.956 | ||
| Culture Type/Time | Fatty Acid Composition of Yeast Lipids (%, w/w) | |||||||||
| Shake-Flasks | ≤C12:0 * | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C20:0 | ≥C22:0 $ | Other × |
| Gly0 ≈ 70 g/L, t ≈ 180 h | 2.5 ± 0.3 | 2.3 ± 0.4 | 21.1 ± 1.7 | 5.1 ± 0.6 | 15.6 ± 2.0 | 30.2 ± 3.5 | 11.5 ± 1.3 | 0.8 ± 0.1 | 2.2 ± 0.3 | 8.7 ± 0.9 |
| Gly0 ≈ 100 g/L, t ≈ 160 h | 0.8 ± 0.1 | 0.7 ± 0.1 | 22.2 ± 3.5 | 5.5 ± 0.4 | 18.5 ± 2.4 | 32.3 ± 4.0 | 12.2 ± 2.0 | 1.2 ± 0.2 | 2.5 ± 0.4 | 4.1 ± 0.3 |
| Gly0 ≈ 100 g/L, t ≈ 180 h | 1.3 ± 0.2 | 0.9 ± 0.2 | 17.5 ± 2.5 | 3.6 ± 0.5 | 25.3 ± 2.4 | 28.4 ± 4.1 | 8.8 ± 1.0 | 5.9 ± 1.1 | 3.0 ± 0.4 | 5.3 ± 0.6 |
| Gly0 ≈ 120 g/L, t ≈ 160 h | 1.8 ± 0.3 | 1.0 ± 0.1 | 19.9 ± 2.0 | 4.6 ± 0.7 | 18.9 ± 3.0 | 30.8 ± 5.0 | 9.0 ± 1.5 | 4.2 ± 0.9 | 2.0 ± 0.3 | 7.8 ± 1.4 |
| Gly0 ≈ 120 g/L, t ≈ 190 h | 0.2 ± 0.1 | 0.4 ± 0.1 | 21.7 ± 2.4 | 6.6 ± 0.8 | 15.9 ± 2.5 | 36.2 ± 5.2 | 12.8 ± 2.0 | 1.1 ± 0.1 | 2.9 ± 0.5 | 2.2 ± 0.4 |
| Gly0 ≈ 150 g/L, t ≈ 190 h | 3.9 ± 0.2 | 2.4 ± 0.2 | 19.5 ± 1.9 | 5.1 ± 0.4 | 18.0 ± 3.2 | 30.0 ± 5.0 | 10.7 ± 1.8 | 2.0 ± 0.2 | 2.0 ± 0.4 | 6.4 ± 0.9 |
| Culture Type/Time | Fatty Acid Composition of Yeast Lipids (%, w/w) | |||||||||
| Bioreactor | ≤C12:0 * | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C20:0 | ≥C22:0 $ | Other × |
| t = 20.5 h | 1.9 ± 0.4 | 3.0 ± 0.4 | 21.1 ± 2.5 | 7.7 ± 0.8 | 17.0 ± 1.8 | 30.5 ± 3.9 | 17.7 ± 2.6 | - | - | 1.1 ± 0.3 |
| t = 41.5 h | - | 1.2 ± 0.2 | 18.4 ± 2.0 | 6.8 ± 0.9 | 12.0 ± 2.0 | 37.3 ± 4.0 | 16.2 ± 3.0 | - | 3.0 ± 0.4 | 5.1 ± 0.3 |
| t = 44.5 h | 1.5 ± 0.4 | 0.7 ± 0.3 | 16.5 ± 2.0 | 8.7 ± 1.0 | 7.7 ± 1.0 | 39.0 ± 5.1 | 15.4 ± 2.9 | 0.5 ± 0.1 | 2.6 ± 0.4 | 7.4 ± 1.2 |
| t = 66.0 h | 2.5 ± 0.2 | 1.2 ± 0.2 | 16.4 ± 2.1 | 7.3 ± 0.9 | 9.2 ± 1.4 | 36.2 ± 6.1 | 14.9 ± 3.0 | 0.5 ± 0.2 | 2.2 ± 0.4 | 9.9 ± 1.5 |
| t = 67.0 h | 1.7 ± 0.1 | 0.8 ± 0.1 | 16.3 ± 2.2 | 8.1 ± 1.2 | 8.1 ± 1.3 | 39.7 ± 5.8 | 16.7 ± 3.1 | 0.4 ± 0.1 | 2.8 ± 0.3 | 5.4 ± 0.9 |
| t = 89.0 h | 0.7 ± 0.1 | 0.6 ± 0.1 | 16.7 ± 2.3 | 9.0 ± 1.4 | 8.1 ± 1.6 | 40.3 ± 6.0 | 16.1 ± 3.2 | 0.4 ± 0.1 | 2.7 ± 0.3 | 5.4 ± 1.0 |
| t = 91.0 h | 2.2 ± 0.3 | 1.1 ± 0.2 | 17.0 ± 1.9 | 8.4 ± 1.7 | 8.4 ± 1.5 | 37.4 ± 5.1 | 16.2 ± 3.0 | 0.3 ± 0.1 | 1.5 ± 0.2 | 7.5 ± 1.3 |
| t = 97.0 h | 0.9 ± 0.1 | 0.7 ± 0.2 | 16.3 ± 2.5 | 8.5 ± 1.8 | 8.0 ± 1.6 | 40.2 ± 6.1 | 16.1 ± 2.7 | 0.5 ± 0.2 | 2.8 ± 0.3 | 6.0 ± 1.0 |
| t = 122.5 h | 0.9 ± 0.1 | 0.5 ± 0.2 | 16.1 ± 2.0 | 9.5 ± 1.8 | 7.0 ± 1.0 | 41.1 ± 4.9 | 16.9 ± 2.0 | 0.7 ± 0.2 | 2.5 ± 0.3 | 4.8 ± 1.2 |
| t = 164.0 h | 1.1 ± 0.2 | 0.8 ± 0.4 | 15.0 ± 1.9 | 9.5 ± 1.5 | 6.0 ± 1.0 | 43.0 ± 4.4 | 17.9 ± 3.1 | 0.8 ± 0.1 | 1.9 ± 0.3 | 4.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Appl. Sci. 2020, 10, 7373. https://doi.org/10.3390/app10207373
Papanikolaou S, Diamantopoulou P, Blanchard F, Lambrinea E, Chevalot I, Stoforos NG, Rondags E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Applied Sciences. 2020; 10(20):7373. https://doi.org/10.3390/app10207373
Chicago/Turabian StylePapanikolaou, Seraphim, Panagiota Diamantopoulou, Fabrice Blanchard, Eleni Lambrinea, Isabelle Chevalot, Nikolaos G. Stoforos, and Emmanuel Rondags. 2020. "Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production" Applied Sciences 10, no. 20: 7373. https://doi.org/10.3390/app10207373
APA StylePapanikolaou, S., Diamantopoulou, P., Blanchard, F., Lambrinea, E., Chevalot, I., Stoforos, N. G., & Rondags, E. (2020). Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Applied Sciences, 10(20), 7373. https://doi.org/10.3390/app10207373








